Excitation-Inhibition Imbalance in Migraine: From Neurotransmitters to Brain Oscillations
Abstract
:1. Introduction
1.1. Background
1.1.1. Migraine Pathophysiology Is Unclear
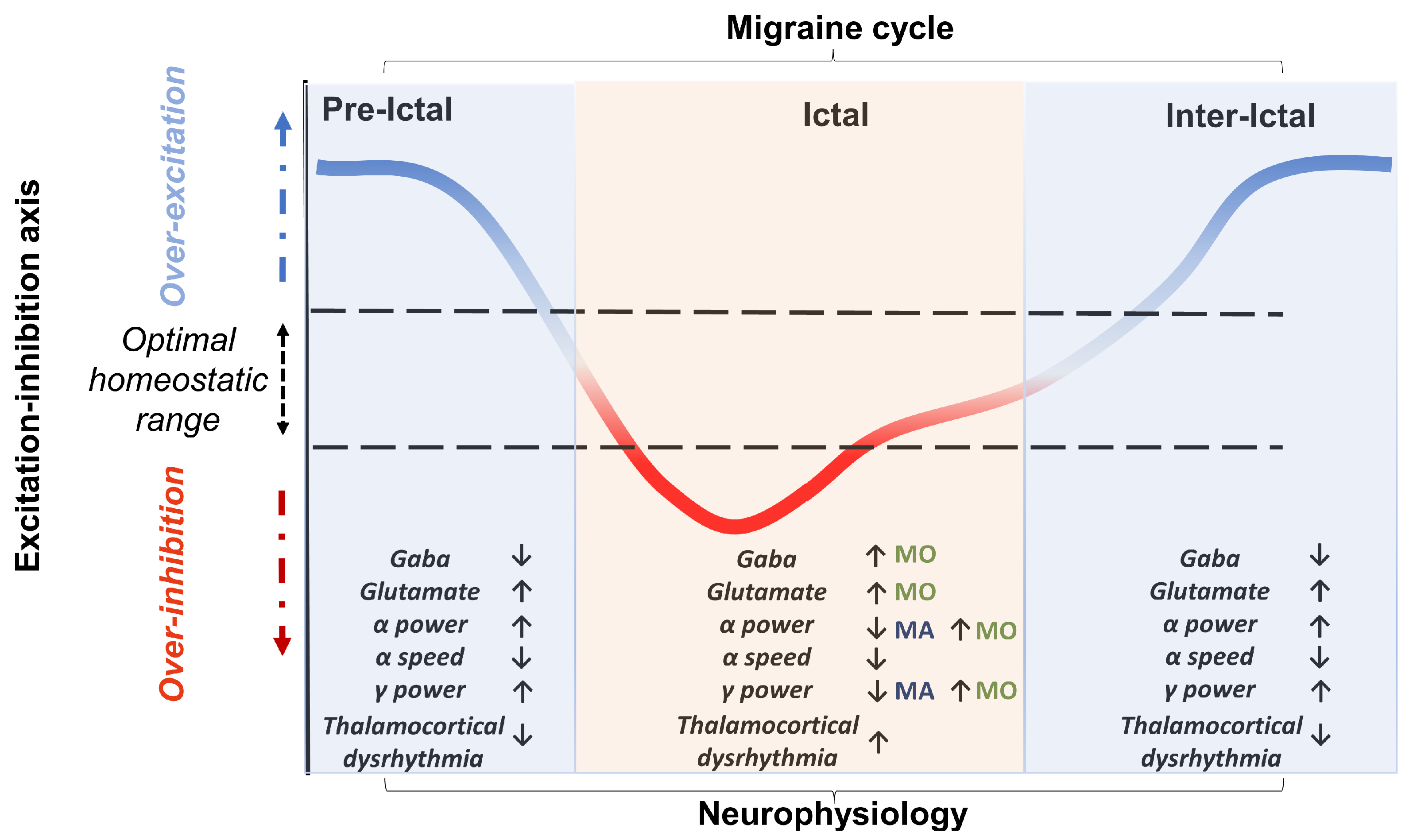
1.1.2. Goals of the Review
2. Differences in Alpha-Band Oscillations
2.1. Introduction to Alpha
2.2. Differences in Alpha during Attacks
2.3. Differences in Alpha between Attacks
2.4. Differences in Alpha during Visual Stimulation
2.5. Differences in Alpha between Migraine Subtypes
2.6. Summary
3. Differences in Gamma-Band Oscillations
3.1. Introduction to Gamma
3.2. The Origins of Gamma Oscillations
3.3. Gamma Activity during Attack
3.4. Gamma Activity in between Attacks
3.5. Summary
4. Integrating Alpha- and Gamma-Band Oscillations
4.1. Thalamocortical Dysrhythmia
4.2. Summary
5. Differences in Neurotransmitters
5.1. Glutamate
5.2. GABA
5.3. Serotonin
5.4. Summary
6. Excitation–Inhibition Balance and Dysrhythmia
6.1. Relationship between Oscillations and Neurotransmitters
6.1.1. Glutamate and Oscillations
6.1.2. GABA and Alpha-Band Oscillations
6.1.3. GABA and Gamma-Band Oscillations
6.1.4. GABAergic Medications
6.2. Summary
7. Models and Behaviour
7.1. Functional and Physiological Models
7.2. Modelling GABA and Glutamate
7.3. Models of Binocular Rivalry
7.4. Models of Surround Suppression
7.5. Modelling Other Visual Processing Differences in Migraine
7.6. Modelling the Effects of Neurostimulation
7.7. Summary
8. Treatments
8.1. Pharmacological Interventions
8.2. Nutritional Interventions
8.3. Neurostimulation Interventions
8.4. Summary
9. Discussion
9.1. Excitatory–Inhibitory Imbalance as a Homeostatic Mechanism
9.2. Excitatory–Inhibitory Imbalance and Sensory Processing
9.3. Imprecise Perception in Migraine
9.4. Potential Role of Rhythmic Neurostimulation Protocol to Restore the Altered Oscillatory Process
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSD | Cortical spreading depression |
| GABA | Gamma-aminobutyric acid |
| Glx | Glutamate + glutamine |
| MO | Migraine without aura |
| MA | Migraine with aura |
| PET | Positron emission tomography |
| SAI | Short latency afferent inhibition |
| SSVEP | Steady-state visual evoked potential |
| tACS | Transcranial alternating current stimulation |
| tDCA | Transcranial direct current stimulation |
| tES | Transcranial electrical stimulation |
| TMS | Transcranial magnetic stimulation |
References
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D. The economic burden of migraine to society. Pharmacoeconomics 1998, 13, 667–676. [Google Scholar] [CrossRef]
- Headache Classification Committee (HCC) of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Liberman, J.N.; Lipton, R.B. Age-dependent prevalence and clinical features of migraine. Neurology 2006, 67, 246–251. [Google Scholar] [CrossRef]
- Lipton, R.B.; Stewart, W.F.; Diamond, S.; Diamond, M.L.; Reed, M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache J. Head Face Pain 2001, 41, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Lyngberg, A.C.; Rasmussen, B.K.; Jørgensen, T.; Jensen, R. Prognosis of migraine and tension-type headache: A population-based follow-up study. Neurology 2005, 65, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Jen, J.C. Familial Hemiplegic Migraine. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2001; pp. 1–19, [Updated 2021 April 29]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1388/ (accessed on 14 May 2012).
