Immunomodulatory Role of Interferons in Viral and Bacterial Infections
Abstract
1. Introduction
2. Results and Discussion
2.1. Classification, Characteristics, and Mechanisms of Action of Interferons
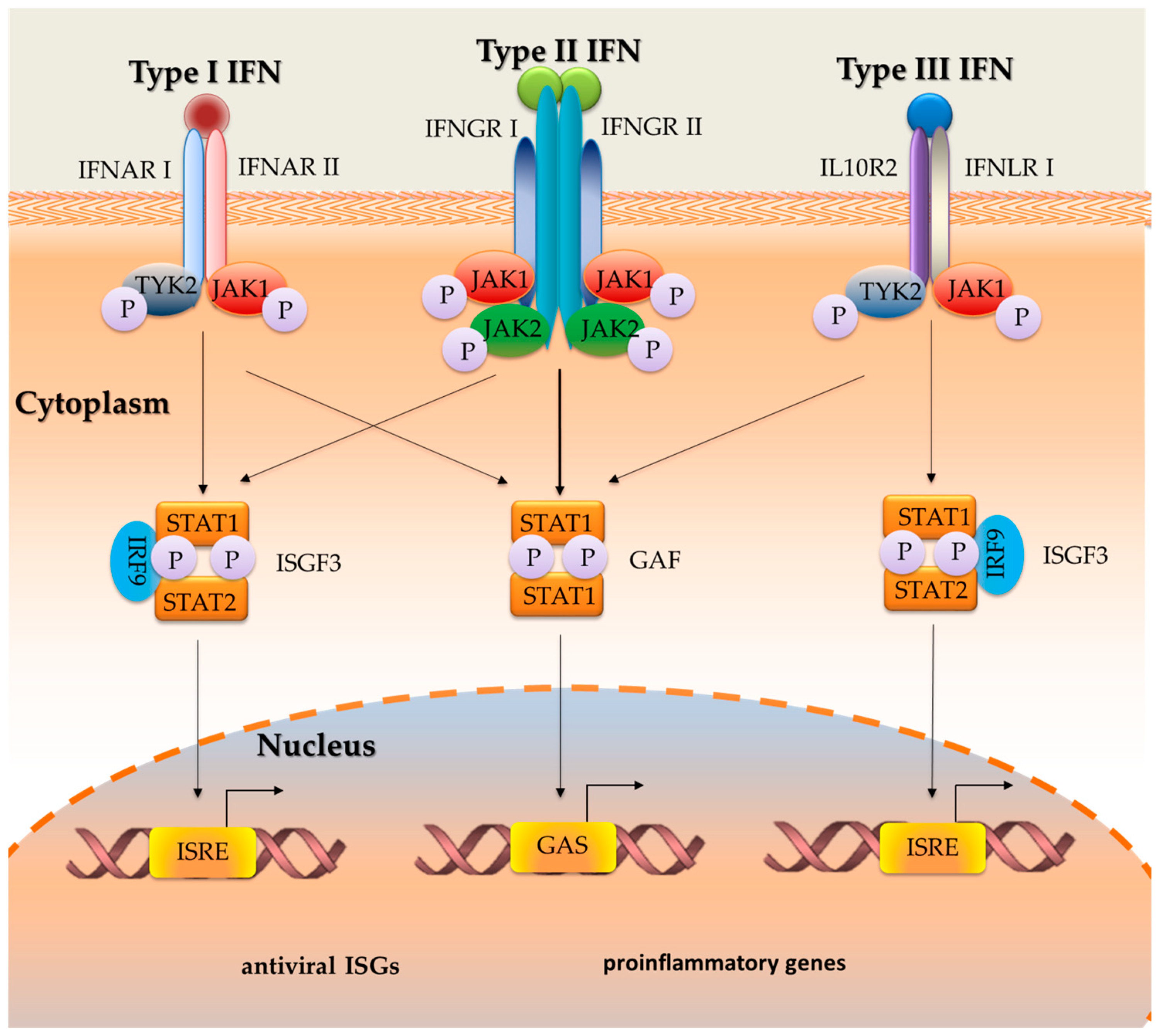
2.1.1. Type I IFNs
| Types of IFNs | Subtypes | Gens | Protein Length (AA) | Molecular Mass (kDa) | Protein ID | PI | Amino Acid Composition | Secondary Structure | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Hydrophilic | % Hydrophobic | α-Helix | β-Strand | ||||||||
| Type I | IFN-α1/13 | IFNA1; IFNA13 | 189 | 21,725 | P01562 | 5.17 | 57.14% | 42.86% | 6 | 0 | [29] |
| IFN-α2 | IFNA2 | 188 | 21,578 | P01563 | 6.05 | 57.14% | 42.86% | 2 | 0 | [30] | |
| IFN-α4 | IFNA4 | 189 | 21,808 | P05014 | 5.54 | 58.20% | 41.80% | 5 | 0 | [31] | |
| IFN-α5 | IFNA5 | 189 | 21,942 | P01569 | 5.48 | 57.14% | 42.86% | 5 | 0 | [32] | |
| IFN-α6 | ITGA6 | 1130 | 126,606 | P23229 | 5.94 | 60.88% | 39.12% | 5 | 61 | [33] | |
| IFN-α7 | CHRNA7 | 502 | 56,449 | P36544 | 5.73 | 52.79% | 47.21% | 12 | 16 | [34] | |
| IFN-α8 | IFNA8 | 189 | 21,989 | P32881 | 5.17 | 57.14% | 42.86% | 5 | 0 | [35] | |
| IFN-α10 | IFNA10 | 189 | 21,835 | P01566 | 5.75 | 57.67% | 42.33% | 5 | 0 | [36] | |
| IFN-α14 | IFNA14 | 189 | 22,063 | P01570 | 6.49 | 56.61% | 43.39% | 5 | 0 | [37] | |
| IFN-α16 | IFI16 | 785 | 88,256 | Q16666 | 8.42 | 61.78% | 38.22% | 15 | 25 | [38] | |
| IFN-α17 | IFNA17 | 189 | 21,728 | P01571 | 5.50 | 57.67% | 42.33% | 5 | 0 | [39] | |
| IFN-α21 | IFNA21 | 189 | 21,741 | P01568 | 6.07 | 57.14% | 42.86% | 5 | 0 | [40] | |
| IFN-β | IFNB1 | 187 | 22,294 | P01574 | 8.06 | 60.43% | 39.57% | 8 | 0 | [41] | |
| IFN-ω | IFNW1 | 195 | 22,319 | P05000 | 8.37 | 56.41% | 43.59% | 5 | 0 | [42] | |
| IFN-ɛ | IFNE | 208 | 24,414 | Q86WN2 | 7.91 | 57.69% | 42.31% | 6 | 0 | [43] | |
| IFN-κ | IFNK | 207 | 25,218 | Q9P0W0 | 7.66 | 60.39% | 39.61% | 7 | 0 | [44] | |
| Type II | IFN-γ | IFNG | 166 | 19,348 | P01579 | 8.74 | 63.85% | 36.15% | 7 | 0 | [45,46] |
| Type III | IFNλ1/IL-29 | IFNL1 | 200 | 21,898 | Q8IU54 | 8.09 | 52.50% | 47.50% | 5 | 0 | [47] |
| IFNλ2/IL-28A | IFNL2 | 200 | 22,288 | Q8IZJ0 | 7.13 | 52.00% | 48.00% | 6 | 0 | [48] | |
| IFNλ3/IL-28B | IFNL3 | 196 | 21,706 | Q8IZI9 | 7.52 | 52.04% | 47.96% | 6 | 0 | [49] | |
| IFN-λ4 | IFNL4 | 179 | 19,675 | K9M1U5 | 11.17 | 50.84% | 49.16% | 4 | 0 | [50,51] | |
2.1.2. Type II IFN
2.1.3. Type III IFN
2.2. The Role of the Signal Transduction Mechanism in the Activity of Interferons
2.3. The Role of Interferons in the Course of Viral Infections
2.3.1. Herpes Simplex Virus (HSV)
2.3.2. Influenza Virus
2.3.3. Hepatitis C Virus (HCV)
2.3.4. Lymphocytic Choroid Meningitis Virus (LCMV)
2.3.5. Human Immunodeficiency Virus (HIV)
2.3.6. Epstein–Barr Virus (EBV)
2.3.7. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
2.4. The Role of Interferons in the Course of Bacterial Infections
3. Materials and Methods
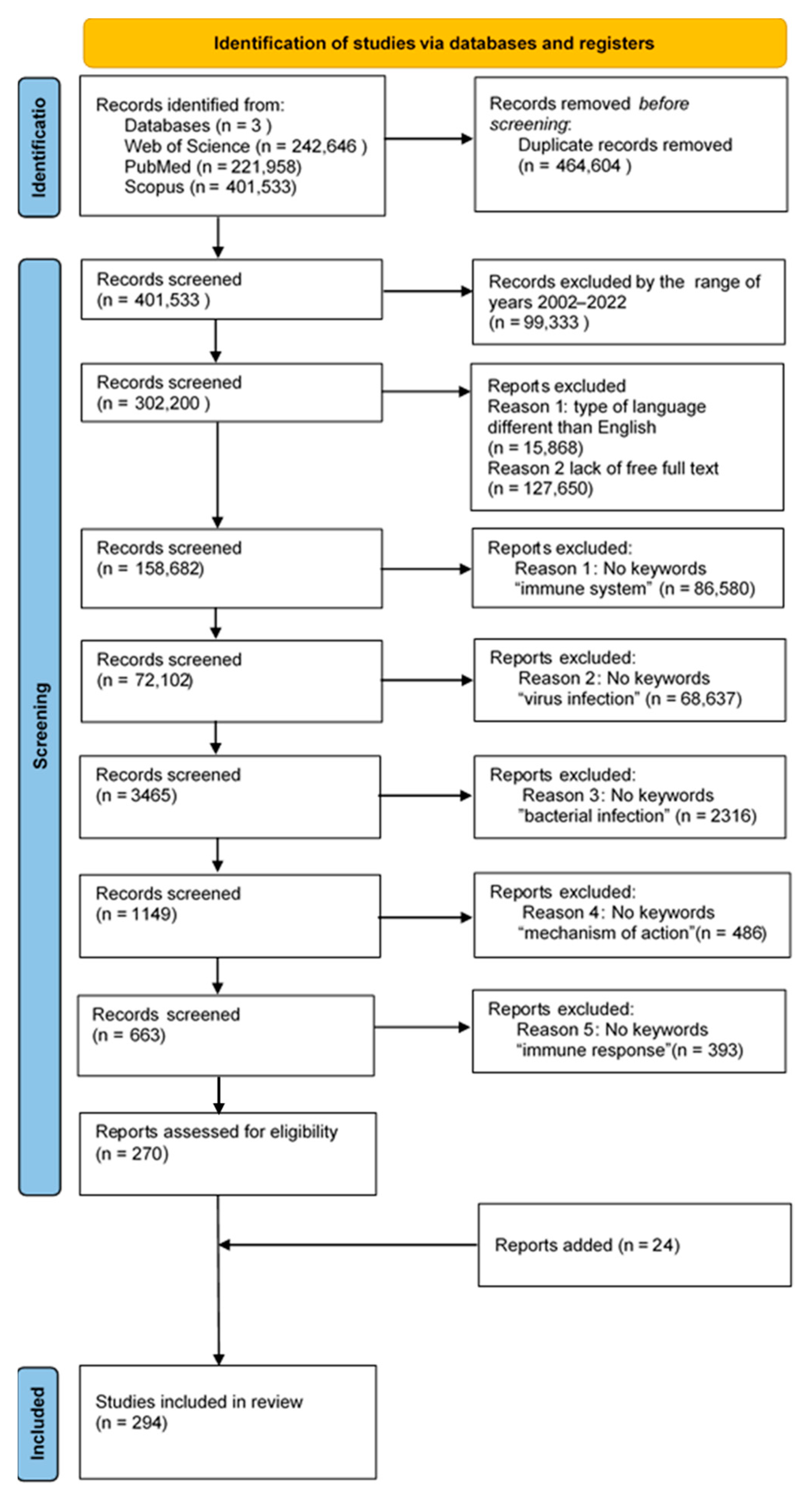
3.1. Search Strategy, Study Selection, and Data Extraction
3.2. Bioinformatics Analyses of the Amino Acid Sequences of IFNs
3.3. Analysis of the Amino Acid Sequence Identity of IFNs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMV | cytomegalovirus |
| DCs | dendritic cells |
| dsRNA | double-stranded RNA |
| EBV | Epstein–Barr virus |
| GAF | gamma-interferon activation factor |
| GAS | gamma ray sequences |
| GBP | guanylate binding proteins |
| HBLV | Human B-lymphotropic virus |
| HCV | Hepatitis C Virus |
| HHV-6 | human herpesvirus type 6 |
| HHV-7 | human herpesvirus type 7 |
| HIV | human immunodeficiency virus |
| HSV | herpes simplex virus |
| IFITM | interferon-induced transmembrane proteins |
| IFN-ɛ | interferon epsilon |
| IFNs | interferons |
| IFN-α | interferon alpha |
| IFN-β | interferon beta |
| IFN-γ | interferon gamma |
| IFN-δ | interferon delta |
| IFN-ζ | interferon zeta |
| IFN-κ | interferon kappa |
| IFN-ν | interferon nu |
| IFN-τ | interferon tau |
| IFN-χ | interferon chi |
| IFN-ω | interferon omega |
| ISGs | interferon-stimulated genes |
| ISRE | interferon regulatory factor |
| JAK2 | Janus kinase 2 |
| LCMV | lymphocytic choriomeningitis virus |
| LCMV | Lymphocytic Choriomeningitis Virus |
| MDA5 | melanoma-differentiation-associated gene 5 |
| MHC | Major histocompatibility complex |
| NOS2 | nitric oxide synthase 2 |
| OAS | oligoadenylate synthases |
| pDCs | plasmacytoid dendritic cells |
| RIG-I | retinoic-acid-inducible protein I |
| SARS-CoV | coronavirus |
| STAT | Signal transducer and activator of transcription |
| TRIM | triremembered motif proteins |
| TYK2 | tyrosine kinase 2 |
| VZV | herpes zoster |
References
