Targeting Adenosine Signalling in Knee Chondropathy: The Combined Action of Polydeoxyribonucleotide and Pulsed Electromagnetic Fields: A Current Concept Review
Abstract
1. Introduction
2. Methods
3. Results
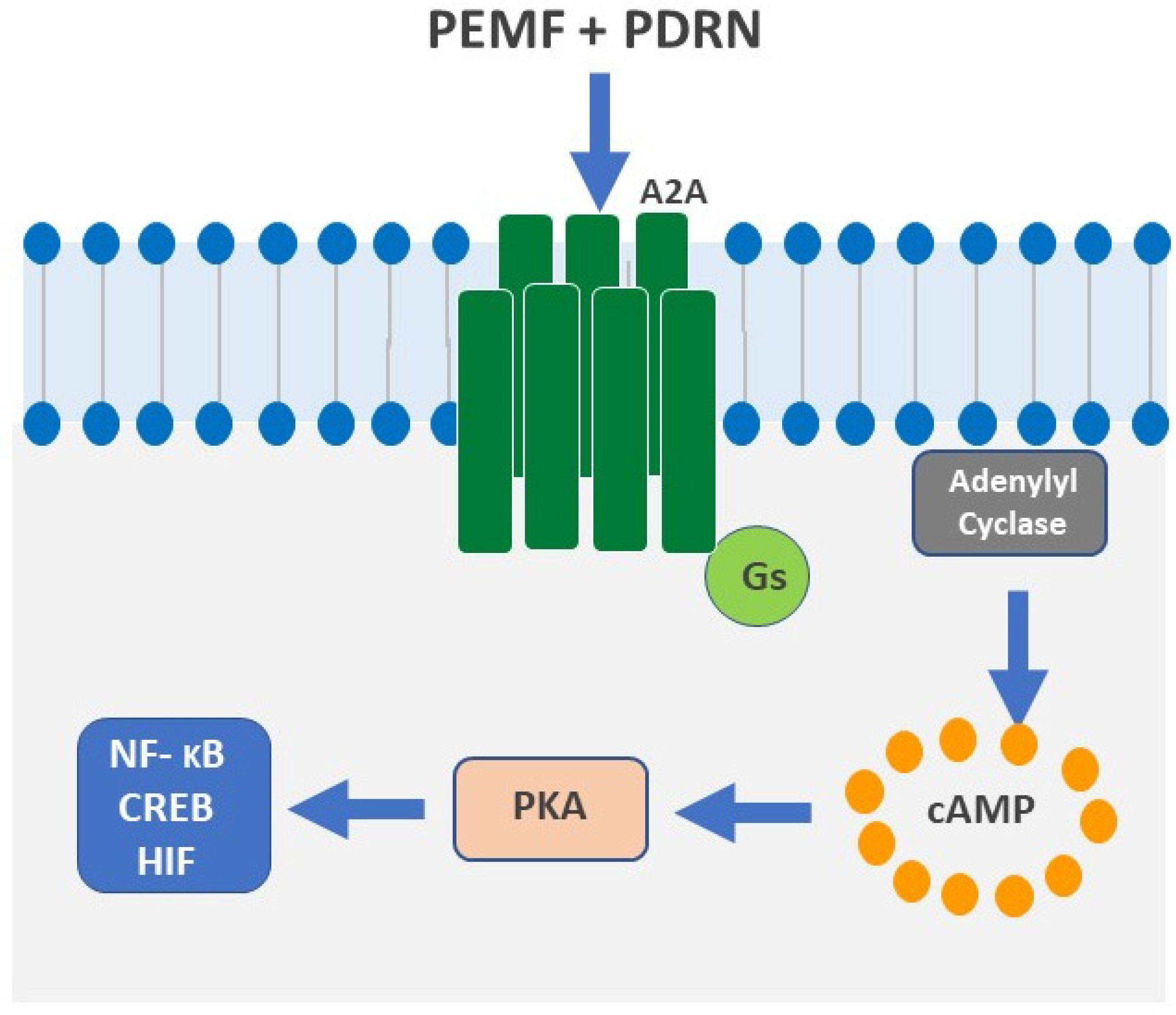
3.1. A2A Receptors
3.2. Polydeoxiriboneucleotides
PDRN in Chondropathy
3.3. PEMF
3.4. PEMF in Chondropathy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legré-Boyer, V. Viscosupplementation: Techniques, indications, results. Orthop. Traumatol. Surg. Res. 2015, 101 (Supp. 1), S101–S108. [Google Scholar] [CrossRef]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Giavaresi, G.; Torricelli, P.; Cavani, F.; Setti, S.; Canè, V.; Giardino, R. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J. Orthop. Res. 2005, 23, 899–908. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a multi-signalling guardian angel in human diseases: When, where and how does it exert its protective effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Fini, M.; Pagani, S.; Giavaresi, G.; De Mattei, M.; Ongaro, A.; Varani, K.; Vincenzi, F.; Massari, L.; Cadossi, M. Functional tissue engineering in articular cartilage repair: Is there a role for electromagnetic biophysical stimulation? Tissue Eng. Part B Rev. 2013, 19, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Capone, F.; Apollonio, F.; Borea, P.A.; Cadossi, R.; Fassina, L.; Grassi, C.; Liberti, M.; Paffi, A.; Parazzini, M.; et al. A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul. 2013, 6, 469–476. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadoss, R. Adenosine receptors as a biological pathway for the anti-inflammatory and beneficial effects of low-frequency low energy pulsed electromagnetic fields. Mediat. Inflamm. 2017, 2017, 27440963. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; De Mattei, M.; Vincenzi, F.; Gessi, S.; Merighi, S.; Pellati, A.; Ongaro, A.; Caruso, A.; Cadossi, R.; Borea, P.A. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthr. Cartil. 2008, 16, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Gessi, S.; Merighi, S.; Iannotta, V.; Cattabriga, E.; Spisani, S.; Cadossi, R.; Borea, P.A. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br. J. Pharmacol. 2002, 136, 57–66. [Google Scholar] [CrossRef]
- Bizzoca, D.; Rocchetti, M.T.; Scacco, S.; Taurino, F.; Vicenti, G.; Spadaccino, F.; Moretti, L.; Ranieri, E.; Gesualdo, L.; Moretti, F.; et al. Beyond pre-analytical and analytical concerns in the study of synovial fluid proteome: Description of an optimized Gel-based protocol. J. Biol. Regul. Homeost. Agents 2021, 35, 827–832. [Google Scholar] [CrossRef]
- De Mattei, M.; Varani, K.; Masieri, F.F.; Pellati, A.; Ongaro, A.; Fini, M.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Caruso, A. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthr. Cartil. 2009, 17, 252–262. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; Masieri, F.F.; Caruso, A.; Setti, S.; Cadossi, R.; Biscione, R.; Massari, L.; Fini, M.; De Mattei, M. Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics 2011, 32, 543–551. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Desai, A.; Chen, J.-F.; Yee, H.; Schwarzschild, M.A.; Fink, J.S.; Cronstein, B.N. Adenosine Promotes Wound Healing and Mediates Angiogenesis in Response to Tissue Injury Via Occupancy of A2A Receptors. Am. J. Pathol. 2002, 160, 2009–2018. [Google Scholar] [CrossRef]
- Effect of Polydeoxyribonucleotides on Human Fibroblasts in Primary Culture-Sini-1999-Cell Biochemistry and Function—Wiley Online Library. Available online: https://onlinelbrary.wiley.com/doi/abs/10.1002/%28SICI%291099-4540844%28199906%2917%3A2%3C107%3A%3AAID-CBF815%3E3.0.CO%3B2-%23 (accessed on 25 October 2022).