- Schott, G.D. Exploring the visual hallucinations of migraine aura: The tacit contribution of illustration. Brain 2007, 130, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, L.; Asher, J.M.; Hibbard, P.B. Migraine Visual Aura and Cortical Spreading Depression—Linking Mathematical Models to Empirical Evidence. Vision 2021, 5, 30. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Sanchez del Rio, M.; Wu, O.; Schwartz, D.; Bakker, D.; Fischl, B.; Kwong, K.K.; Cutrer, F.M.; Rosen, B.R.; Tootell, R.B.; et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl. Acad. Sci. USA 2001, 98, 4687–4692. [Google Scholar] [CrossRef] [Green Version]
- Borgdorff, P. Arguments against the role of cortical spreading depression in migraine. Neurol. Res. 2018, 40, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Cutrer, F.M.; Charles, A. The neurogenic basis of migraine. Headache J. Head Face Pain 2008, 48, 1411–1414. [Google Scholar] [CrossRef]
- Karsan, N.; Goadsby, P.J. Biological insights from the premonitory symptoms of migraine. Nat. Rev. Neurol. 2018, 14, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hallett, M. Photophobia in neurologic disorders. Transl. Neurodegener. 2017, 6, 1–6. [Google Scholar]
- Marcus, D.A.; Soso, M.J. Migraine and stripe-induced visual discomfort. Arch. Neurol. 1989, 46, 1129–1132. [Google Scholar] [CrossRef]
- Harle, D.E.; Shepherd, A.J.; Evans, B.J. Visual stimuli are common triggers of migraine and are associated with pattern glare. Headache J. Head Face Pain 2006, 46, 1431–1440. [Google Scholar] [CrossRef]
- Pietrobon, D. Migraine: New molecular mechanisms. Neuroscientist 2005, 11, 373–386. [Google Scholar] [CrossRef]
- Bigal, M.E.; Lipton, R.B. Concepts and mechanisms of migraine chronification. Headache J. Head Face Pain 2008, 48, 7–15. [Google Scholar] [CrossRef]
- Bille, B. A 40-year follow-up of school children with migraine. Cephalalgia 1997, 17, 488–491. [Google Scholar] [CrossRef]
- Chronicle, E.; Mulleners, W. Might migraine damage the brain? Cephalalgia 1994, 14, 415–418. [Google Scholar] [CrossRef]
- Buse, D.C.; Greisman, J.D.; Baigi, K.; Lipton, R.B. Migraine progression: A systematic review. Headache J. Head Face Pain 2019, 59, 306–338. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Latorre, M.; Roig, M. Natural history of migraine in childhood. Cephalalgia 2000, 20, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, G.; Pierelli, F.; Schoenen, J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007, 27, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Aguggia, M.; Saracco, M. Pathophysiology of migraine chronification. Neurol. Sci. 2010, 31, 15–17. [Google Scholar] [CrossRef]
- Aurora, S.; Wilkinson, F. The brain is hyperexcitable in migraine. Cephalalgia 2007, 27, 1442–1453. [Google Scholar] [CrossRef]
- Coppola, G.; Ambrosini, A.; Clemente, L.D.; Magis, D.; Fumal, A.; Gerard, P.; Pierelli, F.; Schoenen, J. Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia 2007, 27, 1360–1367. [Google Scholar] [CrossRef]
- Roux, F.; Wibral, M.; Singer, W.; Aru, J.; Uhlhaas, P.J. The phase of thalamic alpha activity modulates cortical gamma-band activity: Evidence from resting-state MEG recordings. J. Neurosci. 2013, 33, 17827–17835. [Google Scholar] [CrossRef] [Green Version]
- Rowley, N.M.; Madsen, K.K.; Schousboe, A.; White, H.S. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 2012, 61, 546–558. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Andermann, F. Migraine and epilepsy. In Proceedings of the Seminars in Pediatric Neurology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 17, pp. 117–122. [Google Scholar]
- Peek, A.L.; Leaver, A.M.; Foster, S.; Oeltzschner, G.; Puts, N.A.; Galloway, G.; Sterling, M.; Ng, K.; Refshauge, K.; Aguila, M.E.R.; et al. Increased GABA+ in people with migraine, headache, and pain conditions-a potential marker of pain. J. Pain 2021, 22, 1631–1645. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Clayton, M.S.; Yeung, N.; Cohen Kadosh, R. The effects of 10 Hz transcranial alternating current stimulation on audiovisual task switching. Front. Neurosci. 2018, 12, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romei, V.; Rihs, T.; Brodbeck, V.; Thut, G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport 2008, 19, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Gross, J.; Thut, G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: Correlation or causation? J. Neurosci. 2010, 30, 8692–8697. [Google Scholar] [CrossRef] [Green Version]
- Romei, V.; Brodbeck, V.; Michel, C.; Amedi, A.; Pascual-Leone, A.; Thut, G. Spontaneous fluctuations in posterior α-band EEG activity reflect variability in excitability of human visual areas. Cereb. Cortex 2008, 18, 2010–2018. [Google Scholar] [CrossRef]
- Tarasi, L.; Trajkovic, J.; Diciotti, S.; di Pellegrino, G.; Ferri, F.; Ursino, M.; Romei, V. Predictive waves in the autism-schizophrenia continuum: A novel biobehavioral model. Neurosci. Biobehav. Rev. 2022, 132, 1–22. [Google Scholar] [CrossRef]
- Ippolito, G.; Bertaccini, R.; Tarasi, L.; Di Gregorio, F.; Trajkovic, J.; Battaglia, S.; Romei, V. The Role of Alpha Oscillations among the Main Neuropsychiatric Disorders in the Adult and Developing Human Brain: Evidence from the Last 10 Years of Research. Biomedicines 2022, 10, 3189. [Google Scholar] [CrossRef]
- Stroganova, T.A.; Orekhova, E.V.; Posikera, I.N. EEG alpha rhythm in infants. Clin. Neurophysiol. 1999, 110, 997–1012. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, H.; Schoffelen, J.M.; Oostenveld, R.; Jensen, O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J. Neurosci. 2008, 28, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Limbach, K.; Corballis, P.M. Prestimulus alpha power influences response criterion in a detection task. Psychophysiology 2016, 53, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Tarasi, L.; di Pellegrino, G.; Romei, V. Are you an empiricist or a believer? Neural signatures of predictive strategies in humans. Prog. Neurobiol. 2022, 219, 102367. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.D.; Barnes, G.R.; Hillebrand, A.; Furlong, P.L.; Singh, K.D.; Holliday, I.E. Spatio-temporal imaging of cortical desynchronization in migraine visual aura: A magnetoencephalography case study. Headache J. Head Face Pain 2004, 44, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Bjørk, M.; Sand, T. Quantitative EEG power and asymmetry increase 36 h before a migraine attack. Cephalalgia 2008, 28, 960–968. [Google Scholar] [CrossRef]
- Bjørk, M.; Stovner, L.; Nilsen, B.; Stjern, M.; Hagen, K.; Sand, T. The occipital alpha rhythm related to the “migraine cycle” and headache burden: A blinded, controlled longitudinal study. Clin. Neurophysiol. 2009, 120, 464–471. [Google Scholar] [CrossRef]
- Cao, Z.; Lin, C.T.; Chuang, C.H.; Lai, K.L.; Yang, A.C.; Fuh, J.L.; Wang, S.J. Resting-state EEG power and coherence vary between migraine phases. J. Headache Pain 2016, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Clemens, B.; Bánk, J.; Piros, P.; Bessenyei, M.; Vető, S.; Tóth, M.; Kondákor, I. Three-dimensional localization of abnormal EEG activity in migraine: A low resolution electromagnetic tomography (LORETA) study of migraine patients in the pain-free interval. Brain Topogr. 2008, 21, 36–42. [Google Scholar] [CrossRef]
- Lia, C.; Carenini, L.; Degioz, C.; Bottachi, E. Computerized EEG analysis in migraine patients. Ital. J. Neurol. Sci. 1995, 16, 249–254. [Google Scholar] [CrossRef]
- Gomez-Pilar, J.; García-Azorín, D.; Gomez-Lopez-de San-Roman, C.; Guerrero, Á.L.; Hornero, R. Exploring EEG spectral patterns in episodic and chronic migraine during the interictal state: Determining frequencies of interest in the resting state. Pain Med. 2020, 21, 3530–3538. [Google Scholar] [CrossRef]
- O’Hare, L.; Menchinelli, F.; Durrant, S.J. Resting-state alpha-band oscillations in migraine. Perception 2018, 47, 379–396. [Google Scholar] [CrossRef]
- Van Beijsterveldt, C.; Van Baal, G. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biol. Psychol. 2002, 61, 111–138. [Google Scholar] [CrossRef]
- Angelakis, E.; Lubar, J.F.; Stathopoulou, S.; Kounios, J. Peak alpha frequency: An electroencephalographic measure of cognitive preparedness. Clin. Neurophysiol. 2004, 115, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Schmiedek, F.; Lövdén, M.; Lindenberger, U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 2013, 50, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, Y.; Wang, D.; Zhou, C.; Xu, C. Relationship between individual alpha peak frequency and attentional performance in a multiple object tracking task among ice-hockey players. PLoS ONE 2021, 16, e0251443. [Google Scholar] [CrossRef] [PubMed]
- Samaha, J.; Postle, B.R. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Curr. Biol. 2015, 25, 2985–2990. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Trajkovic, J.; Roperti, C.; Marcantoni, E.; Di Luzio, P.; Avenanti, A.; Thut, G.; Romei, V. Tuning alpha rhythms to shape conscious visual perception. Curr. Biol. 2022, 32, 988–998. [Google Scholar] [CrossRef]