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at age 50: Past, Current and Future Impact on Biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald-Bocarsly, P.; Dai, J.; Singh, S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008, 19, 3–19. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Interferon-γ: A historical perspective. Cytokine Growth Factor Rev. 2009, 20, 97–113. [Google Scholar] [CrossRef] [PubMed]
- De Andrea, M.; Ravera, R.; Gioia, D.; Gariglio, M.; Landolfo, S. The Interferon System: An Overview. Eur. J. Paediatric Neurol. 2002, 6, A41–A46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, H.; Hu, H. Overview of Interferon: Characteristics, signaling and anti-cancer effect. Arch. Biotechnol. Biomed. 2017, 1, 001–016. [Google Scholar] [CrossRef]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Kopitar-Jerala, N. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Zhao, F.-R.; Wang, W.; Zheng, Q.; Zhang, Y.-G.; Chen, J. The regulation of antiviral activity of interferon epsilon. Front. Microbiol. 2022, 13, 6481. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2017, 10, a028423. [Google Scholar] [CrossRef] [PubMed]
- Wack, A. Interfering with transmission. Elife 2018, 7, e37552. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Stubbs, S.; Perez-Gracia, J.L.; Rouzaut, A.; Sanmamed, M.F.; Le Bon, A.; Melero, I. Direct Effects of Type I Interferons on Cells of the Immune System. Clin. Cancer Res. 2011, 17, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Bedsaul, J.R.; Zaritsky, L.A.; Zoon, K.C. Type I Interferon-Mediated Induction of Antiviral Genes and Proteins Fails to Protect Cells from the Cytopathic Effects of Sendai Virus Infection. J. Interf. Cytokine Res. 2016, 36, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Shibabaw, T.; Molla, M.D.; Teferi, B.; Ayelign, B. Role of IFN and Complements System: Innate Immunity in SARS-CoV-2. J. Inflamm. Res. 2020, 13, 507–518. [Google Scholar] [CrossRef]
- Boxx, G.M.; Cheng, G. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef]
- Capobianchi, M.R.; Uleri, E.; Caglioti, C.; Dolei, A. Type I IFN family members: Similarity, differences and interaction. Cytokine Growth Factor Rev. 2014, 26, 103–111. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, L.; Zhang, X.; Liu, J.; Jia, M.-A.; Zhang, J.; Liu, J.; Wang, F. Types of Interferons and Their Expression in Plant Systems. J. Interf. Cytokine Res. 2022, 42, 62–71. [Google Scholar] [CrossRef]
- Borden, E.C. Interferons α and β in cancer: Therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef]
- Fox, L.E.; Locke, M.C.; Lenschow, D.J. Context Is Key: Delineating the Unique Functions of IFNα and IFNβ in Disease. Front. Immunol. 2020, 11, 606874. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Zhuang, Z.; Cai, S.; Zhao, Z.; Zhou, L.; Zhang, J.; Wang, P.-H.; Zhao, J.; Cui, J. Main protease of SARS-CoV-2 serves as a bifunctional molecule in restricting type I interferon antiviral signaling. Signal Transduct. Target. Ther. 2020, 5, 221. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Vosslamber, S.; Mantel, E.; de Ridder, S.; Wesseling, J.G.; van der Pouw Kraan, T.C.T.M.; Leurs, C.; Hegen, H.; Deisenhammer, F.; Killestein, J.; et al. Physiological evidence for diversification of IFNα- and IFNβ-mediated response programs in different autoimmune diseases. Arthritis Res. Ther. 2016, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef]
- Manry, J.; Laval, G.; Patin, E.; Fornarino, S.; Itan, Y.; Fumagalli, M.; Sironi, M.; Tichit, M.; Bouchier, C.; Casanova, J.-L.; et al. Evolutionary genetic dissection of human interferons. J. Exp. Med. 2011, 208, 2747–2759. [Google Scholar] [CrossRef]
- Decker, T.; Müller, M.; Stockinger, S. The Yin and Yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005, 5, 675–687. [Google Scholar] [CrossRef]
- Aggarwal, A.; Stella, A.O.; Walker, G.; Akerman, A.; Esneau, C.; Milogiannakis, V.; Burnett, D.L.; McAllery, S.; Silva, M.R.; Lu, Y.; et al. Platform for isolation and characterization of SARS-CoV-2 variants enables rapid characterization of Omicron in Australia. Nat. Microbiol. 2022, 7, 896–908. [Google Scholar] [CrossRef]
- IFNA1—Interferon Alpha-1/13—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01562/entry (accessed on 16 February 2023).
- Interferon Alpha-2′—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q14645/entry (accessed on 16 February 2023).
- IFNA4—Interferon Alpha-4—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P05014/entry (accessed on 16 February 2023).
- IFNA5—Interferon Alpha-5—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01569/entry (accessed on 16 February 2023).
- ITGA6—Integrin Alpha-6—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P23229/entry (accessed on 16 February 2023).
- CHRNA7—Neuronal Acetylcholine Receptor Subunit Alpha-7—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P36544/entry (accessed on 16 February 2023).