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) Promotes Human Osteoblast Proliferation: A New Proposal for Bone Tissue Repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective Effects of Polydeoxyribonucleotides on Cartilage Degradation in Experimental Cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, A.; Palmieri, A.; Carinci, F.; Trapella, G.; Sollazzo, V.; Lauritano, D. Effects of Glucosamine and Nucleotide Association on Fibroblast: Extracellular Matrix Gene Expression. Int. J. Immunopathol. Pharmacol. 2014, 27, 689–693. [Google Scholar] [CrossRef]
- Bitto, A.; Polito, F.; Altavilla, D.; Minutoli, L.; Migliorato, A.; Squadrito, F. Polydeoxyribonucleotide (PDRN) Restores Blood Flow in an Experimental Model of Peripheral Artery Occlusive Disease. J. Vasc. Surg. 2008, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine Receptor Stimulation by Polynucleotides (PDRN) Reduces Inflammation in Experimental Periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef]
- Minutoli, L.; Arena, S.; Antonuccio, P.; Romeo, C.; Bitto, A.; Magno, C.; Rinaldi, M.; Micali, A.; Irrera, N.; Pizzino, G.; et al. Role of Inhibitors of Apoptosis Proteins in Testicular Function and Male Fertility: Effects of Polydeoxyribonucleotide Administration in Experimental Varicocele. Biomed. Res. Int. 2015, 2015, 248976. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Minutoli, L.; Arena, F.; Nicotina, P.A.; Romeo, C.; Squadrito, F.; Altavilla, D.; Morgia, G.; Magno, C. Polydeoxyribonucleotide Administration Improves the Intra-Testicular Vascularization in Rat Experimental Varicocele. Fertil. Steril. 2012, 97, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.K.; Jang, H.J.; Kim, S.S.; Lee, S.Y.; Oh, M.Y.; Kim, H.J.; Eom, D.W.; Ham, J.Y.; Han, D.J. Protective Effect of Polydeoxyribonucleotide Against Renal Ischemia-Reperfusion Injury in Mice. Transplant. Proc. 2016, 48, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide Stimulates Angiogenesis and Wound Healing in the Genetically Diabetic Mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rubegni, P.; De Aloe, G.; Mazzatenta, C.; Cattarini, L.; Fimiani, M. Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants. A Pilot Study. Curr. Med. Res. Opin. 2001, 17, 128–131. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surg. Sport. Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; In, Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Dante, D.; Giacomo, S.; Nicolandrea, D.P.; Chiara, C.; Francesca, V.; Paola, T.; Milena, F. Efficacy of intra-articular polynucleotides associated with hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Clin. J. Sport Med. 2018, 30, 1. [Google Scholar]
- Zazgyva, A.; Gergely, I.; Russu, O.; Roman, C.; Pop, T. Polynucleotides versus sodium hyaluronate in the local treatment of knee osteoarthritis. Acta Med. Transilv. 2013, 2, 260–263. [Google Scholar]
- Moretti, L.; Bizzoca, D.; Geronimo, A.; Moretti, F.L.; Monaco, E.; Solarino, G.; Moretti, B. Towards Precision Medicine for Osteoarthritis: Focus on the Synovial Fluid Proteome. Int. J. Mol. Sci. 2022, 23, 9731. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Vicenti, G.; Bizzoca, D.; Nappi, V.S.; Moretti, F.; Carrozzo, M.; Belviso, V.; Moretti, B. Biophysical stimulation of the knee with PEMFs: From bench to bedside. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 1), 23–28. [Google Scholar]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e1900155. [Google Scholar] [CrossRef]
- Pasek, J.; Pasek, T.; Sieroń-Stołtny, K.; Cieślar, G.; Sieroń, A. Electromagnetic fields in medicine—The state of art. Electromagn. Biol. Med. 2015, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A. Biolectromagnetics in the service of medicine: Acceptance speech on the occasion of receiving the d’Arsonval Medal. Biolelectromagnetics 1992, 13, 7–17. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fróhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef] [PubMed]
- De Mattei, M.; Caruso, A.; Pezzetti, F.; Pellati, A.; Stabellini, G.; Sollazzo, V.; Traina, G.C. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect. Tissue Res. 2001, 42, 269–279. [Google Scholar] [CrossRef]
- Nicolin, V.; Ponti, C.; Baldini, G.; Gibellini, D.; Bortul, R.; Zweyer, M.; Martinelli, B.; Narducci, P. In vitro exposure of human chondrocytes to pulsed electromagnetic fields. Eur. J. Histochem. 2007, 51, 203–212. [Google Scholar] [PubMed]