- Cecere, R.; Rees, G.; Romei, V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr. Biol. 2015, 25, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, M.; Treves, T.; Korczyn, A. EEG and topographic frequency analysis in common and classic migraine. Headache J. Head Face Pain 1991, 31, 232–236. [Google Scholar] [CrossRef]
- Bjørk, M.; Stovner, L.; Hagen, K.; Sand, T. What initiates a migraine attack? Conclusions from four longitudinal studies of quantitative EEG and steady-state visual-evoked potentials in migraineurs. Acta Neurol. Scand. 2011, 124, 56–63. [Google Scholar] [CrossRef]
- Furman, A.J.; Prokhorenko, M.; Keaser, M.L.; Zhang, J.; Chen, S.; Mazaheri, A.; Seminowicz, D.A. Sensorimotor peak alpha frequency is a reliable biomarker of prolonged pain sensitivity. Cereb. Cortex 2020, 30, 6069–6082. [Google Scholar] [CrossRef] [PubMed]
- Valentini, E.; Halder, S.; McInnerney, D.; Cooke, J.; Gyimes, I.L.; Romei, V. Assessing the specificity of the relationship between brain alpha oscillations and tonic pain. NeuroImage 2022, 255, 119143. [Google Scholar] [CrossRef]
- Freschl, J.; Al Azizi, L.; Balboa, L.; Kaldy, Z.; Blaser, E. The development of peak alpha frequency from infancy to adolescence and its role in visual temporal processing: A meta-analysis. Dev. Cogn. Neurosci. 2022, 57, 101146. [Google Scholar] [CrossRef]
- Clayton, M.S.; Yeung, N.; Cohen Kadosh, R. The many characters of visual alpha oscillations. Eur. J. Neurosci. 2018, 48, 2498–2508. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, M.L.; Kékesi, K.A.; Juhász, G.; Crunelli, V.; Hughes, S.W. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron 2009, 63, 683–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyrke, T.; Kangasniemi, P.; Lang, H. Alpha rhythm in classical migraine (migraine with aura): Abnormalities in the headache-free interval. Cephalalgia 1990, 10, 177–181. [Google Scholar] [CrossRef]
- Fong, C.Y.; Law, W.H.C.; Fahrenfort, J.J.; Braithwaite, J.J.; Mazaheri, A. Attenuated alpha oscillation and hyperresponsiveness reveals impaired perceptual learning in migraineurs. J. Headache Pain 2022, 23, 44. [Google Scholar] [CrossRef]
- Angelini, L.; De Tommaso, M.; Guido, M.; Hu, K.; Ivanov, P.C.; Marinazzo, D.; Nardulli, G.; Nitti, L.; Pellicoro, M.; Pierro, C.; et al. Steady-state visual evoked potentials and phase synchronization in migraine patients. Phys. Rev. Lett. 2004, 93, 038103. [Google Scholar] [CrossRef] [Green Version]
- De Tommaso, M.; Stramaglia, S.; Marinazzo, D.; Guido, M.; Lamberti, P.; Livrea, P. Visually evoked phase synchronisation changes of alpha rhythm in migraine. Correlations with clinical features. Neurol. Sci. 2004, 25, s283–s284. [Google Scholar] [CrossRef]
- De Tommaso, M.; Marinazzo, D.; Guido, M.; Libro, G.; Stramaglia, S.; Nitti, L.; Lattanzi, G.; Angelini, L.; Pellicoro, M. Visually evoked phase synchronization changes of alpha rhythm in migraine: Correlations with clinical features. Int. J. Psychophysiol. 2005, 57, 203–210. [Google Scholar] [CrossRef]
- De Tommaso, M.; Marinazzo, D.; Nitti, L.; Pellicoro, M.; Guido, M.; Serpino, C.; Stramaglia, S. Effects of levetiracetam vs topiramate and placebo on visually evoked phase synchronization changes of alpha rhythm in migraine. Clin. Neurophysiol. 2007, 118, 2297–2304. [Google Scholar] [CrossRef]
- De Tommaso, M.; Stramaglia, S.; Brighina, F.; Fierro, B.; Francesco, V.D.; Todarello, O.; Serpino, C.; Pellicoro, M. Lack of effects of low frequency repetitive transcranial magnetic stimulation on alpha rhythm phase synchronization in migraine patients. Neurosci. Lett. 2011, 488, 143–147. [Google Scholar] [CrossRef]
- Surges, R.; Volynski, K.E.; Walker, M.C. Is levetiracetam different from other antiepileptic drugs? Levetiracetam and its cellular mechanism of action in epilepsy revisited. Ther. Adv. Neurol. Disord. 2008, 1, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boroojerdi, B.; Prager, A.; Muellbacher, W.; Cohen, L.G. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology 2000, 54, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Caparelli, E.; Backus, W.; Telang, F.; Wang, G.; Maloney, T.; Goldstein, R.; Henn, F. Is 1 Hz rTMS always inhibitory in healthy individuals? Open Neuroimaging J. 2012, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fierro, B.; Ricci, R.; Piazza, A.; Scalia, S.; Giglia, G.; Vitello, G.; Brighina, F. 1 Hz rTMS enhances extrastriate cortex activity in migraine: Evidence of a reduced inhibition? Neurology 2003, 61, 1446–1448. [Google Scholar] [CrossRef]
- De Tommaso, M.; Stramaglia, S.; Marinazzo, D.; Trotta, G.; Pellicoro, M. Functional and effective connectivity in EEG alpha and beta bands during intermittent flash stimulation in migraine with and without aura. Cephalalgia 2013, 33, 938–947. [Google Scholar] [CrossRef]
- Chamanzar, A.; Haigh, S.M.; Grover, P.; Behrmann, M. Abnormalities in cortical pattern of coherence in migraine detected using ultra high-density EEG. Brain Commun. 2021, 3, fcab061. [Google Scholar] [CrossRef]
- Hansen, J.M.; Charles, A. Differences in treatment response between migraine with aura and migraine without aura: Lessons from clinical practice and RCTs. J. Headache Pain 2019, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, O.; Tallon-Baudry, C. Oscillatory gamma activity in humans: A possible role for object representation. Int. J. Psychophysiol. 2000, 38, 211–223. [Google Scholar] [CrossRef]
- Van Pelt, S.; Boomsma, D.I.; Fries, P. Magnetoencephalography in twins reveals a strong genetic determination of the peak frequency of visually induced gamma-band synchronization. J. Neurosci. 2012, 32, 3388–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 407. [Google Scholar] [CrossRef] [PubMed]
- Csibra, G.; Davis, G.; Spratling, M.; Johnson, M. Gamma oscillations and object processing in the infant brain. Science 2000, 290, 1582–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, K.; Seymour, R.A.; Rippon, G. Brain oscillations and connectivity in autism spectrum disorders (ASD): New approaches to methodology, measurement and modelling. Neurosci. Biobehav. Rev. 2016, 71, 601–620. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Kucewicz, M.T.; Cimbalnik, J.; Matsumoto, J.Y.; Brinkmann, B.H.; Hu, W.; Marsh, W.R.; Meyer, F.B.; Stead, S.M.; Worrell, G.A. Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology 2015, 84, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Hoogenboom, N.; Schoffelen, J.M.; Oostenveld, R.; Parkes, L.M.; Fries, P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 2006, 29, 764–773. [Google Scholar] [CrossRef]
- Steriade, M.; Contreras, D.; Amzica, F.; Timofeev, I. Synchronization of fast (30-40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J. Neurosci. 1996, 16, 2788–2808. [Google Scholar] [CrossRef] [Green Version]
- Buchner, H.; Gobbele, R.; Waberski, T.D.; Wagner, M.; Fuchs, M. Evidence for independent thalamic and cortical sources involved in the generation of the visual 40 Hz response in humans. Neurosci. Lett. 1999, 269, 59–62. [Google Scholar] [CrossRef]
- Orekhova, E.V.; Sysoeva, O.V.; Schneiderman, J.F.; Lundström, S.; Galuta, I.A.; Goiaeva, D.E.; Prokofyev, A.O.; Riaz, B.; Keeler, C.; Hadjikhani, N.; et al. Input-dependent modulation of MEG gamma oscillations reflects gain control in the visual cortex. Sci. Rep. 2018, 8, 8451. [Google Scholar] [CrossRef] [Green Version]
- Orekhova, E.V.; Stroganova, T.A.; Schneiderman, J.F.; Lundström, S.; Riaz, B.; Sarovic, D.; Sysoeva, O.V.; Brant, G.; Gillberg, C.; Hadjikhani, N. Neural gain control measured through cortical gamma oscillations is associated with sensory sensitivity. Hum. Brain Mapp. 2019, 40, 1583–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ge, H.; Xiang, J.; Miao, A.; Tang, L.; Wu, T.; Chen, Q.; Yang, L.; Wang, X. Resting state brain activity in patients with migraine: A magnetoencephalography study. J. Headache Pain 2015, 16, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Xiang, J.; Wu, T.; Zhu, D.; Shi, J. Abnormal resting-state brain activity in headache-free migraine patients: A magnetoencephalography study. Clin. Neurophysiol. 2016, 127, 2855–2861. [Google Scholar] [CrossRef]
- Bassez, I.; Ricci, K.; Vecchio, E.; Delussi, M.; Gentile, E.; Marinazzo, D.; de Tommaso, M. The effect of painful laser stimuli on EEG gamma-band activity in migraine patients and healthy controls. Clin. Neurophysiol. 2020, 131, 1755–1766. [Google Scholar] [CrossRef]
- Ren, J.; Xiang, J.; Chen, Y.; Li, F.; Wu, T.; Shi, J. Abnormal functional connectivity under somatosensory stimulation in migraine: A multi-frequency magnetoencephalography study. J. Headache Pain 2019, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Barlow, H.B. Possible principles underlying the transformation of sensory messages. Sens. Commun. 1961, 1, 217–233. [Google Scholar]
- Hibbard, P.B.; O’Hare, L. Uncomfortable images produce non-sparse responses in a model of primary visual cortex. R. Soc. Open Sci. 2015, 2, 140535. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, L.; Clarke, A.D.; Pollux, P.M. VEP responses to op-art stimuli. PLoS ONE 2015, 10, e0139400. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, L. Steady-state VEP responses to uncomfortable stimuli. Eur. J. Neurosci. 2017, 45, 410–422. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, L.; Hird, E.; Whybrow, M. Steady-state visual evoked potential responses predict visual discomfort judgements. Eur. J. Neurosci. 2021, 54, 7575–7598. [Google Scholar] [CrossRef]