- IFNA8—Interferon Alpha-8—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P32881/entry (accessed on 16 February 2023).
- IFNA10—Interferon Alpha-10—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01566/entry (accessed on 16 February 2023).
- IFNA14—Interferon Alpha-14—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01570/entry (accessed on 16 February 2023).
- IFI16—Gamma-Interferon-Inducible Protein 16—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q16666/entry (accessed on 16 February 2023).
- IFNA17—Interferon Alpha-17—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01571/entry (accessed on 16 February 2023).
- Virion Membrane Protein A21—Variola Virus|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P0DSR8/entry (accessed on 16 February 2023).
- IFNB1—Interferon Beta—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01574/entry (accessed on 16 February 2023).
- IFNW1—Interferon Omega-1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P05000/entry (accessed on 16 February 2023).
- IFNE—Interferon Epsilon—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q86WN2/entry (accessed on 16 February 2023).
- IFNK—Interferon Kappa—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9P0W0/entry (accessed on 16 February 2023).
- IFNG—Interferon Gamma—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P01579/entry (accessed on 16 February 2023).
- Razaghi, A.; Owens, L.; Heimann, K. Review of the recombinant human interferon gamma as an immunotherapeutic: Impacts of production platforms and glycosylation. J. Biotechnol. 2016, 240, 48–60. [Google Scholar] [CrossRef]
- IFNL1—Interferon Lambda-1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q8IU54/entry (accessed on 16 February 2023).
- IFNL2—Interferon Lambda-2—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q8IZJ0/entry (accessed on 16 February 2023).
- IFNL3—Interferon Lambda-3—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q8IZI9/entry (accessed on 16 February 2023).
- IFNL4—Interferon Lambda-4—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/K9M1U5/entry (accessed on 16 February 2023).
- Takaoka, A.; Yanai, H. Interferon signalling network in innate defence. Cell. Microbiol. 2006, 8, 907–922. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, M.L.; Pervolaraki, K.; Boulant, S. Differential Regulation of Type I and Type III Interferon Signaling. Int. J. Mol. Sci. 2019, 20, 1445. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- IFNG Gene—GeneCards|IFNG Protein|IFNG Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=IFNG (accessed on 16 February 2023).
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef]
- Ping, Z.; Qi, J.; Sun, Y.; Lu, G.; Shi, Y.; Wang, X.; Gao, G.F.; Wang, M. Crystal Structure of the Interferon Gamma Receptor Alpha Chain from Chicken Reveals an Undetected Extra Helix Compared with the Human Counterparts. J. Interf. Cytokine Res. 2014, 34, 41–51. [Google Scholar] [CrossRef]
- Gad, H.H.; Dellgren, C.; Hamming, O.J.; Vends, S.; Paludan, S.R.; Hartmann, R. Interferon-λ Is Functionally an Interferon but Structurally Related to the Interleukin-10 Family. J. Biol. Chem. 2009, 284, 20869–20875. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Rivera, A.; Parker, D.; Durbin, J.E. Type III IFNs: Beyond antiviral protection. Semin. Immunol. 2019, 43, 101303. [Google Scholar] [CrossRef]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I.; et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013, 45, 164–171. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kienes, I.; Weidl, T.; Mirza, N.; Chamaillard, M.; Kufer, T.A. Role of NLRs in the Regulation of Type I Interferon Signaling, Host Defense and Tolerance to Inflammation. Int. J. Mol. Sci. 2021, 22, 1301. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, C.; Liu, Y.; Rao, Y.; Feng, P. Herpes Simplex Virus and Pattern Recognition Receptors: An Arms Race. Front. Immunol. 2021, 11, 3799. [Google Scholar] [CrossRef] [PubMed]
- Jounai, N.; Kobiyama, K.; Takeshita, F.; Ishii, K.J. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front. Cell Infect. Microbiol. 2013, 2, 168. [Google Scholar] [CrossRef]
- Danastas, K.; Miranda-Saksena, M.; Cunningham, A.L. Herpes Simplex Virus Type 1 Interactions with the Interferon System. Int. J. Mol. Sci. 2020, 21, 5150. [Google Scholar] [CrossRef]
- Schenten, D.; Medzhitov, R. The Control of Adaptive Immune Responses by the Innate Immune System. Adv. Immunol. 2011, 109, 87–124. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef]
- Abe, T.; Barber, G.N. Cytosolic-DNA-Mediated, STING-Dependent Proinflammatory Gene Induction Necessitates Canonical NF-ΚB Activation through TBK1. J. Virol. 2014, 88, 5328–5341. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Favoreel, H.W. Herpesviruses and the Type III Interferon System. Virol. Sin. 2021, 36, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016, 16, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.L.; Tiu, R.V. Biological Rationale and Clinical Use of Interferon in the Classical BCR-ABL-Negative Myeloproliferative Neoplasms. J. Interf. Cytokine Res. 2013, 33, 145–153. [Google Scholar] [CrossRef]
- Ding, S.; Khoury-Hanold, W.; Iwasaki, A.; Robek, M.D. Epigenetic Reprogramming of the Type III Interferon Response Potentiates Antiviral Activity and Suppresses Tumor Growth. PLoS Biol. 2014, 12, e1001758. [Google Scholar] [CrossRef]
- Wu, W.; Metcalf, J.P. The Role of Type I IFNs in Influenza: Antiviral Superheroes or Immunopathogenic Villains? J. Innate Immun. 2020, 12, 437–447. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Rojas, M.; Luz-Crawford, P.; Soto-Rifo, R.; Reyes-Cerpa, S.; Toro-Ascuy, D. The Landscape of IFN/ISG Signaling in HIV-1-Infected Macrophages and Its Possible Role in the HIV-1 Latency. Cells 2021, 10, 2378. [Google Scholar] [CrossRef]
- Yang, E.; Li, M.M.H. All About the RNA: Interferon-Stimulated Genes That Interfere With Viral RNA Processes. Front. Immunol. 2020, 11, 5024. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Stertz, S.; Kochs, G. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect. 2007, 9, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx Proteins: Antiviral Gatekeepers That Restrain the Uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Braun, B.A.; Marcovitz, A.; Camp, J.G.; Jia, R.; Bejerano, G. Mx1 and Mx2 key antiviral proteins are surprisingly lost in toothed whales. Proc. Natl. Acad. Sci. USA 2015, 112, 8036–8040. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Vidal, S.M. Functional Diversity of Mx Proteins: Variations on a Theme of Host Resistance to Infection. Genome Res. 2002, 12, 527–530. [Google Scholar] [CrossRef]
- Silverman, R.H. Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef]
- Ogden, K.M.; Hu, L.; Jha, B.K.; Sankaran, B.; Weiss, S.R.; Silverman, R.H.; Patton, J.T.; Prasad, B.V.V. Structural Basis for 2′-5′-Oligoadenylate Binding and Enzyme Activity of a Viral RNase L Antagonist. J. Virol. 2015, 89, 6633–6645. [Google Scholar] [CrossRef] [PubMed]
- Pindel, A.; Sadler, A. The Role of Protein Kinase R in the Interferon Response. J. Interf. Cytokine Res. 2011, 31, 59–70. [Google Scholar] [CrossRef] [PubMed]
- McAllister, C.S.; Taghavi, N.; Samuel, C.E. Protein Kinase PKR Amplification of Interferon β Induction Occurs through Initiation Factor eIF-2α-mediated Translational Control. J. Biol. Chem. 2012, 287, 36384–36392. [Google Scholar] [CrossRef]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2019, 11, 480. [Google Scholar] [CrossRef]
- Zhao, C.; Collins, M.N.; Hsiang, T.-Y.; Krug, R.M. Interferon-induced ISG15 pathway: An ongoing virus–host battle. Trends Microbiol. 2013, 21, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; McGilvray, I. The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in Hepatitis C Virus infection and resistance to interferon therapy. Int. J. Biochem. Cell Biol. 2011, 43, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-G.; Yan, X.-Z.; Xie, Y.-Y.; Gao, X.-C.; Song, A.-X.; Zhang, D.-E.; Hu, H.-Y. Different Roles for Two Ubiquitin-like Domains of ISG15 in Protein Modification*. J. Biol. Chem. 2008, 283, 13370–13377. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-C.; Young, H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef]
- Subramanian, G.; Kuzmanovic, T.; Zhang, Y.; Peter, C.B.; Veleeparambil, M.; Chakravarti, R.; Sen, G.C.; Chattopadhyay, S. A new mechanism of interferon’s antiviral action: Induction of autophagy, essential for paramyxovirus replication, is inhibited by the interferon stimulated gene, TDRD7. PLoS Pathog. 2018, 14, e1006877. [Google Scholar] [CrossRef]
- Fritsch, S.D.; Weichhart, T. Effects of Interferons and Viruses on Metabolism. Front. Immunol. 2016, 7, 630. [Google Scholar] [CrossRef]
- Fensterl, V.; Sen, G.C. Interferons and viral infections. Biofactors 2009, 35, 14–20. [Google Scholar] [CrossRef]
- Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 16 February 2023).
- Herpes Simplex Virus (HSV) Infections. Available online: https://empendium.com/mcmtextbook/social/chapter/B31.II.18.1.8. (accessed on 16 February 2023).