- Chang, C.H.; Loo, S.T.; Liu, H.L. Can low frequency electromagnetic field help cartilage tissue engineering? J. Biomed. Mater. Res. A 2010, 92, 843–851. [Google Scholar] [CrossRef]
- Aaron, R.K.; Boyan, B.D.; Ciombor, D.M.; Schwartz, Z.; Simon, B.J. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin. Orthop. Relat. Res. 2004, 419, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, D.M.; Lester, G.; Aaron, R.K.; Neame, P.; Caterson, B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J. Orthop. Res. 2002, 20, 40–50. [Google Scholar] [CrossRef]
- Schmidt-Rohlfing, B.; Silny, J.; Woodruff, S.; Gavenis, K. Effects of pulsed and sinusoid electromagnetic fields on human chondrocytes cultivated in a collagen matrix. Rheumatol. Int. 2008, 28, 971–977. [Google Scholar] [CrossRef]
- Veronesi, F.; Torricelli, P.; Giavaresi, G.; Sartori, M.; Cavani, F.; Setti, S.; Cadossi, M.; Ongaro, A.; Fini, M. In vivo effect of two different pulsed electromagnetic field frequencies on osteoarthritis. J. Orthop. Res. 2014, 32, 677–685. [Google Scholar] [CrossRef]
- Veronesi, F.; Cadossi, M.; Giavaresi, G.; Martini, L.; Setti, S.; Buda, R.; Giannini, S.; Fini, M. Pulsed electromagnetic fields combined with a collagenous scaffold and bone marrow concentrate enhance osteochondral regeneration: An in vivo study. BMC Musculoskelet. Disord. 2015, 16, 233. [Google Scholar] [CrossRef]
- Adravanti, P.; Nicoletti, S.; Setti, S.; Ampollini, A.; de Girolamo, L. Effect of pulsed electromagnetic field therapy in patients undergoing total knee arthroplasty: A randomised controlled trial. Int. Orthop. 2014, 38, 397–403. [Google Scholar] [CrossRef]
- Bizzoca, D.; Brunetti, G.; Moretti, L.; Piazzolla, A.; Vicenti, G.; Moretti, F.L.; Solarino, G.; Moretti, B. Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4582. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Bizzoca, D.; Moretti, L.; Gnoni, A.; Moretti, F.L.; Scacco, S.; Banfi, G.; Piazzolla, A.; Solarino, G.; Moretti, B. The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. J. Funct. Morphol. Kinesiol. 2022, 7, 97. [Google Scholar] [CrossRef]
- Mandelbaum, B.T.; Browne, J.E.; Fu, F.; Micheli, J.; Mosley, J.B.; Erggelet, C.; Anderson, A.F. Articular Cartilage lesions of the knee. Am. J. Sport. Med. 2000, 26, 853–861. [Google Scholar] [CrossRef]
- Vavken, P.; Arrich, F.; Schuhfried, O.; Dorotka, R. Effectiveness of pulsed electromagnetic field therapy in the management of osteoarthritis of the knee: A meta-analysis of randomized controlled trials. J. Rehabil. Med. 2009, 41, 406–411. [Google Scholar] [CrossRef]
- Gobbi, A.; Lad, D.; Petrera, M.; Karnatzikos, G. Symptomatic Early Osteoarthritis of the Knee Treated with Pulsed Electromagnetic Fields: Two-Year Follow-up. Cartilage 2014, 5, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Gobbi, A.; Nakamura, N.; Peretti, G.M. Stem Cells for Cartilage Regeneration: A Roadmap to the Clinic. Stem Cells Int. 2018, 2018, 7348560. [Google Scholar] [CrossRef] [PubMed]
- Servodio Iammarrone, C.; Cadossi, M.; Sambri, A.; Grosso, E.; Corrado, B.; Servodio Iammarrone, F. Is there a role of pulsed electromagnetic fields in management of patellofemoral pain syndrome? Randomized controlled study at one year follow-up. Bioelectromagnetics 2016, 37, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Marcheggiani Muccioli, G.M.; Grassi, A.; Setti, S.; Filardo, G.; Zambelli, L.; Bonanzinga, T.; Rimondi, E.; Busacca, M.; Zaffagnini, S. Conservative treatment of spontaneous osteonecrosis of the knee in the early stage: Pulsed electromagnetic fields therapy. Eur. J. Radiol. 2013, 82, 530–537. [Google Scholar] [CrossRef]
- Collarile, M.; Sambri, A.; Lullini, G.; Cadossi, M.; Zorzi, C. Biophysical stimulation improves clinical results of matrix-assisted autologous chondrocyte implantation in the treatment of chondral lesions of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Cadossi, M.; Buda, R.E.; Ramponi, L.; Sambri, A.; Natali, S.; Giannini, S. Bone marrow-derived cells and biophysical stimulation for talar osteochondral lesions: A randomized controlled study. Foot Ankle Int. 2014, 35, 981–987. [Google Scholar] [CrossRef]