- Vanagaite, J.; Pareja, J.; Støren, O.; White, L.; Sanc, T.; Stovner, L. Light-induced discomfort and pain in migraine. Cephalalgia 1997, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Hine, T.J.; Beaumont, H.M. Color and spatial frequency are related to visual pattern sensitivity in migraine. Headache J. Head Face Pain 2013, 53, 1087–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orekhova, E.V.; Rostovtseva, E.N.; Manyukhina, V.O.; Prokofiev, A.O.; Obukhova, T.S.; Nikolaeva, A.Y.; Schneiderman, J.F.; Stroganova, T.A. Spatial suppression in visual motion perception is driven by inhibition: Evidence from MEG gamma oscillations. NeuroImage 2020, 213, 116753. [Google Scholar] [CrossRef]
- Lisicki, M.; D’Ostilio, K.; Coppola, G.; Nonis, R.; de Noordhout, A.M.; Parisi, V.; Magis, D.; Schoenen, J. Headache related alterations of visual processing in migraine patients. J. Pain 2020, 21, 593–602. [Google Scholar] [CrossRef]
- Coppola, G.; Vandenheede, M.; Di Clemente, L.; Ambrosini, A.; Fumal, A.; De Pasqua, V.; Schoenen, J. Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain 2005, 128, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, C.E.; Lakatos, P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009, 32, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osipova, D.; Hermes, D.; Jensen, O. Gamma power is phase-locked to posterior alpha activity. PLoS ONE 2008, 3, e3990. [Google Scholar] [CrossRef] [Green Version]
- Herring, J.D.; Esterer, S.; Marshall, T.R.; Jensen, O.; Bergmann, T.O. Low-frequency alternating current stimulation rhythmically suppresses gamma-band oscillations and impairs perceptual performance. Neuroimage 2019, 184, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, E.V.; Prokofyev, A.O.; Nikolaeva, A.Y.; Schneiderman, J.F.; Stroganova, T.A. Additive effect of contrast and velocity suggests the role of strong excitatory drive in suppression of visual gamma response. PLoS ONE 2020, 15, e0228937. [Google Scholar] [CrossRef]
- Jensen, O.; Colgin, L.L. Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 2007, 11, 267–269. [Google Scholar] [CrossRef]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreckenberger, M.; Lange-Asschenfeld, C.; Lochmann, M.; Mann, K.; Siessmeier, T.; Buchholz, H.G.; Bartenstein, P.; Gründer, G. The thalamus as the generator and modulator of EEG alpha rhythm: A combined PET/EEG study with lorazepam challenge in humans. Neuroimage 2004, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Bracaglia, M.; Di Lenola, D.; Iacovelli, E.; Di Lorenzo, C.; Serrao, M.; Evangelista, M.; Parisi, V.; Schoenen, J.; Pierelli, F. Lateral inhibition in the somatosensory cortex during and between migraine without aura attacks: Correlations with thalamocortical activity and clinical features. Cephalalgia 2016, 36, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, D.J.; Wilcox, S.L.; Veggeberg, R.; Noseda, R.; Burstein, R.; Borsook, D.; Becerra, L. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J. Neurosci. 2016, 36, 8026–8036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Fu, Z.; Zeng, F.; Maleki, N.; Lan, L.; Li, Z.; Park, J.; Wilson, G.; Gao, Y.; Liu, M.; et al. Abnormal thalamocortical network dynamics in migraine. Neurology 2019, 92, e2706–e2716. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.P.; Westerfield, M.; Lopes, M.; Maruta, C.; Gil-da Costa, R. Brain state monitoring for the future prediction of migraine attacks. Cephalalgia 2020, 40, 255–265. [Google Scholar] [CrossRef]
- Coppola, G.; Di Renzo, A.; Tinelli, E.; Di Lorenzo, C.; Di Lorenzo, G.; Parisi, V.; Serrao, M.; Schoenen, J.; Pierelli, F. Thalamo-cortical network activity during spontaneous migraine attacks. Neurology 2016, 87, 2154–2160. [Google Scholar] [CrossRef]
- Coppola, G.; Di Lenola, D.; Abagnale, C.; Ferrandes, F.; Sebastianelli, G.; Casillo, F.; Di Lorenzo, C.; Serrao, M.; Evangelista, M.; Schoenen, J.; et al. Short-latency afferent inhibition and somato-sensory evoked potentials during the migraine cycle: Surrogate markers of a cycling cholinergic thalamo-cortical drive? J. Headache Pain 2020, 21, 34. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, D.; Castellazzi, G.; De Icco, R.; Bacila, A.; Allena, M.; Faggioli, A.; Sances, G.; Pichiecchio, A.; Borsook, D.; Gandini Wheeler-Kingshott, C.A.; et al. Thalamocortical connectivity in experimentally-induced migraine attacks: A pilot study. Brain Sci. 2021, 11, 165. [Google Scholar] [CrossRef]
- Meneghetti, N.; Cerri, C.; Vannini, E.; Tantillo, E.; Tottene, A.; Pietrobon, D.; Caleo, M.; Mazzoni, A. Synaptic alterations in visual cortex reshape contrast-dependent gamma oscillations and inhibition-excitation ratio in a genetic mouse model of migraine. J. Headache Pain 2022, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Prescot, A.; Becerra, L.; Pendse, G.; Tully, S.; Jensen, E.; Hargreaves, R.; Renshaw, P.; Burstein, R.; Borsook, D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol. Pain 2009, 5, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arngrim, N.; Schytz, H.W.; Britze, J.; Amin, F.M.; Vestergaard, M.B.; Hougaard, A.; Wolfram, F.; de Koning, P.J.; Olsen, K.S.; Secher, N.H.; et al. Migraine induced by hypoxia: An MRI spectroscopy and angiography study. Brain 2016, 139, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrusic, I.; Zidverc-Trajkovic, J. Cortical spreading depression: Origins and paths as inferred from the sequence of events during migraine aura. Funct. Neurol. 2014, 29, 207. [Google Scholar] [PubMed]
- Gonzalez de la Aleja, J.; Ramos, A.; Mato-Abad, V.; Martínez-Salio, A.; Hernández-Tamames, J.A.; Molina, J.A.; Hernández-Gallego, J.; Álvarez-Linera, J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache J. Head Face Pain 2013, 53, 365–375. [Google Scholar] [CrossRef]
- Bathel, A.; Schweizer, L.; Stude, P.; Glaubitz, B.; Wulms, N.; Delice, S.; Schmidt-Wilcke, T. Increased thalamic glutamate/glutamine levels in migraineurs. J. Headache Pain 2018, 19, 55. [Google Scholar] [CrossRef]
- Siniatchkin, M.; Sendacki, M.; Moeller, F.; Wolff, S.; Jansen, O.; Siebner, H.; Stephani, U. Abnormal changes of synaptic excitability in migraine with aura. Cereb. Cortex 2012, 22, 2207–2216. [Google Scholar] [CrossRef] [Green Version]
- Zielman, R.; Wijnen, J.P.; Webb, A.; Onderwater, G.L.; Ronen, I.; Ferrari, M.D.; Kan, H.E.; Terwindt, G.M.; Kruit, M.C. Cortical glutamate in migraine. Brain 2017, 140, 1859–1871. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Bai, X.; Zhang, Y.; Yuan, Z.; Tang, H.; Li, Z.; Hu, Z.; Zhang, Y.; Yu, X.; et al. Gamma-aminobutyric acid and glutamate/glutamine levels in the dentate nucleus and periaqueductal gray with episodic and chronic migraine: A proton magnetic resonance spectroscopy study. J. Headache Pain 2022, 23, 83. [Google Scholar] [CrossRef]
- Niddam, D.M.; Lai, K.L.; Tsai, S.Y.; Lin, Y.R.; Chen, W.T.; Fuh, J.L.; Wang, S.J. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain 2018, 141, 377–390. [Google Scholar] [CrossRef]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.; Watson, J.; Aguila, M.E.R.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020, 210, 116532. [Google Scholar] [CrossRef] [PubMed]
- Bridge, H.; Stagg, C.J.; Near, J.; Lau, C.i.; Zisner, A.; Cader, M.Z. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 2015, 35, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Fayed, N.; Andrés, E.; Viguera, L.; Modrego, P.J.; Garcia-Campayo, J. Higher glutamate+ glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad. Radiol. 2014, 21, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Perenboom, M.; Najafabadi, A.Z.; Zielman, R.; Tolner, E.; Carpay, J.; Ferrari, M. Visual sensitivity is more enhanced in migraineurs with aura than in migraineurs without aura. Cephalalgia 2015, 35, 1224. [Google Scholar]
- Evans, B.; Stevenson, S. The Pattern Glare Test: A review and determination of normative values. Ophthalmic Physiol. Opt. 2008, 28, 295–309. [Google Scholar] [CrossRef]
- Bigal, M.; Hetherington, H.; Pan, J.; Tsang, A.; Grosberg, B.; Avdievich, N.; Friedman, B.; Lipton, R. Occipital levels of GABA are related to severe headaches in migraine. Neurology 2008, 70, 2078–2080. [Google Scholar] [CrossRef] [Green Version]
- Onderwater, G.L.; Wijnen, J.P.; Najac, C.; van Dongen, R.M.; Ronen, I.; Webb, A.; Zielman, R.; van Zwet, E.W.; Ferrari, M.D.; Kan, H.E.; et al. Cortical glutamate and gamma-aminobutyric acid over the course of a provoked migraine attack, a 7 Tesla magnetic resonance spectroscopy study. Neuroimage Clin. 2021, 32, 102889. [Google Scholar] [CrossRef]
- Wu, X.; Han, S.; Yang, Y.; Dai, H.; Wu, P.; Zhao, H.; Jin, X.; Li, Y. Decreased brain GABA levels in patients with migraine without aura: An exploratory proton magnetic resonance spectroscopy study. Neuroscience 2022, 488, 10–19. [Google Scholar] [CrossRef]