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- Gerada, C.; Steain, M.; Campbell, T.M.; McSharry, B.; Slobedman, B.; Abendroth, A. Granzyme B Cleaves Multiple Herpes Simplex Virus 1 and Varicella-Zoster Virus (VZV) Gene Products, and VZV ORF4 Inhibits Natural Killer Cell Cytotoxicity. J. Virol. 2019, 93, e01140-19. [Google Scholar] [CrossRef]
- Nagda, M. The Role of Granzyme B in the Pathogenesis of Ocular HSV—1 Infection. Dissertation Thesis, University of nevada Honors College, Las Vegas, NV, USA, 2018. [Google Scholar]
- Cartier, A.; Broberg, E.; Komai, T.; Henriksson, M.; Masucci, M.G. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 2003, 10, 1320–1328. [Google Scholar] [CrossRef]
- You, H.; Lin, Y.; Lin, F.; Yang, M.; Li, J.; Zhang, R.; Huang, Z.; Shen, Q.; Tang, R.; Zheng, C. β-Catenin Is Required for the cGAS/STING Signaling Pathway but Antagonized by the Herpes Simplex Virus 1 US3 Protein. J. Virol. 2020, 94, e01847-19. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Gong, M.-J.; Zhao, F.-R.; Shao, J.-J.; Xie, Y.-L.; Zhang, Y.-G.; Chang, H.-Y. Type I Interferons: Distinct Biological Activities and Current Applications for Viral Infection. Cell Physiol. Biochem. 2018, 51, 2377–2396. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, B.; Liu, S.-Y.; Iyer, S.S.; Yu, Y.; Wu, A.; Cheng, G. Positive Feedback Regulation of Type I IFN Production by the IFN-Inducible DNA Sensor cGAS. J. Immunol. 2015, 194, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Sirén, J.; Pirhonen, J.; Julkunen, I.; Matikainen, S. IFN-Alpha Regulates TLR-Dependent Gene Expression of IFN-Alpha, IFN-Beta, IL-28, and IL-29. J. Immunol. 2005, 174, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Gary, H.; Lebon, P.; Dalloul, A.H. Type I Interferon Production by Plasmacytoid Dendritic Cells and Monocytes Is Triggered by Viruses, but the Level of Production Is Controlled by Distinct Cytokines. J. Interf. Cytokine Res. 2002, 22, 653–659. [Google Scholar] [CrossRef]
- Swiecki, M.; Wang, Y.; Gilfillan, S.; Colonna, M. Plasmacytoid Dendritic Cells Contribute to Systemic but Not Local Antiviral Responses to HSV Infections. PLoS Pathog. 2013, 9, e1003728. [Google Scholar] [CrossRef]
- Schuster, P.; Thomann, S.; Werner, M.; Vollmer, J.; Schmidt, B. A Subset of Human Plasmacytoid Dendritic Cells Expresses CD8α upon Exposure to Herpes Simplex Virus Type 1. Front. Microbiol. 2015, 6, 557. [Google Scholar] [CrossRef]
- Donaghy, H.; Bosnjak, L.; Harman, A.N.; Marsden, V.; Tyring, S.K.; Meng, T.-C.; Cunningham, A.L. Role for Plasmacytoid Dendritic Cells in the Immune Control of Recurrent Human Herpes Simplex Virus Infection. J. Virol. 2009, 83, 1952–1961. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Zhu, L.; Wang, X.; Richers, B.; Leung, D.Y.M.; Bin, L. Interferon Kappa Is Important for Keratinocyte Host Defense against Herpes Simplex Virus-1. J. Immunol. Res. 2020, 2020, 5084682. [Google Scholar] [CrossRef]
- Posavad, C.M.; Zhao, L.; Mueller, D.E.; Stevens, C.E.; Huang, M.L.; Wald, A.; Corey, L. Persistence of Mucosal T-Cell Responses to Herpes Simplex Virus Type 2 in the Female Genital Tract. Mucosal Immunol. 2014, 8, 115–126. [Google Scholar] [CrossRef]
- Kim, M.; Truong, N.R.; James, V.; Bosnjak, L.; Sandgren, K.J.; Harman, A.N.; Nasr, N.; Bertram, K.M.; Olbourne, N.; Sawleshwarkar, S.; et al. Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin. PLoS Pathog. 2015, 11, e1004812. [Google Scholar] [CrossRef] [PubMed]
- Harpur, C.M.; Kato, Y.; Dewi, S.T.; Stankovic, S.; Johnson, D.N.; Bedoui, S.; Whitney, P.G.; Lahoud, M.H.; Caminschi, I.; Heath, W.R.; et al. Classical Type 1 Dendritic Cells Dominate Priming of Th1 Responses to Herpes Simplex Virus Type 1 Skin Infection. J. Immunol. 2019, 202, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Koelle, D.; Cao, J.; Vazquez, J.; Huang, M.L.; Hladik, F.; Wald, A.; Corey, L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007, 204, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hladik, F.; Woodward, A.L.; Klock, A.; Peng, T.; Johnston, C.; Remington, M.; Magaret, A.; Koelle, D.; Wald, A.; et al. Persistence of HIV-1 receptor–positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 2009, 15, 886–892. [Google Scholar] [CrossRef]
- Ellermann-Eriksen, S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol. J. 2005, 2, 59. [Google Scholar] [CrossRef]
- Sainz, B.; Halford, W.P. Alpha/Beta Interferon and Gamma Interferon Synergize To Inhibit the Replication of Herpes Simplex Virus Type 1. J. Virol. 2002, 76, 11541–11550. [Google Scholar] [CrossRef]
- Mikloska, Z.; Cunningham, A.L. Alpha and Gamma Interferons Inhibit Herpes Simplex Virus Type 1 Infection and Spread in Epidermal Cells after Axonal Transmission. J. Virol. 2001, 75, 11821–11826. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, J.; Hwangbo, Y.; Corey, L.; Bumgarner, R.E. Independent and Cooperative Antiviral Actions of Beta Interferon and Gamma Interferon against Herpes Simplex Virus Replication in Primary Human Fibroblasts. J. Virol. 2008, 82, 1934–1945. [Google Scholar] [CrossRef]
- Nturibi, E.; Bhagwat, A.R.; Coburn, S.; Myerburg, M.M.; Lakdawala, S.S. Intracellular Colocalization of Influenza Viral RNA and Rab11A Is Dependent upon Microtubule Filaments. J. Virol. 2017, 91, e01179-17. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. JAMA J. Am. Med. Assoc. 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; von der Malsburg, A.; Dick, A.; Faelber, K.; Schröder, G.F.; Haller, O.; Kochs, G.; Daumke, O. Structure of Myxovirus Resistance Protein A Reveals Intra- and Intermolecular Domain Interactions Required for the Antiviral Function. Immunity 2011, 35, 514–525. [Google Scholar] [CrossRef]
- Turan, K.; Mibayashi, M.; Sugiyama, K.; Saito, S.; Numajiri, A.; Nagata, K. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 2004, 32, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Killip, M.J.; Staeheli, P.; Randall, R.; Jackson, D. The Human Interferon-Induced MxA Protein Inhibits Early Stages of Influenza A Virus Infection by Retaining the Incoming Viral Genome in the Cytoplasm. J. Virol. 2013, 87, 13053–13058. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hinson, E.R.; Cresswell, P. The Interferon-Inducible Protein Viperin Inhibits Influenza Virus Release by Perturbing Lipid Rafts. Cell Host Microbe 2007, 2, 96–105. [Google Scholar] [CrossRef]
- Li, K.; Markosyan, R.M.; Zheng, Y.-M.; Golfetto, O.; Bungart, B.; Li, M.; Ding, S.; He, Y.; Liang, C.; Lee, J.C.; et al. IFITM Proteins Restrict Viral Membrane Hemifusion. PLoS Pathog. 2013, 9, e1003124. [Google Scholar] [CrossRef]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-Like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis E Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef]
- Kato, H.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Uematsu, S.; Matsui, K.; Tsujimura, T.; Takeda, K.; Fujita, T.; Takeuchi, O.; et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity 2005, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ishii, K.J.; Kumar, H.; Tanimoto, T.; Coban, C.; Uematsu, S.; Kawai, T.; Akira, S. Differential Role of TLR- and RLR-Signaling in the Immune Responses to Influenza A Virus Infection and Vaccination. J. Immunol. 2007, 179, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.K.; Muehmer, M.; Mages, J.; Gueinzius, K.; Hess, C.; Heeg, K.; Bals, R.; Lang, R.; Dalpke, A.H. Differential Recognition of TLR-Dependent Microbial Ligands in Human Bronchial Epithelial Cells. J. Immunol. 2007, 178, 3134–3142. [Google Scholar] [CrossRef]
- Guillot, L.; Le Goffic, R.; Bloch, S.; Escriou, N.; Akira, S.; Chignard, M.; Si-Tahar, M. Involvement of Toll-like Receptor 3 in the Immune Response of Lung Epithelial Cells to Double-stranded RNA and Influenza A Virus. J. Biol. Chem. 2005, 280, 5571–5580. [Google Scholar] [CrossRef]
- Le Goffic, R.; Pothlichet, J.; Vitour, D.; Fujita, T.; Meurs, E.; Chignard, M.; Si-Tahar, M. Cutting Edge: Influenza A Virus Activates TLR3-Dependent Inflammatory and RIG-I-Dependent Antiviral Responses in Human Lung Epithelial Cells. J. Immunol. 2007, 178, 3368–3372. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.A.; García-Sastre, A.; Katze, M.G.; et al. Distinct RIG-I and MDA5 Signaling by RNA Viruses in Innate Immunity. J. Virol. 2008, 82, 335–345. [Google Scholar] [CrossRef]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef]
- García-Sastre, A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011, 162, 12–18. [Google Scholar] [CrossRef]
- Engel, D.A. The influenza virus NS1 protein as a therapeutic target. Antivir. Res. 2013, 99, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Donelan, N.R.; Basler, C.F.; García-Sastre, A. A Recombinant Influenza A Virus Expressing anRNA-Binding-Defective NS1 Protein Induces High Levels of BetaInterferon and Is Attenuated inMice. J. Virol. 2003, 77, 13257–13266. [Google Scholar] [CrossRef]
- Steidle, S.; Martínez-Sobrido, L.; Mordstein, M.; Lienenklaus, S.; García-Sastre, A.; Stäheli, P.; Kochs, G. Glycine 184 in Nonstructural Protein NS1 Determines the Virulence of Influenza A Virus Strain PR8 without Affecting the Host Interferon Response. J. Virol. 2010, 84, 12761–12770. [Google Scholar] [CrossRef]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.-S.; Huang, I.-C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; García-Sastre, A. Influenza A Virus NS1 Targets the Ubiquitin Ligase TRIM25 to Evade Recognition by the Host Viral RNA Sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef]
- Hayman, A.; Comely, S.; Lackenby, A.; Murphy, S.; McCauley, J.; Goodbourn, S.; Barclay, W. Variation in the Ability of Human Influenza A Viruses to Induce and Inhibit the IFN-Beta Pathway. Virology 2006, 347, 52–64. [Google Scholar] [CrossRef]
- Satterly, N.; Tsai, P.-L.; van Deursen, J.; Nussenzveig, D.R.; Wang, Y.; Faria, P.A.; Levay, A.; Levy, D.E.; Fontoura, B.M.A. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. USA 2007, 104, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Killip, M.J.; Fodor, E.; Randall, R.E. Influenza virus activation of the interferon system. Virus Res. 2015, 209, 11–22. [Google Scholar] [CrossRef]
- Blach, S.; A Terrault, N.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Hepatitis, C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 16 February 2023).