- Benazzo, F.; Zanon, G.; Pederzini, L.; Modonesi, F.; Cardile, C.; Falez, F.; Ciolli, L.; La Cava, F.; Giannini, S.; Buda, R.; et al. Effects of biophysical stimulation in patients undergoing arthroscopic reconstruction of anterior cruciate ligament: Prospective, randomized and double-blind study. Knee Surg. Sport. Traumatol. Arthrosc. 2008, 16, 595–601. [Google Scholar] [CrossRef]
- Gremion, G.; Gaillard, D.; Leyvraz, P.F.; Jolles, B.M. Effect of biomagnetic therapy versus physiotherapy for treatment of knee osteoarthritis: A randomized controlled trial. J. Rehabil. Med. 2009, 41, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Ozgüçlü, E.; Cetin, A.; Cetin, M.; Calp, E. Additional effect of pulsed electromagnetic field therapy on knee osteoarthritis treatment: A randomized, placebo-controlled study. Clin. Rheumatol. 2010, 29, 927–931. [Google Scholar] [CrossRef]
- Nelson, F.R.; Zvirbulis, R.; Pilla, A.A. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: A randomized double-blind pilot study. Rheumatol. Int. 2013, 33, 2169–2173. [Google Scholar] [CrossRef]
- Bagnato, G.L.; Miceli, G.; Marino, N.; Sciortino, D.; Bagnato, G.F. Pulsed electromagnetic fields in knee osteoarthritis: A double blind, placebo-controlled, randomized clinical trial. Rheumatol. Oxf. 2016, 55, 755–762. [Google Scholar] [CrossRef]
- Wuschech, H.; von Hehn, U.; Mikus, E.; Funk, R.H. Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics 2015, 36, 576–585. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Solarino, G.; Moretti, F.; Ottaviani, G.; Simone, F.; Zavattini, G.; Maccagnano, G.; Noia, G.; Moretti, B. The role of biophysical stimulation with pemfs in fracture healing: From bench to bedside. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 1), 131–135. [Google Scholar] [PubMed]
- Moretti, L.; Bizzoca, D.; Giancaspro, G.A.; Cassano, G.D.; Moretti, F.; Setti, S.; Moretti, B. Biophysical Stimulation in Athletes’ Joint Degeneration: A Narrative Review. Medicina 2021, 57, 1206. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H. Health effects of electromagnetic fields on children. Clin. Exp. Pediatr. 2020, 63, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Bizzoca, D.; Moretti, L.; Ragni, E.; Moretti, F.L.; Vicenti, G.; Solarino, G.; Rizzello, A.; Petruzzella, V.; Palese, L.L.; et al. A Gel-Based Proteomic Analysis Reveals Synovial α-Enolase and Fibrinogen β-Chain Dysregulation in Knee Osteoarthritis: A Controlled Trial. J. Pers. Med. 2023, 13, 916. [Google Scholar] [CrossRef]
- Bizzoca, D.; Vicenti, G.; Solarino, G.; Moretti, F.; Gnoni, A.; Maccagnano, G.; Noia, G.; Moretti, B. Gut microbiota and osteoarthritis: A deep insight into a new vision of the disease. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 1), 51–55. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, L.; Bizzoca, D.; Geronimo, A.; Abbaticchio, A.M.; Moretti, F.L.; Carlet, A.; Fischetti, F.; Moretti, B. Targeting Adenosine Signalling in Knee Chondropathy: The Combined Action of Polydeoxyribonucleotide and Pulsed Electromagnetic Fields: A Current Concept Review. Int. J. Mol. Sci. 2023, 24, 10090. https://doi.org/10.3390/ijms241210090
Moretti L, Bizzoca D, Geronimo A, Abbaticchio AM, Moretti FL, Carlet A, Fischetti F, Moretti B. Targeting Adenosine Signalling in Knee Chondropathy: The Combined Action of Polydeoxyribonucleotide and Pulsed Electromagnetic Fields: A Current Concept Review. International Journal of Molecular Sciences. 2023; 24(12):10090. https://doi.org/10.3390/ijms241210090
Chicago/Turabian StyleMoretti, Lorenzo, Davide Bizzoca, Alessandro Geronimo, Andrea Michele Abbaticchio, Francesco Luca Moretti, Arianna Carlet, Francesco Fischetti, and Biagio Moretti. 2023. "Targeting Adenosine Signalling in Knee Chondropathy: The Combined Action of Polydeoxyribonucleotide and Pulsed Electromagnetic Fields: A Current Concept Review" International Journal of Molecular Sciences 24, no. 12: 10090. https://doi.org/10.3390/ijms241210090
APA StyleMoretti, L., Bizzoca, D., Geronimo, A., Abbaticchio, A. M., Moretti, F. L., Carlet, A., Fischetti, F., & Moretti, B. (2023). Targeting Adenosine Signalling in Knee Chondropathy: The Combined Action of Polydeoxyribonucleotide and Pulsed Electromagnetic Fields: A Current Concept Review. International Journal of Molecular Sciences, 24(12), 10090. https://doi.org/10.3390/ijms241210090