- Chan, Y.M.; Pitchaimuthu, K.; Wu, Q.Z.; Carter, O.L.; Egan, G.F.; Badcock, D.R.; McKendrick, A.M. Relating excitatory and inhibitory neurochemicals to visual perception: A magnetic resonance study of occipital cortex between migraine events. PLoS ONE 2019, 14, e0208666. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.M.; Glarin, R.; Moffat, B.A.; Bode, S.; McKendrick, A.M. Relating the cortical visual contrast gain response to spectroscopy-measured excitatory and inhibitory metabolites in people who experience migraine. PLoS ONE 2022, 17, e0266130. [Google Scholar] [CrossRef]
- Stærmose, T.G.; Knudsen, M.K.; Kasch, H.; Blicher, J.U. Cortical GABA in migraine with aura-an ultrashort echo magnetic resonance spectroscopy study. J. Headache Pain 2019, 20, 110. [Google Scholar] [CrossRef]
- Pohl, H.; Wyss, P.; Sandor, P.S.; Schoenen, J.; Luechinger, R.; O’Gorman, R.; Riederer, F.; Gantenbein, A.R.; Michels, L. The longitudinal influence of tDCS on occipital GABA and glutamate/glutamine levels in episodic migraineurs. J. Neurosci. Res. 2023, 101, 815–825. [Google Scholar] [CrossRef]
- Vieira, D.; Naffah-Mazacoratti, M.; Zukerman, E.; Soares, C.S.; Alonso, E.; Faulhaber, M.; Cavalheiro, E.; Peres, M. Cerebrospinal fluid GABA levels in chronic migraine with and without depression. Brain Res. 2006, 1090, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Stokoe, M.; Khaira, A.; Webb, M.; Noel, M.; Amoozegar, F.; Harris, A.D. GABA and glutamate in pediatric migraine. Pain 2021, 162, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Bai, X.; Zhang, Y.; Yuan, Z.; Tang, H.; Zhang, X.; Li, Z.; Zhang, P.; Hu, Z.; et al. Changes in gamma-aminobutyric acid and glutamate/glutamine levels in the right thalamus of patients with episodic and chronic migraine: A proton magnetic resonance spectroscopy study. Headache J. Head Face Pain 2023, 63, 104–113. [Google Scholar] [CrossRef]
- Mathers, D.A.; Wan, X.; Puil, E. Barbiturate activation and modulation of GABAA receptors in neocortex. Neuropharmacology 2007, 52, 1160–1168. [Google Scholar] [CrossRef]
- Bigal, M.E.; Lipton, R.; Tepper, S.; Rapoport, A.; Sheftell, F. Primary chronic daily headache and its subtypes in adolescents and adults. Neurology 2004, 63, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Aguila, M.E.R.; Lagopoulos, J.; Leaver, A.M.; Rebbeck, T.; Hübscher, M.; Brennan, P.C.; Refshauge, K.M. Elevated levels of GABA+ in migraine detected using 1H-MRS. NMR Biomed. 2015, 28, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Zhang, Z.; Cao, Z. Altered metabolites in the occipital lobe in migraine without aura during the attack and the interictal period. Front. Neurol. 2021, 12, 656349. [Google Scholar] [CrossRef]
- Peek, A.L.; Leaver, A.M.; Foster, S.; Puts, N.A.; Oeltzschner, G.; Henderson, L.; Galloway, G.; Ng, K.; Refshauge, K.; Rebbeck, T. Increase in ACC GABA+ levels correlate with decrease in migraine frequency, intensity and disability over time. J. Headache Pain 2021, 22, 150. [Google Scholar] [CrossRef]
- Burch, R. Antidepressants for preventive treatment of migraine. Curr. Treat. Options Neurol. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Drummond, P. Tryptophan depletion increases nausea, headache and photophobia in migraine sufferers. Cephalalgia 2006, 26, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Khorsha, F.; Rafiee, P.; Shirani, P.; Nourmohammadi, M.; Ansari, H. The association between dietary tryptophan intake and migraine. Neurol. Sci. 2019, 40, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Odink, J.; Tapparelli, C.; Van Kempen, G.; Pennings, E.; Bruyn, G. Serotonin metabolism in migraine. Neurology 1989, 39, 1239. [Google Scholar] [CrossRef]
- Deen, M.; Correnti, E.; Kamm, K.; Kelderman, T.; Papetti, L.; Rubio-Beltrán, E.; Vigneri, S.; Edvinsson, L.; Maassen Van Den Brink, A. Blocking CGRP in migraine patients–a review of pros and cons. J. Headache Pain 2017, 18, 86. [Google Scholar] [CrossRef] [Green Version]
- Shields, K.G.; Goadsby, P.J. Serotonin receptors modulate trigeminovascular responses in ventroposteromedial nucleus of thalamus: A migraine target? Neurobiol. Dis. 2006, 23, 491–501. [Google Scholar] [CrossRef]
- Supornsilpchai, W.; Sanguanrangsirikul, S.; Maneesri, S.; Srikiatkhachorn, A. Serotonin depletion, cortical spreading depression, and trigeminal nociception. Headache J. Head Face Pain 2006, 46, 34–39. [Google Scholar] [CrossRef]
- Jacobs, G.; Van der Grond, J.; Teeuwisse, W.; Langeveld, T.; Van Pelt, J.; Verhagen, J.; de Kam, M.; Cohen, A.; Zitman, F.; van Gerven, J. Hypothalamic glutamate levels following serotonergic stimulation: A pilot study using 7-Tesla magnetic resonance spectroscopy in healthy volunteers. Prog. -Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 486–491. [Google Scholar] [CrossRef]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Krystal, J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 2002, 159, 663–665. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Wylezinska, M.; Taylor, M.; Jezzard, P.; Matthews, P.M.; Cowen, P.J. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry 2004, 161, 368–370. [Google Scholar] [CrossRef]
- Silva, L.R.; Amitai, Y.; Connors, B.W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 1991, 251, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Kopell, N.J. Thalamic model of awake alpha oscillations and implications for stimulus processing. Proc. Natl. Acad. Sci. USA 2012, 109, 18553–18558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukumaraswamy, S.D.; Shaw, A.D.; Jackson, L.E.; Hall, J.; Moran, R.; Saxena, N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 2015, 35, 11694–11706. [Google Scholar] [CrossRef] [Green Version]
- De la Salle, S.; Choueiry, J.; Shah, D.; Bowers, H.; McIntosh, J.; Ilivitsky, V.; Knott, V. Effects of ketamine on resting-state EEG activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front. Pharmacol. 2016, 7, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Soldevilla, D. On the physiological modulation and potential mechanisms underlying parieto-occipital alpha oscillations. Front. Comput. Neurosci. 2018, 12, 23. [Google Scholar] [CrossRef]
- Lozano-Soldevilla, D.; ter Huurne, N.; Cools, R.; Jensen, O. GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Curr. Biol. 2014, 24, 2878–2887. [Google Scholar] [CrossRef] [Green Version]
- Baumgarten, T.J.; Neugebauer, J.; Oeltzschner, G.; Füllenbach, N.D.; Kircheis, G.; Häussinger, D.; Lange, J.; Wittsack, H.J.; Butz, M.; Schnitzler, A. Connecting occipital alpha band peak frequency, visual temporal resolution, and occipital GABA levels in healthy participants and hepatic encephalopathy patients. Neuroimage Clin. 2018, 20, 347–356. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [Green Version]
- Whittington, M.A.; Traub, R.D.; Jefferys, J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 1995, 373, 612–615. [Google Scholar] [CrossRef]
- Volman, V.; Behrens, M.M.; Sejnowski, T.J. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J. Neurosci. 2011, 31, 18137–18148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukumaraswamy, S.D.; Edden, R.A.; Jones, D.K.; Swettenham, J.B.; Singh, K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8356–8361. [Google Scholar] [CrossRef] [Green Version]
- Gaetz, W.; Edgar, J.C.; Wang, D.; Roberts, T.P. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage 2011, 55, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousijn, H.; Haegens, S.; Wallis, G.; Near, J.; Stokes, M.G.; Harrison, P.J.; Nobre, A.C. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc. Natl. Acad. Sci. USA 2014, 111, 9301–9306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyss, C.; Tse, D.H.; Kometer, M.; Dammers, J.; Achermann, R.; Shah, N.J.; Kawohl, W.; Neuner, I. GABA metabolism and its role in gamma-band oscillatory activity during auditory processing: An MRS and EEG study. Hum. Brain Mapp. 2017, 38, 3975–3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujala, J.; Jung, J.; Bouvard, S.; Lecaignard, F.; Lothe, A.; Bouet, R.; Ciumas, C.; Ryvlin, P.; Jerbi, K. Gamma oscillations in V1 are correlated with GABAA receptor density: A multi-modal MEG and Flumazenil-PET study. Sci. Rep. 2015, 5, 16347. [Google Scholar] [CrossRef] [Green Version]
- Storer, R.J.; Akerman, S.; Goadsby, P.J. GABA receptors modulate trigeminovascular nociceptive neurotransmission in the trigeminocervical complex. Br. J. Pharmacol. 2001, 134, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Linde, M.; Mulleners, W.M.; Chronicle, E.P.; McCrory, D.C. Valproate for preventing migraine attacks in adults. Cochrane Database Syst. Rev. 2013, 6, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Rad, R.E.; Ghaffari, F.; Fotokian, Z.; Ramezani, A. The effectiveness of ibuprofen and lorazepam combination therapy in treating the symptoms of acute Migraine: A randomized clinical trial. Electron. Physician 2017, 9, 3912. [Google Scholar]
- Harnod, T.; Wang, Y.C.; Lin, C.L.; Tseng, C.H. Association between use of short-acting benzodiazepines and migraine occurrence: A nationwide population-based case–control study. Curr. Med Res. Opin. 2017, 33, 511–517. [Google Scholar] [CrossRef]