- Alter, M.J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 2436–2441. [Google Scholar] [CrossRef]
- Simmonds, P. The Origin of Hepatitis C Virus. In Hepatitis C Virus: From Molecular Virology to Antiviral Therapy; Bartenschlager, R., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–15. ISBN 978-3-642-27340-7. [Google Scholar]
- Afzal, M.S.; Iqbal, M.A.; Ramamoorthy, V.; Campa, A.; Rubens, M.; Martinez, S.S.; Fleetwood, C.; Stewart, T.; Liuzzi, J.P.; George, F.; et al. Hepatitis C Virus and Interferon-Free Antiviral Therapeutics Revolution: Implications for Pakistan. Viral Immunol. 2017, 30, 252–257. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, A.; Hotta, H. Impact of hepatitis C virus heterogeneity on interferon sensitivity: An overview. World J. Gastroenterol. 2014, 20, 7555–7569. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Perelson, A.S. Treatment of Hepatitis C Virus Infection With Interferon and Small Molecule Direct Antivirals: Viral Kinetics and Modeling. Crit. Rev. Immunol. 2010, 30, 131–148. [Google Scholar] [CrossRef]
- Mazana, J. Interferon Treatment of Chronic Hepatitis C. Basic Imunobiology and Imunopathology of the Liver. Rev. Española De Sanid. Penit. 2013, 15, 16–22. [Google Scholar] [CrossRef]
- Badr, G.; Bédard, N.; Abdel-Hakeem, M.S.; Trautmann, L.; Willems, B.; Villeneuve, J.-P.; Haddad, E.K.; Sékaly, R.P.; Bruneau, J.; Shoukry, N.H. Early Interferon Therapy for Hepatitis C Virus Infection Rescues Polyfunctional, Long-Lived CD8+ Memory T Cells. J. Virol. 2008, 82, 10017–10031. [Google Scholar] [CrossRef]
- Chen, S.L.; Morgan, T.R. The Natural History of Hepatitis C Virus (HCV) Infection. Int. J. Med. Sci. 2006, 3, 47–52. [Google Scholar] [CrossRef]
- Rauch, A.; Gaudieri, S.; Thio, C.; Bochud, P.-Y. Host genetic determinants of spontaneous hepatitis C clearance. Pharmacogenomics 2009, 10, 1819–1837. [Google Scholar] [CrossRef]
- Ge, D.; Fellay, J.; Thompson, A.J.; Simon, J.S.; Shianna, K.V.; Urban, T.J.; Heinzen, E.L.; Qiu, P.; Bertelsen, A.H.; Muir, A.J.; et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009, 461, 399–401. [Google Scholar] [CrossRef]
- Suppiah, V.; Moldovan, M.; Ahlenstiel, G.; Berg, T.; Weltman, M.; Abate, M.L.; Bassendine, M.; Spengler, U.; Dore, G.J.; Powell, E.; et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat. Genet. 2009, 41, 1100–1104. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishida, N.; Sugiyama, M.; Kurosaki, M.; Matsuura, K.; Sakamoto, N.; Nakagawa, M.; Korenaga, M.; Hino, K.; Hige, S.; et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009, 41, 1105–1109. [Google Scholar] [CrossRef]
- Heim, M.H. Innate immunity and HCV. J. Hepatol. 2013, 58, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Thimme, R.; Binder, M.; Bartenschlager, R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol. Rev. 2012, 36, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Baral, S.; Dixit, N.M. Interferon at the cellular, individual, and population level in hepatitis C virus infection: Its role in the interferon-free treatment era. Immunol. Rev. 2018, 285, 55–71. [Google Scholar] [CrossRef]
- Wieland, S.; Makowska, Z.; Campana, B.; Calabrese, D.; Dill, M.T.; Chung, J.; Chisari, F.V.; Heim, M.H. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology 2013, 59, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Perales, C.; Beach, N.M.; Gallego, I.; Soria, M.E.; Quer, J.; Esteban, J.I.; Rice, C.; Domingo, E.; Sheldon, J. Response of Hepatitis C Virus to Long-Term Passage in the Presence of Alpha Interferon: Multiple Mutations and a Common Phenotype. J. Virol. 2013, 87, 7593–7607. [Google Scholar] [CrossRef]
- Rowlands, A.G.; Panniers, R.; Henshaw, E.C. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J. Biol. Chem. 1988, 263, 5526–5533. [Google Scholar] [CrossRef]
- Kau, A.; Vermehren, J.; Sarrazin, C. Treatment predictors of a sustained virologic response in hepatitis B and C. J. Hepatol. 2008, 49, 634–651. [Google Scholar] [CrossRef]
- Frese, M.; Pietschmann, T.; Moradpour, D.; Haller, O.; Bartenschlager, R. Interferon-α inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J. Gen. Virol. 2001, 82, 723–733. [Google Scholar] [CrossRef]
- Shi, X.; Jiao, B.; Chen, Y.; Li, S.; Chen, L. MxA is a positive regulator of type I IFN signaling in HCV infection. J. Med. Virol. 2017, 89, 2173–2180. [Google Scholar] [CrossRef]
- Itsui, Y.; Sakamoto, N.; Kurosaki, M.; Kanazawa, N.; Tanabe, Y.; Koyama, T.; Takeda, Y.; Nakagawa, M.; Kakinuma, S.; Sekine, Y.; et al. Expressional screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J. Viral Hepat. 2006, 13, 690–700. [Google Scholar] [CrossRef]
- Lymphocytic Choriomeningitis (LCM)|CDC. Available online: https://www.cdc.gov/vhf/lcm/index.html (accessed on 16 February 2023).