- Rivolta, D.; Heidegger, T.; Scheller, B.; Sauer, A.; Schaum, M.; Birkner, K.; Singer, W.; Wibral, M.; Uhlhaas, P.J. Ketamine dysregulates the amplitude and connectivity of high-frequency oscillations in cortical–subcortical networks in humans: Evidence from resting-state magnetoencephalography-recordings. Schizophr. Bull. 2015, 41, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Lally, N.; Mullins, P.G.; Roberts, M.V.; Price, D.; Gruber, T.; Haenschel, C. Glutamatergic correlates of gamma-band oscillatory activity during cognition: A concurrent ER-MRS and EEG study. Neuroimage 2014, 85, 823–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, D.; Wilson, S.; Lingford-Hughes, A.; Myers, J.; Papadopoulos, A.; Muthukumaraswamy, S. Differences between magnetoencephalographic (MEG) spectral profiles of drugs acting on GABA at synaptic and extrasynaptic sites: A study in healthy volunteers. Neuropharmacology 2015, 88, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, E.; Frankel, S. γ-Aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.R.; Vieira, P.G.; Pack, C.C. Transcranial electrical stimulation: How can a simple conductor orchestrate complex brain activity? PLoS Biol. 2023, 21, e3001973. [Google Scholar] [CrossRef] [PubMed]
- Zhaoping, L.; May, K.A. Psychophysical tests of the hypothesis of a bottom-up saliency map in primary visual cortex. PLoS Comput. Biol. 2007, 3, e62. [Google Scholar] [CrossRef]
- Zhang, X.; Zhaoping, L.; Zhou, T.; Fang, F. Neural activities in V1 create a bottom-up saliency map. Neuron 2012, 73, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Glomb, K.; Cabral, J.; Cattani, A.; Mazzoni, A.; Raj, A.; Franceschiello, B. Computational models in electroencephalography. Brain Topogr. 2021, 35, 142–161. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef]
- Destexhe, A.; Bal, T.; McCormick, D.A.; Sejnowski, T.J. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J. Neurophysiol. 1996, 76, 2049–2070. [Google Scholar] [CrossRef]
- Destexhe, A.; Sejnowski, T.J. Synchronized oscillations in thalamic networks: Insights from modeling studies. Thalamus 1997, 2, 331–371. [Google Scholar]
- Da Silva, F.L.; Van Leeuwen, W.S. The cortical source of the alpha rhythm. Neurosci. Lett. 1977, 6, 237–241. [Google Scholar] [CrossRef]
- Teleńczuk, M.; Teleńczuk, B.; Destexhe, A. Modelling unitary fields and the single-neuron contribution to local field potentials in the hippocampus. J. Physiol. 2020, 598, 3957–3972. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Cowan, J.D. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 1973, 13, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Börgers, C.; Börgers, C. A Wilson-Cowan Model of an Oscillatory EI Network. In An Introduction to Modeling Neuronal Dynamics; Springer: Berlin, Germany, 2017; pp. 175–180. [Google Scholar]
- Bell, T.; Khaira, A.; Stokoe, M.; Webb, M.; Noel, M.; Amoozegar, F.; Harris, A.D. Age-related differences in resting state functional connectivity in pediatric migraine. J. Headache Pain 2021, 22, 65. [Google Scholar] [CrossRef]
- Colon, E.; Ludwick, A.; Wilcox, S.L.; Youssef, A.M.; Danehy, A.; Fair, D.A.; Lebel, A.A.; Burstein, R.; Becerra, L.; Borsook, D. Migraine in the young brain: Adolescents vs. young adults. Front. Hum. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, R.; Rocca, M.A.; Colombo, B.; Valsasina, P.; Meani, A.; Falini, A.; Filippi, M. Dysregulation of multisensory processing stands out from an early stage of migraine: A study in pediatric patients. J. Neurol. 2020, 267, 760–769. [Google Scholar] [CrossRef]
- Silvestro, M.; Tessitore, A.; Caiazzo, G.; Scotto di Clemente, F.; Trojsi, F.; Cirillo, M.; Esposito, F.; Tedeschi, G.; Russo, A. Disconnectome of the migraine brain: A “connectopathy” model. J. Headache Pain 2021, 22, 102. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Steriade, M.; Gloor, P.; Llinas, R.R.; Da Silva, F.L.; Mesulam, M.M. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 481–508. [Google Scholar] [CrossRef]
- Da Silva, F.L. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alamancos, M.A.; Connors, B.W. Cellular mechanisms of the augmenting response: Short-term plasticity in a thalamocortical pathway. J. Neurosci. 1996, 16, 7742–7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, A.C.; Connors, B.W. Two types of network oscillations in neocortex mediated by distinct glutamate receptor subtypes and neuronal populations. J. Neurophysiol. 1996, 75, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alais, D.; Blake, R. Binocular Rivalry; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Blake, R. A neural theory of binocular rivalry. Psychol. Rev. 1989, 96, 145. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R. Minimal physiological conditions for binocular rivalry and rivalry memory. Vis. Res. 2007, 47, 2741–2750. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, F.; Karanovic, O.; Wilson, H. Binocular rivalry in migraine. Cephalalgia 2008, 28, 1327–1338. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Battista, J.; Snyder, J.S.; Carter, O.L. Visual and auditory perceptual rivalry in migraine. Cephalalgia 2011, 31, 1158–1169. [Google Scholar] [CrossRef]
- Robertson, C.E.; Ratai, E.M.; Kanwisher, N. Reduced GABAergic action in the autistic brain. Curr. Biol. 2016, 26, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, A.M.; Knapen, T.; Scholte, H.S.; John-Saaltink, E.S.; Donner, T.H.; Lamme, V.A. GABA shapes the dynamics of bistable perception. Curr. Biol. 2013, 23, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Mentch, J.; Spiegel, A.; Ricciardi, C.; Robertson, C.E. GABAergic inhibition gates perceptual awareness during binocular rivalry. J. Neurosci. 2019, 39, 8398–8407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katyal, S.; He, S.; He, B.; Engel, S.A. Frequency of alpha oscillation predicts individual differences in perceptual stability during binocular rivalry. Hum. Brain Mapp. 2019, 40, 2422–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battista, J.; Badcock, D.R.; McKendrick, A.M. Migraine increases centre-surround suppression for drifting visual stimuli. PLoS ONE 2011, 6, e18211. [Google Scholar] [CrossRef]
- Field, D.T.; Cracknell, R.O.; Eastwood, J.R.; Scarfe, P.; Williams, C.M.; Zheng, Y.; Tavassoli, T. High-dose Vitamin B6 supplementation reduces anxiety and strengthens visual surround suppression. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2852. [Google Scholar] [CrossRef]
- Martin, D.L. Pyridoxal Phosphate, GABA and Seizure Susceptibility. In Proceedings of the Biochemistry of Vitamin B 6 and PQQ; Springer: Berlin, Germany, 1994; pp. 343–347. [Google Scholar]
- Bertalmío, M.; Calatroni, L.; Franceschi, V.; Franceschiello, B.; Gomez Villa, A.; Prandi, D. Visual illusions via neural dynamics: Wilson–Cowan-type models and the efficient representation principle. J. Neurophysiol. 2020, 123, 1606–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, A. Visual contrast processing in migraine. Cephalalgia 2000, 20, 865–880. [Google Scholar] [CrossRef]
- Shepherd, A.J. Local and global motion after-effects are both enhanced in migraine, and the underlying mechanisms differ across cortical areas. Brain 2006, 129, 1833–1843. [Google Scholar] [CrossRef] [Green Version]
- Tibber, M.S.; Guedes, A.; Shepherd, A.J. Orientation discrimination and contrast detection thresholds in migraine for cardinal and oblique angles. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5599–5604. [Google Scholar] [CrossRef]
- Tibber, M.S.; Kelly, M.G.; Jansari, A.; Dakin, S.C.; Shepherd, A.J. An inability to exclude visual noise in migraine. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2539–2546. [Google Scholar] [CrossRef] [Green Version]
- Krause, M.R.; Vieira, P.G.; Thivierge, J.P.; Pack, C.C. Brain stimulation competes with ongoing oscillations for control of spike timing in the primate brain. PLoS Biol. 2022, 20, e3001650. [Google Scholar] [CrossRef]
- Bigal, M.; Rapoport, A.; Aurora, S.; Sheftell, F.; Tepper, S.; Dahlof, C. Satisfaction with current migraine therapy: Experience from 3 centers in US and Sweden. Headache J. Head Face Pain 2007, 47, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Preventive migraine treatment. Contin. Lifelong Learn. Neurol. 2015, 21, 973. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.P.; Fuccaro, M.; Lambru, G. The role of erenumab in the treatment of migraine. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420927119. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the trigeminal system in migraine. Headache J. Head Face Pain 2019, 59, 659–681. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br. J. Clin. Pharmacol. 2015, 80, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.L. Regulatory properties of brain glutamate decarboxylase. Cell. Mol. Neurobiol. 1987, 7, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.K.; Wade, A.R.; Penkman, K.E.; Baker, D.H. Dietary modulation of cortical excitation and inhibition. J. Psychopharmacol. 2017, 31, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Azuma, S.; Inoué, S. Vitamin B12 enhances GABA content but reduces glutamate content in the rat suprachiasmatic nucleus. Am. J. -Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R359–R363. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, H.F.; Tsai, C.H.; Hsu, Y.Y.; Fang, C.J.; Chen, C.J.; Hung, Y.H.; Hu, F.W. Effect of Vitamin B2 supplementation on migraine prophylaxis: A systematic review and meta-analysis. Nutr. Neurosci. 2022, 25, 1801–1812. [Google Scholar] [CrossRef]