- Knust, B.; Holman, R.C.; Redd, J.; Mehal, J.M.; Grube, S.M.; MacNeil, A.; Cheek, J.; Rollin, P. Lymphocytic Choriomeningitis Virus Infections among American Indians. Emerg. Infect. Dis. 2013, 19, 328–329. [Google Scholar] [CrossRef]
- Ledesma, J.; Fedele, C.G.; Carro, F.; Lledó, L.; Sánchez-Seco, M.P.; Tenorio, A.; Soriguer, R.C.; Saz, J.V.; Domínguez, G.; Rosas, M.F.; et al. Independent Lineage of Lymphocytic Choriomeningitis Virus in Wood Mice (Apodemus sylvaticus), Spain. Emerg. Infect. Dis. 2009, 15, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Clingan, J.M.; Ostrow, K.; Hosiawa, K.A.; Chen, Z.J.; Matloubian, M. Differential Roles for RIG-I–like Receptors and Nucleic Acid-Sensing TLR Pathways in Controlling a Chronic Viral Infection. J. Immunol. 2012, 188, 4432–4440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kurt-Jones, E.A.; Mandell, L.; Cerny, A.; Chan, M.; Golenbock, D.T.; Finberg, R.W. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 2005, 35, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Kawai, T.; Sato, S.; Ishii, K.J.; Coban, C.; Hemmi, H.; Yamamoto, M.; Terai, K.; Matsuda, M.; Inoue, J.-I.; Uematsu, S.; et al. Interferon-Alpha Induction through Toll-like Receptors Involves a Direct Interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004, 5, 1061–1068. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Zhou, S.; Cerny, A.M.; Zacharia, A.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Finberg, R.W. Induction and Inhibition of Type I Interferon Responses by Distinct Components of Lymphocytic Choriomeningitis Virus. J. Virol. 2010, 84, 9452–9462. [Google Scholar] [CrossRef]
- Marié, I.; Durbin, J.E.; Levy, D.E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998, 17, 6660–6669. [Google Scholar] [CrossRef]
- Suprunenko, T.; Hofer, M.J. Complexities of Type I Interferon Biology: Lessons from LCMV. Viruses 2019, 11, 172. [Google Scholar] [CrossRef]
- Ni Lee, L.; Burke, S.; Montoya, M.; Borrow, P. Multiple Mechanisms Contribute to Impairment of Type 1 Interferon Production during Chronic Lymphocytic Choriomeningitis Virus Infection of Mice1. J. Immunol. 2009, 182, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Murira, A.; Mindt, B.C.; Germain, A.; Tarrab, E.; Lapierre, P.; Fritz, J.H.; Lamarre, A. Type I Interferon Impairs Specific Antibody Responses Early during Establishment of LCMV Infection. Front. Immunol. 2016, 7, 564. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.; Teijaro, J.R.; De La Torre, J.C.; Oldstone, M.B.A. Early Virus-Host Interactions Dictate the Course of a Persistent Infection. PLoS Pathog. 2015, 11, e1004588. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riaño, E.; Cheng, B.Y.; de la Torre, J.C.; Martínez-Sobrido, L. D471G Mutation in LCMV-NP Affects Its Ability to Self-associate and Results in a Dominant Negative Effect in Viral RNA Synthesis. Viruses 2012, 4, 2137–2161. [Google Scholar] [CrossRef]
- Martínez-Sobrido, L.; Emonet, S.; Giannakas, P.; Cubitt, B.; García-Sastre, A.; de la Torre, J.C. Identification of Amino Acid Residues Critical for the Anti-Interferon Activity of the Nucleoprotein of the Prototypic Arenavirus Lymphocytic Choriomeningitis Virus. J. Virol. 2009, 83, 11330–11340. [Google Scholar] [CrossRef]
- Doyle, T.; Goujon, C.; Malim, M.H. HIV-1 and interferons: Who’s interfering with whom? Nat. Rev. Genet. 2015, 13, 403–413. [Google Scholar] [CrossRef]
- Bosinger, S.E.; Utay, N.S. Type I Interferon: Understanding Its Role in HIV Pathogenesis and Therapy. Curr. HIV/AIDS Rep. 2015, 12, 41–53. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, J.; Li, J.; Li, D.; Li, G.; Li, F.; Zhang, Q.; Yu, H.; Yasui, F.; Ye, C.; et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Investig. 2016, 127, 269–279. [Google Scholar] [CrossRef]
- Collaboration, T.H.-C. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids 2010, 24, 123–137. [Google Scholar] [CrossRef]
- Ortega, M.; Brier, M.R.; Ances, B.M. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. Aids 2015, 29, 703–712. [Google Scholar] [CrossRef]
- Zhen, A.; Rezek, V.; Youn, C.; Lam, B.; Chang, N.; Rick, J.; Carrillo, M.; Martin, H.; Kasparian, S.; Syed, P.; et al. Targeting type I interferon–mediated activation restores immune function in chronic HIV infection. J. Clin. Investig. 2016, 127, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gargan, S.; Ahmed, S.; Mahony, R.; Bannan, C.; Napoletano, S.; O’Farrelly, C.; Borrow, P.; Bergin, C.; Stevenson, N.J. HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-viral ISG Induction. Ebiomedicine 2018, 30, 203–216. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Tran, J.T.; Sanchez, D.J. HIV blocks Type I IFN signaling through disruption of STAT1 phosphorylation. J. Endotoxin Res. 2018, 24, 490–500. [Google Scholar] [CrossRef]
- Ezeonwumelu, I.J.; Garcia-Vidal, E.; Ballana, E. JAK-STAT Pathway: A Novel Target to Tackle Viral Infections. Viruses 2021, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Grechko, A.V.; Orekhov, A.N.; Bobryshev, Y.V. An immunoregulatory role of dendritic cell-derived exosomes versus HIV-1 infection: Take it easy but be warned. Ann. Transl. Med. 2017, 5, 362. [Google Scholar] [CrossRef] [PubMed]
- Harman, A.N.; Nasr, N.; Feetham, A.; Galoyan, A.; Alshehri, A.A.; Rambukwelle, D.; Botting, R.A.; Hiener, B.M.; Diefenbach, E.; Diefenbach, R.J.; et al. HIV Blocks Interferon Induction in Human Dendritic Cells and Macrophages by Dysregulation of TBK1. J. Virol. 2015, 89, 6575–6584. [Google Scholar] [CrossRef]
- Okumura, A.; Alce, T.; Lubyova, B.; Ezelle, H.; Strebel, K.; Pitha, P.M. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology 2008, 373, 85–97. [Google Scholar] [CrossRef]
- Khan, H.; Sumner, R.P.; Rasaiyaah, J.; Tan, C.P.; Rodriguez-Plata, M.T.; Van Tulleken, C.; Fink, D.; Zuliani-Alvarez, L.; Thorne, L.; Stirling, D.; et al. HIV-1 Vpr antagonizes innate immune activation by targeting karyopherin-mediated NF-κB/IRF3 nuclear transport. Elife 2020, 9, e60821. [Google Scholar] [CrossRef]
- Martinelli, E.; Cicala, C.; Van Ryk, D.; Goode, D.J.; Macleod, K.; Arthos, J.; Fauci, A.S. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3396–3401. [Google Scholar] [CrossRef]
- Wie, S.-H.; Du, P.; Luong, T.Q.; Rought, S.E.; Beliakova-Bethell, N.; Lozach, J.; Corbeil, J.; Kornbluth, R.S.; Richman, D.D.; Woelk, C.H. HIV Downregulates Interferon-Stimulated Genes in Primary Macrophages. J. Interf. Cytokine Res. 2013, 33, 90–95. [Google Scholar] [CrossRef]
- He, B.; Tran, J.T.; Sanchez, D.J. Manipulation of Type I Interferon Signaling by HIV and AIDS-Associated Viruses. J. Immunol. Res. 2019, 2019, 8685312. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Scagnolari, C.; Antonelli, G. Type I interferon and HIV: Subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev. 2018, 40, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Voigt, S.; Tagawa, T.; Albanese, M.; Chen, Y.-F.A.; Chen, Y.; Fachko, D.N.; Pich, D.; Göbel, C.; Skalsky, R.L.; et al. Multiple Viral microRNAs Regulate Interferon Release and Signaling Early during Infection with Epstein-Barr Virus. mBio 2021, 12, e03440-20. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N. EBV-Induced Oncogenesis. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-82714-0. [Google Scholar]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef] [PubMed]
- Mui, U.N.; Haley, C.T.; Tyring, S.K. Viral Oncology: Molecular Biology and Pathogenesis. J. Clin. Med. 2017, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Chen, K.; Young, K.H. Epstein–Barr virus-positive T/NK-cell lymphoproliferative disorders. Exp. Mol. Med. 2015, 47, e133. [Google Scholar] [CrossRef]
- Liu, X.; Sadaoka, T.; Krogmann, T.; Cohen, J.I. Epstein-Barr Virus (EBV) Tegument Protein BGLF2 Suppresses Type I Interferon Signaling To Promote EBV Reactivation. J. Virol. 2020, 94, e00258-20. [Google Scholar] [CrossRef]
- Mui, U.N.; Haley, C.T.; Vangipuram, R.; Tyring, S.K. Human oncoviruses: Mucocutaneous manifestations, pathogenesis, therapeutics, and prevention: Hepatitis viruses, human T-cell leukemia viruses, herpesviruses, and Epstein-Barr virus. J. Am. Acad. Dermatol. 2019, 81, 23–41. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Al Zaidan, L.; Malki, A. Epstein–Barr Virus-Associated Malignancies: Roles of Viral Oncoproteins in Carcinogenesis. Front. Oncol. 2018, 8, 265. [Google Scholar] [CrossRef]
- Yin, H.; Qu, J.; Peng, Q.; Gan, R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med. Microbiol. Immunol. 2018, 208, 573–583. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Wang, C.; Gan, R. Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int. 2021, 21, 93. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Bowie, A.G. Sensing and Signaling in Antiviral Innate Immunity. Curr. Biol. 2010, 20, R328–R333. [Google Scholar] [CrossRef]
- Barber, G.N. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr. Opin. Immunol. 2011, 23, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, A.G.; Young, L.S. LMP1 structure and signal transduction. Semin. Cancer Biol. 2001, 11, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Negishi, H.; Taniguchi, T. The IRF Family Transcription Factors at the Interface of Innate and Adaptive Immune Responses. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Brumm, K.; Zhang, L. The Latent Membrane Protein 1 of Epstein-Barr Virus (EBV) Primes EBV Latency Cells for Type I Interferon Production. J. Biol. Chem. 2006, 281, 9163–9169. [Google Scholar] [CrossRef]
- Zhang, L.; Pagano, J.S.; Servant, M.J.; Tenoever, B.; Lin, R.; Barnes, B.; Lubyova, B.; Pitha, P.M.; Levy, D.E.; Marié, I.; et al. Review: Structure and Function of IRF-7. J. Interf. Cytokine Res. 2002, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.E.; Roman, R.M.; Rudenga, B.J.; Holers, V.M.; Craft, J.E. Epstein-Barr virus promotes interferon-α production by plasmacytoid dendritic cells. Arthritis Rheum. 2010, 62, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Yuen, K.-S.; Botelho, M.G.; Jin, D.-Y. Epstein–Barr Virus and Innate Immunity: Friends or Foes? Microorganisms 2019, 7, 183. [Google Scholar] [CrossRef]
- Chemudupati, M.; Kenney, A.D.; Smith, A.C.; Fillinger, R.J.; Zhang, L.; Zani, A.; Liu, S.-L.; Anderson, M.Z.; Sharma, A.; Yount, J.S. Butyrate Reprograms Expression of Specific Interferon-Stimulated Genes. J. Virol. 2020, 94, e00326-20. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Inoue, K.; Adachi-Takasawa, K.; Takada, K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt’s lymphoma. EMBO J. 2002, 21, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, N.; Ravanshad, M.; Asadi, B.; Kianfar, R.; Maleki, A. Investigation of IL-2 and IFN-γ to EBV Peptides in Stimulated Whole Blood among Multiple Sclerosis Patients and Healthy Individuals. Intervirology 2021, 64, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lutz, C.T.; Jones, K.; Wockner, L.; Brennan, R.M.; Keane, C.; Chattopadhyay, P.K.; Roederer, M.; Price, D.A.; Cole, D.K.; et al. Interferon-γControl of EBV-Transformed B Cells: A Role for CD8+T Cells That Poorly Kill EBV-Infected Cells. Viral Immunol. 2002, 15, 213–225. [Google Scholar] [CrossRef]
- Christian, M. Dendritic cells during Epstein Barr virus infection. Front. Microbiol. 2014, 5, 308. [Google Scholar] [CrossRef]
- Petersen, T.; Møller-Larsen, A.; Ellermann-Eriksen, S.; Thiel, S.; Christensen, T. Effects of interferon-beta therapy on elements in the antiviral immune response towards the human herpesviruses EBV, HSV, and VZV, and to the human endogenous retroviruses HERV-H and HERV-W in multiple sclerosis. J. Neuroimmunol. 2012, 249, 105–108. [Google Scholar] [CrossRef]
- Siegel, A.M.; Heimall, J.; Freeman, A.F.; Hsu, A.P.; Brittain, E.; Brenchley, J.M.; Douek, D.C.; Fahle, G.H.; Cohen, J.I.; Holland, S.M.; et al. A Critical Role for STAT3 Transcription Factor Signaling in the Development and Maintenance of Human T Cell Memory. Immunity 2011, 35, 806–818. [Google Scholar] [CrossRef]
- Gaglia, M.M. Anti-viral and pro-inflammatory functions of Toll-like receptors during gamma-herpesvirus infections. Virol. J. 2021, 18, 218. [Google Scholar] [CrossRef]
- Valente, R.M.; Ehlers, E.; Xu, D.; Ahmad, H.; Steadman, A.; Blasnitz, L.; Zhou, Y.; Kastanek, L.; Meng, B.; Zhang, L. Toll-like Receptor 7 Stimulates the Expression of Epstein-Barr Virus Latent Membrane Protein 1. PLoS ONE 2012, 7, e43317. [Google Scholar] [CrossRef]
- Severa, M.; Giacomini, E.; Gafa, V.; Anastasiadou, E.; Rizzo, F.; Corazzari, M.; Romagnoli, A.; Trivedi, P.; Fimia, G.M.; Coccia, E.M. EBV stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: Implications for viral immune escape. Eur. J. Immunol. 2012, 43, 147–158. [Google Scholar] [CrossRef]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human Coronaviruses: A Review of Virus–Host Interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef]
- Ribero, M.S.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Min, Y.-Q.; Huang, M.; Sun, X.; Deng, F.; Wang, H.; Ning, Y.-J. Immune evasion of SARS-CoV-2 from interferon antiviral system. Comput. Struct. Biotechnol. J. 2021, 19, 4217–4225. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Näslundslund, T.I.; Liljeström, P.; Weber, F.; Reis e Sousa, C. RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5’-Phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef]
- Setaro, A.C.; Gaglia, M.M. All hands on deck: SARS-CoV-2 proteins that block early anti-viral interferon responses. Curr. Res. Virol. Sci. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Rashid, F.; Xie, Z.; Suleman, M.; Shah, A.; Khan, S.; Luo, S. Roles and Functions of SARS-CoV-2 Proteins in Host Immune Evasion. Front. Immunol. 2022, 13, 4122. [Google Scholar] [CrossRef] [PubMed]
- Raj, R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochem. Biophys. Rep. 2020, 25, 100847. [Google Scholar] [CrossRef] [PubMed]
- Lokugamage, K.G.; Hage, A.; de Vries, M.; Valero-Jimenez, A.M.; Schindewolf, C.; Dittmann, M.; Rajsbaum, R.; Menachery, V.D. Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. J. Virol. 2020, 94, e01410-20. [Google Scholar] [CrossRef] [PubMed]
- Baczenas, J.J.; Andersen, H.; Rashid, S.; Yarmosh, D.; Puthuveetil, N.; Parker, M.; Bradford, R.; Florence, C.; Stemple, K.J.; Lewis, M.G.; et al. Propagation of SARS-CoV-2 in Calu-3 Cells to Eliminate Mutations in the Furin Cleavage Site of Spike. Viruses 2021, 13, 2434. [Google Scholar] [CrossRef]
- Busnadiego, I.; Fernbach, S.; Pohl, M.O.; Karakus, U.; Huber, M.; Trkola, A.; Stertz, S.; Hale, B.G. Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio 2020, 11, e01928-20. [Google Scholar] [CrossRef]
- Rebendenne, A.; Valadão, A.L.C.; Tauziet, M.; Maarifi, G.; Bonaventure, B.; McKellar, J.; Planès, R.; Nisole, S.; Arnaud-Arnould, M.; Moncorgé, O.; et al. SARS-CoV-2 Triggers an MDA-5-Dependent Interferon Response Which Is Unable To Control Replication in Lung Epithelial Cells. J. Virol. 2021, 95, e02415-20. [Google Scholar] [CrossRef]
- Grossegesse, M.; Bourquain, D.; Neumann, M.; Schaade, L.; Schulze, J.; Mache, C.; Wolff, T.; Nitsche, A.; Doellinger, J. Deep Time Course Proteomics of SARS-CoV- and SARS-CoV-2-Infected Human Lung Epithelial Cells (Calu-3) Reveals Strong Induction of Interferon-Stimulated Gene Expression by SARS-CoV-2 in Contrast to SARS-CoV. J. Proteome Res. 2022, 21, 459–469. [Google Scholar] [CrossRef]
- Felgenhauer, U.; Schoen, A.; Gad, H.H.; Hartmann, R.; Schaubmar, A.R.; Failing, K.; Drosten, C.; Weber, F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020, 295, 13958–13964. [Google Scholar] [CrossRef]
- Schroeder, S.; Pott, F.; Niemeyer, D.; Veith, T.; Richter, A.; Muth, D.; Goffinet, C.; A Müller, M.; Drosten, C. Interferon antagonism by SARS-CoV-2: A functional study using reverse genetics. Lancet Microbe 2021, 2, e210–e218. [Google Scholar] [CrossRef]
- Taylor, J.K.; Coleman, C.M.; Postel, S.; Sisk, J.M.; Bernbaum, J.G.; Venkataraman, T.; Sundberg, E.J.; Frieman, M.B. Severe Acute Respiratory Syndrome Coronavirus ORF7a Inhibits Bone Marrow Stromal Antigen 2 Virion Tethering through a Novel Mechanism of Glycosylation Interference. J. Virol. 2015, 89, 11820–11833. [Google Scholar] [CrossRef]
- Kopecky-Bromberg, S.A.; Martínez-Sobrido, L.; Frieman, M.; Baric, R.A.; Palese, P. Severe Acute Respiratory Syndrome Coronavirus Open Reading Frame (ORF) 3b, ORF 6, and Nucleocapsid Proteins Function as Interferon Antagonists. J. Virol. 2007, 81, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Shin, E.-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, P.; Castiglia, V.; Ivin, M.; Ebner, F. Type I Interferons in Bacterial Infections: A Balancing Act. Front. Immunol. 2016, 7, 652. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, N.; Dickenson, R.E.; Odendall, C. Interferons: Tug of War Between Bacteria and Their Host. Front. Cell Infect. Microbiol. 2021, 11, 624094. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.V.; Hsu, C.-Y.; Matsuo, K.; Buzun, E.; Terrazas, M.C.; Loomis, L.R.; Lu, H.-H.; Park, J.H.; Rivaud, P.; Thomson, M.; et al. Commensal Bacteria Promote Type I Interferon Signaling to Maintain Immune Tolerance. bioRxiv 2021, 2021, 464743. [Google Scholar]
- Alphonse, N.; Dickenson, R.E.; Alrehaili, A.; Odendall, C. Functions of IFNλs in Anti-Bacterial Immunity at Mucosal Barriers. Front. Immunol. 2022, 13, 7639. [Google Scholar] [CrossRef]
- Peignier, A.; Parker, D. Impact of Type I Interferons on Susceptibility to Bacterial Pathogens. Trends Microbiol. 2021, 29, 823–835. [Google Scholar] [CrossRef]
- Perry, A.K.; Chen, G.; Zheng, D.; Tang, H.; Cheng, G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005, 15, 407–422. [Google Scholar] [CrossRef]
- An, J.; Woodward, J.J.; Sasaki, T.; Minie, M.; Elkon, K.B. Cutting Edge: Antimalarial Drugs Inhibit IFN-β Production through Blockade of Cyclic GMP-AMP Synthase–DNA Interaction. J. Immunol. 2015, 194, 4089–4093. [Google Scholar] [CrossRef]
- Fu, Q.; He, Q.; Dong, Q.; Xie, J.; Geng, Y.; Han, H.; Huang, Y.; Lu, J.; Zeng, Z.; Wang, W.; et al. The role of cyclic GMP-AMP synthase and Interferon-I-inducible protein 16 as candidatebiomarkers of systemic lupus erythematosus. Clin. Chim. Acta 2021, 524, 69–77. [Google Scholar] [CrossRef]
- Nagarajan, U. Induction and Function of IFNβ During Viral and Bacterial Infection. Crit. Rev. Immunol. 2011, 31, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Pan, M.; Yin, Y.; Wang, C.; Cui, Y.; Wang, Q. The Regulatory Network of Cyclic GMP-AMP Synthase-Stimulator of Interferon Genes Pathway in Viral Evasion. Front. Microbiol. 2021, 12, 3635. [Google Scholar] [CrossRef] [PubMed]
- Andrade, W.A.; Agarwal, S.; Mo, S.; Shaffer, S.A.; Dillard, J.P.; Schmidt, T.; Hornung, V.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Golenbock, D.T. Type I Interferon Induction by Neisseria gonorrhoeae: Dual Requirement of Cyclic GMP-AMP Synthase and Toll-like Receptor 4. Cell Rep. 2016, 15, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Andrade, W.A.; Firon, A.; Schmidt, T.; Hornung, V.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Trieu-Cuot, P.; Golenbock, D.T.; Kaminski, P.-A. Group B Streptococcus Degrades Cyclic-di-AMP to Modulate STING-Dependent Type I Interferon Production. Cell Host Microbe 2016, 20, 49–59. [Google Scholar] [CrossRef]
- Collins, A.C.; Cai, H.; Li, T.; Franco, L.H.; Li, X.-D.; Nair, V.R.; Scharn, C.R.; Stamm, C.E.; Levine, B.; Chen, Z.J.; et al. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium tuberculosis. Cell Host Microbe 2015, 17, 820–828. [Google Scholar] [CrossRef]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; E Jørgensen, S.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; A Horan, K.; et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. cGAS and Ifi204 Cooperate To Produce Type I IFNs in Response to Francisella Infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef]
- Wassermann, R.; Gulen, M.F.; Sala, C.; Perin, S.G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, J.L.; Schmidt, T.; Hornung, V.; Cole, S.T.; et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef]
- Watson, R.O.; Bell, S.L.; MacDuff, D.A.; Kimmey, J.M.; Diner, E.J.; Olivas, J.; Vance, R.E.; Stallings, C.L.; Virgin, H.W.; Cox, J.S. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 2015, 17, 811–819. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, D.; Gautam, A.; Lee, H.; Kwak, M.H.; Park, M.C.; Park, S.; Wu, G.; Lee, B.L.; Lee, Y.; et al. CpG-DNA exerts antibacterial effects by protecting immune cells and producing bacteria-reactive antibodies. Sci. Rep. 2018, 8, 16236. [Google Scholar] [CrossRef]
- Van Seventer, J. Type I Interferon in LPS/CpG-Induced Lethal Toxic Shock. Available online: https://grantome.com/grant/NIH/R21-AI061433-02 (accessed on 16 February 2023).