- Colombo, B.; Saraceno, L.; Comi, G. Riboflavin and migraine: The bridge over troubled mitochondria. Neurol. Sci. 2014, 35, 141–144. [Google Scholar] [CrossRef]
- Holton, K.F. Micronutrients may Be a unique weapon against the neurotoxic triad of excitotoxicity, oxidative stress and neuroinflammation: A perspective. Front. Neurosci. 2021, 15, 726457. [Google Scholar] [CrossRef] [PubMed]
- Kuriemann, G.; Löscher, W.; Dominick, H.; Palm, G. Disappearance of neonatal seizures and low CSF GABA levels after treatment with vitamin B6. Epilepsy Res. 1987, 1, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. Migraine and epilepsy—Shared mechanisms within the family of episodic disorders. In Jasper’s Basic Mechanisms of the Epilepsies [Internet], 4th ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Kretsch, M.J.; Sauberlich, H.E.; Newbrun, E. Electroencephalographic changes and periodontal status during short-term vitamin B-6 depletion of young, nonpregnant women. Am. J. Clin. Nutr. 1991, 53, 1266–1274. [Google Scholar] [CrossRef]
- Plecko, B.; Stöckler, S. Vitamin B6 dependent seizures. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2009, 36, S73–S77. [Google Scholar]
- Ghin, F.; O’Hare, L.; Pavan, A. Electrophysiological aftereffects of high-frequency transcranial random noise stimulation (hf-tRNS): An EEG investigation. Exp. Brain Res. 2021, 239, 2399–2418. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.C.; Huang, C.C.Y.; Chung, Y.A.; Im, J.J.; Lin, Y.Y.; Ma, C.C.; Tzeng, N.S.; Chang, H.A. High-frequency transcranial random noise stimulation modulates gamma-band EEG source-based large-scale functional network connectivity in patients with schizophrenia: A randomized, double-blind, sham-controlled clinical trial. J. Pers. Med. 2022, 12, 1617. [Google Scholar] [CrossRef]
- Bhola, R.; Kinsella, E.; Giffin, N.; Lipscombe, S.; Ahmed, F.; Weatherall, M.; Goadsby, P.J. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: Evaluation of outcome data for the UK post market pilot program. J. Headache Pain 2015, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, L.; Griffiths, R. Transcranial Electrical Stimulation in Migraine–How Does It Work and What Can We Learn from It? OBM Neurobiol. 2022, 6, 145. [Google Scholar] [CrossRef]
- Antal, A.; Kriener, N.; Lang, N.; Boros, K.; Paulus, W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 2011, 31, 820–828. [Google Scholar] [CrossRef]
- Mansour, A.G.; Ahdab, R.; Khazen, G.; El-Khoury, C.; Sabbouh, T.M.; Salem, M.; Yamak, W.; Chalah, M.A.; Ayache, S.S.; Riachi, N. Transcranial direct current stimulation of the occipital cortex in medication overuse headache: A pilot randomized controlled cross-over study. J. Clin. Med. 2020, 9, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auvichayapat, P.; Janyacharoen, T.; Rotenberg, A.; Tiamkao, S.; Krisanaprakornkit, T.; Sinawat, S.; Punjaruk, W.; Thinkhamrop, B.; Auvichayapat, N. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J. Med. Assoc. Thai. 2012, 95, 1003–1012. [Google Scholar] [PubMed]
- Andrade, S.M.; de Brito Aranha, R.E.L.; de Oliveira, E.A.; de Mendonça, C.T.P.L.; Martins, W.K.N.; Alves, N.T.; Fernández-Calvo, B. Transcranial direct current stimulation over the primary motor vs prefrontal cortex in refractory chronic migraine: A pilot randomized controlled trial. J. Neurol. Sci. 2017, 378, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dalla Volta, G.; Marceglia, S.; Zavarise, P.; Antonaci, F. Cathodal tDCS guided by thermography as adjunctive therapy in chronic migraine patients: A sham-controlled pilot study. Front. Neurol. 2020, 11, 121. [Google Scholar] [CrossRef]
- Rahimi, M.D.; Fadardi, J.S.; Saeidi, M.; Bigdeli, I.; Kashiri, R. Effectiveness of cathodal tDCS of the primary motor or sensory cortex in migraine: A randomized controlled trial. Brain Stimul. 2020, 13, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koninck, B.P.; Brazeau, D.; Guay, S.; Babiloni, A.H.; De Beaumont, L. Transcranial Alternating Current Stimulation to Modulate Alpha Activity: A Systematic Review. Neuromodul. Technol. Neural Interface 2023, 1–36. [Google Scholar] [CrossRef]
- Antal, A.; Bischoff, R.; Stephani, C.; Czesnik, D.; Klinker, F.; Timäus, C.; Chaieb, L.; Paulus, W. Low intensity, transcranial, alternating current stimulation reduces migraine attack burden in a home application set-up: A double-blinded, randomized feasibility study. Brain Sci. 2020, 10, 888. [Google Scholar] [CrossRef]
- Ward, L.M. Physics of neural synchronisation mediated by stochastic resonance. Contemp. Phys. 2009, 50, 563–574. [Google Scholar] [CrossRef]
- Pavan, A.; Ghin, F.; Contillo, A.; Milesi, C.; Campana, G.; Mather, G. Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS): A combined stochastic resonance and equivalent noise approach. Brain Stimul. 2019, 12, 967–977. [Google Scholar] [CrossRef]
- O’Hare, L.; Goodwin, P.; Sharp, A.; Contillo, A.; Pavan, A. Improvement in visual perception after high-frequency transcranial random noise stimulation (hf-tRNS) in those with migraine: An equivalent noise approach. Neuropsychologia 2021, 161, 107990. [Google Scholar] [CrossRef]
- Garg, S.; Williams, S.; Jung, J.; Pobric, G.; Nandi, T.; Lim, B.; Vassallo, G.; Green, J.; Evans, D.G.; Stagg, C.J.; et al. Non-invasive brain stimulation modulates GABAergic activity in Neurofibromatosis 1. Sci. Rep. 2022, 12, 18297. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, V.; Near, J.; Johansen-Berg, H.; Stagg, C.J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 2015, 4, e08789. [Google Scholar] [CrossRef] [PubMed]
- Benwell, C.S.; Coldea, A.; Harvey, M.; Thut, G. Low pre-stimulus EEG alpha power amplifies visual awareness but not visual sensitivity. Eur. J. Neurosci. 2022, 55, 3125–3140. [Google Scholar] [CrossRef]
- Iemi, L.; Busch, N.A.; Laudini, A.; Haegens, S.; Samaha, J.; Villringer, A.; Nikulin, V.V. Multiple mechanisms link prestimulus neural oscillations to sensory responses. Elife 2019, 8, e43620. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Lundqvist, M.; Waite, A.S.; Kopell, N.; Miller, E.K. Layer and rhythm specificity for predictive routing. Proc. Natl. Acad. Sci. USA 2020, 117, 31459–31469. [Google Scholar] [CrossRef] [PubMed]
- Kitzbichler, M.G.; Khan, S.; Ganesan, S.; Vangel, M.G.; Herbert, M.R.; Hämäläinen, M.S.; Kenet, T. Altered development and multifaceted band-specific abnormalities of resting state networks in autism. Biol. Psychiatry 2015, 77, 794–804. [Google Scholar] [CrossRef] [Green Version]
- Tarasi, L.; Magosso, E.; Ricci, G.; Ursino, M.; Romei, V. The directionality of fronto-posterior brain connectivity is associated with the degree of individual autistic traits. Brain Sci. 2021, 11, 1443. [Google Scholar] [CrossRef]
- Wang, J.; Barstein, J.; Ethridge, L.E.; Mosconi, M.W.; Takarae, Y.; Sweeney, J.A. Resting state EEG abnormalities in autism spectrum disorders. J. Neurodev. Disord. 2013, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Ursino, M.; Serra, M.; Tarasi, L.; Ricci, G.; Magosso, E.; Romei, V. Bottom-up vs. top-down connectivity imbalance in individuals with high-autistic traits: An electroencephalographic study. Front. Syst. Neurosci. 2022, 16, 932128. [Google Scholar] [CrossRef]
- Tarasi, L.; Borgomaneri, S.; Romei, V. Antivax attitude in the general population along the autism-schizophrenia continuum and the impact of socio-demographic factors. Front. Psychol. 2023, 14, 1059676. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Miller, L.J.; Nielsen, D.M.; Schoen, S.A. The presence of migraines and its association with sensory hyperreactivity and anxiety symptomatology in children with autism spectrum disorder. Autism 2014, 18, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Dickinson, J.E.; Battista, J.; McKendrick, A.M.; Badcock, D.R. Evidence for increased internal noise in migraineurs for contrast and shape processing. Cephalalgia 2012, 32, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, J.; Di Gregorio, F.; Avenanti, A.; Thut, G.; Romei, V. Two oscillatory correlates of attention control in the alpha-band with distinct consequences on perceptual gain and metacognition. J. Neurosci. 2023, 43, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Coldea, A.; Veniero, D.; Morand, S.; Trajkovic, J.; Romei, V.; Harvey, M.; Thut, G. Effects of Rhythmic Transcranial Magnetic Stimulation in the Alpha-Band on Visual Perception Depend on Deviation From Alpha-Peak Frequency: Faster Relative Transcranial Magnetic Stimulation Alpha-Pace Improves Performance. Front. Neurosci. 2022, 16, 886342. [Google Scholar] [CrossRef]
- Ronconi, L.; Busch, N.A.; Melcher, D. Alpha-band sensory entrainment alters the duration of temporal windows in visual perception. Sci. Rep. 2018, 8, 11810. [Google Scholar] [CrossRef] [PubMed]
- Wutz, A.; Melcher, D.; Samaha, J. Frequency modulation of neural oscillations according to visual task demands. Proc. Natl. Acad. Sci. USA 2018, 115, 1346–1351. [Google Scholar] [CrossRef] [Green Version]
- Cooke, J.; Poch, C.; Gillmeister, H.; Costantini, M.; Romei, V. Oscillatory properties of functional connections between sensory areas mediate cross-modal illusory perception. J. Neurosci. 2019, 39, 5711–5718. [Google Scholar] [CrossRef] [Green Version]