- Carrero, J.A. Confounding roles for type I interferons during bacterial and viral pathogenesis. Int. Immunol. 2013, 25, 663–669. [Google Scholar] [CrossRef]
- Castiglia, V.; Piersigilli, A.; Ebner, F.; Janos, M.; Goldmann, O.; Damböck, U.; Kröger, A.; Weiss, S.; Knapp, S.; Jamieson, A.M.; et al. Type I Interferon Signaling Prevents IL-1β-Driven Lethal Systemic Hyperinflammation during Invasive Bacterial Infection of Soft Tissue. Cell Host Microbe 2016, 19, 375–387. [Google Scholar] [CrossRef]
- Fieber, C.; Janos, M.; Koestler, T.; Gratz, N.; Li, X.-D.; Castiglia, V.; Aberle, M.; Sauert, M.; Wegner, M.; Alexopoulou, L.; et al. Innate Immune Response to Streptococcus pyogenes Depends on the Combined Activation of TLR13 and TLR2. PLoS ONE 2015, 10, e0119727. [Google Scholar] [CrossRef]
- Hidmark, A.; Paul, A.V.S.; Dalpke, A.H. Cutting Edge: TLR13 Is a Receptor for Bacterial RNA. J. Immunol. 2012, 189, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Chen, Z.J. Sequence specific detection of bacterial 23S ribosomal RNA by TLR13. Elife 2012, 1, e00102. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Krüger, A.; Ferstl, R.; Kaufmann, A.; Nees, G.; Sigmund, A.; Bathke, B.; Lauterbach, H.; Suter, M.; Dreher, S.; et al. TLR13 Recognizes Bacterial 23S RRNA Devoid of Erythromycin Resistance–Forming Modification. Science 2012, 337, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Bergstrøm, B.; Aune, M.H.; Awuh, J.A.; Kojen, J.F.; Blix, K.J.; Ryan, L.; Flo, T.H.; Mollnes, T.E.; Espevik, T.; Stenvik, J. TLR8 Senses Staphylococcus aureus RNA in Human Primary Monocytes and Macrophages and Induces IFN-β Production via a TAK1–IKKβ–IRF5 Signaling Pathway. J. Immunol. 2015, 195, 1100–1111. [Google Scholar] [CrossRef]
- Krüger, A.; Oldenburg, M.; Chebrolu, C.; Beisser, D.; Kolter, J.; Sigmund, A.M.; Steinmann, J.; Schäfer, S.; Hochrein, H.; Rahmann, S.; et al. Human TLR 8 senses UR/URR motifs in bacterial and mitochondrial RNA. EMBO Rep. 2015, 16, 1656–1663. [Google Scholar] [CrossRef]
- Kagan, J.C.; Su, T.; Horng, T.; Chow, A.; Akira, S.; Medzhitov, R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 2008, 9, 361–368. [Google Scholar] [CrossRef]
- Aubry, C.; Corr, S.C.; Wienerroither, S.; Goulard, C.; Jones, R.; Jamieson, A.M.; Decker, T.; O’Neill, L.A.J.; Dussurget, O.; Cossart, P. Both TLR2 and TRIF Contribute to Interferon-β Production during Listeria Infection. PLoS ONE 2012, 7, e33299. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 16 February 2023).
- Kozlowski, L.P. IPC—Isoelectric Point Calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Services. Available online: https://services.healthtech.dtu.dk (accessed on 16 February 2023).
- Align|UniProt. Available online: https://www.uniprot.org/align (accessed on 16 February 2023).
| Name | Isoform | Protein ID | Characteristic | Reference |
|---|---|---|---|---|
| IFN-α6 | Alpha-6X1A | P23229-2 | 259-297: Missing 1084-1130:RYDDSVPRYHAVRIRKEEREIKDEKYIDNLEKKQWITKWNENESYS → NKKDHYDATYHKAEIHAQPSDKERLTSDA | [33] |
| Alpha-6X1B | P23229-3 | 259-297: Missing | ||
| Alpha-6X2A | P23229-4 | 215-258: Missing 1084-1130:SRYDDSVPRYHAVRIRKEEREIKDEKYIDNLEKKQWITKWNENESYS → NKKDHYDATYHKAEIHAQPSDKERLTSDA | ||
| Alpha-6X2B | P23229-5 | 215-258: Missing | ||
| Alpha-6X1X2A | P23229-6 | 1084-1130:SRYDDSVPRYHAVRIRKEEREIKDEKYIDNLEKKQWITKWNENESYS → NKKDHYDATYHKAEIHAQPSDKERLTSDA | ||
| 7 | P23229-7 | 1-114: Missing 215-258: Missing 1084-1130:SRYDDSVPRYHAVRIRKEEREIKDEKYIDNLEKKQWITKWNENESYS → NKKDHYDATYHKAEIHAQPSDKERLTSDA | ||
| 9 | P23229-9 | 918-932: Missing 1084-1130:SRYDDSVPRYHAVRIRKEEREIKDEKYIDNLEKKQWITKWNENESYS → NKKDHYDATYHKAEIHAQPSDKERLTSDA | ||
| IFN-α7 | 2 | P36544-2 | 18-18: H → HGKATASPPSTPPWDPGHIPGASVRPAPGP | [33] |
| 3 | P36544-3 | 81-102: SWTDHYLQWNVSEYPGVKTVRF → AYSRVPATSMYAGFPLMCSTAN 103-502: Missing | ||
| IFN-α16 | IFI 16B | Q16666-2 | 444-499: Missing | [38] |
| IFI 16C | Q16666-3 | 444-555: Missing | ||
| 4 | Q16666-6 | 128-183: Missing | ||
| IFN-λ4 | p170 | K9M1U5-2 | 123-179:LELARPGSSRKVPGAQKRRHKPRRADSPRCRKASVVFNLLRLLTWELRLAAHSGPCL → VSDGRAPPPLSPASFSASSGPRRAPALCQSVLLSGKTHPDRSRVLWVS | [50] |
| p131 | K9M1U5-3 | 75-122: Missing | ||
| p107 | K9M1U5-4 | 51-122: Missing |
| Name | Characteristics | Reference |
|---|---|---|
| Mx antiviral protein pathway |
| [84,85,86,87] |
| Ribonuclease L pathway targeting 2’,5’-oligoadenylate synthetase |
| [88,89] |
| Protein kinase R pathway |
| [90,91,92] |
| Pathway similar to ISG15 |
| [93,94,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertowska, P.; Smolak, K.; Mertowski, S.; Grywalska, E. Immunomodulatory Role of Interferons in Viral and Bacterial Infections. Int. J. Mol. Sci. 2023, 24, 10115. https://doi.org/10.3390/ijms241210115
Mertowska P, Smolak K, Mertowski S, Grywalska E. Immunomodulatory Role of Interferons in Viral and Bacterial Infections. International Journal of Molecular Sciences. 2023; 24(12):10115. https://doi.org/10.3390/ijms241210115
Chicago/Turabian StyleMertowska, Paulina, Konrad Smolak, Sebastian Mertowski, and Ewelina Grywalska. 2023. "Immunomodulatory Role of Interferons in Viral and Bacterial Infections" International Journal of Molecular Sciences 24, no. 12: 10115. https://doi.org/10.3390/ijms241210115
APA StyleMertowska, P., Smolak, K., Mertowski, S., & Grywalska, E. (2023). Immunomodulatory Role of Interferons in Viral and Bacterial Infections. International Journal of Molecular Sciences, 24(12), 10115. https://doi.org/10.3390/ijms241210115