| Study | Summary of Findings | Paradigm | Sample |
|---|---|---|---|
| Hall et al. [45] | Strong desynchronisation of occipital and temporal alpha band during the aura period, terminating abruptly upon disappearance of scintillations. | Analysis of MEG activity during an episode of scintillating scotoma | Patient with aura |
| Bjørk and Sand [46] | Increased occipital alpha power during the attack phase. | 5 min eyes-closed EEG-videometry | 41 migraine patients (33 MO, 8 MA) |
| Bjørk et al. [47] | Alpha peak frequency reduction correlated with increasing disease- and attack-duration. Frequency variability increased before the attack, while peak alpha power increased during the attack. | 5 min eyes-closed EEG-videometry | 41 migraine patients (33 MO, 8 MA) and 32 controls |
| Clemens et al. [49] | Increased alpha power in the migraineurs than in the control group in the right occipital region. | Resting-state EEG | 20 MO patients and 17 controls. |
| Lia et al. [50] | Increased relative alpha power in migraineurs, particularly in posterior regions. | Resting-state EEG | 17 MO and 11 MA patients and 28 controls. |
| Gomez-Pilar et al. [51] | Difference in the upper-alpha band in migraineurs, with differences evident in the central and left parietal regions. | Resting-state EEG | 87 patients with migraine (45 with episodic and 42 with chronic migraine) and 39 controls. |
| O’Hare et al. [52] | Increased occipital resting-state alpha power in the mixed migraine group compared to controls. | Resting-state EEG | 13 patients with migraine and 17 controls |
| Neufeld et al. [61] | Faster peak alpha frequency over posterior areas in migraineurs compared to control groups. The peak alpha power was lower among patients than controls. | Resting-state EEG | 42 migraineurs and 20 controls. |
| Bjørk et al. [62] | Absolute power and alpha peak frequency were similar between groups. | EEG with photic stimulation on different days. | 41 migraineurs (33 MO, 8 MA), 32 controls. |
| Fong et al. [69] | Migraineurs had significantly less posterior alpha power prior to the onset of the stimulus relative to controls. Migraineurs showed greater poststimulus alpha desynchronisation. | EEG activity during visual stimulation | 28 migraineurs (11 MO, 17 MA) and 29 controls. |
| de Tommaso et al. [73] | Increased alpha synchronisation in migraineurs compared to controls using flash stimulation. | EEG activity during flash stimuli presentation | 45 MO patients and 24 controls. |
| Study | Summary of Findings | Paradigm | Sample |
|---|---|---|---|
| Hall et al. [45] | Strong gamma-band desynchronisation in temporal areas for the 1 min of the migraine aura, slowly returning to baseline levels over a 16-min period. | Analysis of MEG activity during an episode of scintillating scotoma | Patient with aura |
| Liu et al. [94] | Increase in gamma power in the lateral cortical regions in those with acute migraine (both MA and MO) compared to controls. | Analysis of MEG activity during the headache attack. | 22 patients with an acute migraine and 22 controls. |
| Li et al. [95] | Gamma-band oscillations in left frontal and temporal areas have higher power in migraineurs compared to control groups. | Resting-state MEG | 25 migraine patients during the headache-free phase and 25 controls. |
| Bassez et al. [96] | No difference between controls and MO groups in the gamma-band response to painful laser stimulation. | EEG during laser stimulation | 23 MO patients and 23 controls |
| Coppola et al. [28] | Evoked gamma-band amplitude recorded over the occipital region is increased in between attacks in MA. | EEG during visual stimulation | 15 MO and 15 MA patients |
| Lisicki et al. [106] | Ictal and chronic migraine patients showed increased gamma-band power. | EEG during visual stimulation | 70 migraine patients (30 interictal, 20 ictal episodic migraineurs, 20 chronic migraineurs), and 20 controls. |
| Coppola et al. [107] | Early high-frequency oscillations were smaller in the mixed migraine (MO and MA) during the interictal phase compared to control group | EEG activity of parietal area during somatosensory stimulation | 29 migraineurs (14 MO, 15 MA) during the interictal phase, 13 migraineurs (9 MO, 4 MA) during the ictal phase, and 15 controls. |
| Study | Summary of Findings | Paradigm | Sample |
|---|---|---|---|
| Prescot et al. [124] | No differences in glutamate levels between migraineurs and control participants in the anterior cingulate cortex and insula. | MRS | 10 migraine patients, and 8 controls |
| Gonzalez de la Aleja et al. [127] | Higher glutamate levels in migraineurs. Higher glutamate/glutamine ratio in the occipital cortex of migraineurs compared with controls. | MRS | 27 patients with migraine (19 MO, 8 MA) and 19 controls. |
| Bathel et al. [128] | Increased Glx concentration in the thalamus and occipital regions in MO. | MRS | 15 MO patients and 15 controls. |
| Siniatchkin et al. [129] | Migraineurs showed significantly higher glutamate/creatine ratios (Glx/Cr). | MRS | 10 MA patients and 10 controls. |
| Zielman et al. [130] | Higher glutamate concentration in MO compared to controls, but not MA compared to controls | MRS | 63 patients with migraine (36 MO, 27 MA) and 27 controls. |
| Wang et al. [131] | Higher Glx/water ratios in chronic but not episodic migraine in the periaqueductal gray. | MRS | 25 chronic migraine patients, 24 episodic migraine patients and 16 controls. |
| Niddam et al. [132] | No group differences in the concentration of glutamate and glutamine. | MRS | 25 chronic migraine patients, 24 episodic migraine patients and 25 controls. |
| Peek et al. [133] | Higher levels of glutamate in migraineurs compared to controls. | Meta-Analysis | 35 studies were included investigating combinations of migraine (n = 11), musculoskeletal pain (n = 8), chronic pain syndromes (n = 9) and miscellaneous pain (n = 10). |
| Bridge et al. [134] | Glutamate levels in migraineurs, but not controls, correlated with BOLD signal in the primary visual cortex during visual stimulation. | MRS | 13 MA patients and 13 controls. |
| Study | Summary of Findings | Paradigm | Sample |
|---|---|---|---|
| Bridge et al. [134] | GABA levels in the occipital cortex were lower in migraineurs than controls. | MRS | 13 MA patients and 13 controls. |
| Bigal et al. [138] | GABA levels were not significantly different in migraineurs and controls. When pooling the MO and MA patients, GABA concentration was lower in individuals with severe headaches in the previous month. | MRS | 9 MA patients, 10 MO patients, and 9 controls. |
| Onderwater et al. [139] | GABA levels increased from interictal towards the preictal state in migraineurs compared with controls in a provoked migraine attack. | MRS | 24 MO patients and 13 controls. |
| Wang et al. [131] | Chronic migraineurs had significantly lower levels of GABA/water and GABA/creatine in the dentate nucleus. | MRS | 25 chronic migraine patients, 24 episodic migraine patients, and 16 controls. |
| Wu et al. [140] | Lower GABA concentration in the anterior cingulate cortex and medial prefrontal lobe in migraineurs than controls. | MRS | 28 MO patients and 28 controls. |
| Chan et al. [141] | Occipital GABA levels were similar between groups. | MRS | 9 MA patients, 7 MO patients, and 16 controls. |
| Zhang et al. [147] | Lower GABA concentration in chronic migraine.GABA/Glx ratio was lower in the chronic than in the episodic group. | MRS | 26 patients (episodic migraine = 11; chronic migraine = 15) and 16 controls. |
| Chan et al. [142] | No significant difference in GABA and glutamate levels were found between groups. | MRS | 18 patients with migraine and 18 controls |
| Vieira et al. [145] | Chronic migraineurs with comorbid depression showed reduced GABA levels. | Cerebrospinal fluid (CSF) analysis | 14 chronic migraine patients, with or without depression and 14 controls. |
| Strmose et al. [143] | No difference was found in GABA/total creatine levels in either the occipital cortex or in the somatosensory cortex. | MRS | 14 MA patients and 16 controls. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hare, L.; Tarasi, L.; Asher, J.M.; Hibbard, P.B.; Romei, V. Excitation-Inhibition Imbalance in Migraine: From Neurotransmitters to Brain Oscillations. Int. J. Mol. Sci. 2023, 24, 10093. https://doi.org/10.3390/ijms241210093
O’Hare L, Tarasi L, Asher JM, Hibbard PB, Romei V. Excitation-Inhibition Imbalance in Migraine: From Neurotransmitters to Brain Oscillations. International Journal of Molecular Sciences. 2023; 24(12):10093. https://doi.org/10.3390/ijms241210093
Chicago/Turabian StyleO’Hare, Louise, Luca Tarasi, Jordi M. Asher, Paul B. Hibbard, and Vincenzo Romei. 2023. "Excitation-Inhibition Imbalance in Migraine: From Neurotransmitters to Brain Oscillations" International Journal of Molecular Sciences 24, no. 12: 10093. https://doi.org/10.3390/ijms241210093
APA StyleO’Hare, L., Tarasi, L., Asher, J. M., Hibbard, P. B., & Romei, V. (2023). Excitation-Inhibition Imbalance in Migraine: From Neurotransmitters to Brain Oscillations. International Journal of Molecular Sciences, 24(12), 10093. https://doi.org/10.3390/ijms241210093











