Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease
Abstract
1. Introduction
2. Pathogenesis of Plaque in PD Cases
2.1. Role of the Immune System in Inflammatory Response
2.2. Differentiation and Proliferation of Myofibroblasts in the Profibrotic Environment
2.3. Extracellular Matrix Deposition
2.4. Progression of Fibrosis in Environment with Continuous Inflammation
3. Core Signaling Pathways and Their Crosstalk Involved in Development of Peyronie’s Disease
3.1. TGF-β1 Synthesis and Aberrant Activation of Latent TGF-β1
3.2. Aberrant TGF-β Signaling Pathway in the Pathogenesis of Peyronie’s Disease
3.3. Another Core Signaling Pathway and Crosstalk in Peyronie’s Disease
4. Risk Factors for Pathogenesis of Peyronie’s Disease
4.1. Intrinsic Risk Factors
4.1.1. Genetics of Peyronie’s Disease
4.1.2. Aging
4.1.3. Testosterone Level
4.1.4. ABO Blood Type
4.1.5. Congenital Penile Curvature
4.2. Extrinsic Risk Factors
4.2.1. Smoking
4.2.2. Alcohol
4.2.3. Perineal and Penile Trauma
4.3. Comorbidities
Hypertension, Diabetes Mellitus, Dyslipidemia, and Obesity
5. Other Conditions Possibly Associated with Peyronie’s Disease
5.1. Erectile Dysfunction
5.2. Dupuytren’s Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Maida, F.; Cito, G.; Lambertini, L.; Valastro, F.; Morelli, G.; Mari, A.; Carini, M.; Minervini, A.; Cocci, A. The natural history of Peyronie’s disease. World J. Mens Health 2021, 39, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Tefekli, A.; Erol, B.; Oktar, T.; Tunc, M.; Tellaloglu, S. A retrospective review of 307 men with Peyronie’s disease. J. Urol. 2002, 168, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P.; Schiff, J.; Guhring, P. An analysis of the natural history of Peyronie’s disease. J. Urol. 2006, 175, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Berookhim, B.M.; Choi, J.; Alex, B.; Mulhall, J.P. Deformity stabilization and improvement in men with untreated Peyronie’s disease. BJU. Int. 2014, 113, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Mulhall, J.P. Psychological impact of Peyronie’s disease: A review. J. Sex. Med. 2013, 10, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Terrier, J.E.; Nelson, C.J. Psychological aspects of Peyronie’s disease. Transl. Androl. Urol. 2016, 5, 290–295. [Google Scholar] [CrossRef]
- Nelson, C.J.; Diblasio, C.; Kendirci, M.; Hellstrom, W.; Guhring, P.; Mulhall, J.P. The chronology of depression and distress in men with Peyronie’s disease. J. Sex. Med. 2008, 5, 1985–1990. [Google Scholar] [CrossRef]
- Kuja-Halkola, R.; Henningsohn, L.; D’Onofrio, B.M.; Mills, J.; Adolfsson, A.; Larsson, H.; Cederlöf, M. Mental disorders in Peyronie’s disease: A Swedish cohort study of 3.5 million men. J. Urol. 2021, 205, 864–870. [Google Scholar] [CrossRef]
- Cilio, S.; Fallara, G.; Capogrosso, P.; Candela, L.; Belladelli, F.; Pozzi, E.; Corsini, C.; Raffo, M.; Schifano, N.; Boeri, L.; et al. The symptomatic burden of Peyronie’s disease at presentation according to patient age: A critical analysis of the Peyronie’s disease questionnaire (PDQ) domains. Andrology 2023, 11, 501–507. [Google Scholar] [CrossRef]
- Spirito, L.; Manfredi, C.; La Rocca, R.; Napolitano, L.; Di Girolamo, A.; Capece, M.; Trama, F.; Sciorio, C.; Sokolakis, I.; Creta, M.; et al. Daily low dose tadalafil may reduce the penile curvature progression rate in patients with acute Peyronie’s disease: A retrospective comparative analysis. Int. J. Impot. Res. 2022. [Google Scholar] [CrossRef]
- Capece, M.; Arcaniolo, D.; Manfredi, C.; Palmieri, A.; De Sio, M.; Verze, P.; Fusco, F.; Longo, N.; Mirone, V. Second cycle of intralesional Collagenase Clostridium histolyticum for Peyronie’s disease using the modified shortened protocol: Results from a retrospective analysis. Andrologia 2020, 52, e13527. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Arcaniolo, D.; Amicuzi, U.; Spirito, L.; Napolitano, L.; Crocerossa, F.; Paoletta, M.; Gisone, S.; Cirillo, P.; Crocetto, F.; et al. Impact of extracorporeal shockwave therapy for erectile dysfunction and Peyronie’s disease on reproductive and hormonal testicular function. Andrology 2022, 10, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.M.; El-Sakka, A.I. Extracorporeal shockwave therapy in Peyronie’s disease: Systematic review and meta-analysis. J. Sex. Med. 2021, 18, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Krishnappa, P.; Manfredi, C.; Sinha, M.; Arcaniolo, D.; Matippa, P.; Moncada, I. Penile modeling in Peyronie’s disease: A systematic review of the literature. Sex. Med. Rev. 2022, 10, 434–450. [Google Scholar] [CrossRef]
- Verze, P.; Sokolakis, I.; Manfredi, C.; Collà Ruvolo, C.; Hatzichristodoulou, G.; Romero-Otero, J. Penile prosthesis implant in the management of Peyronies’ disease. Minerva. Urol. Nephrol. 2021, 73, 196–214. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Manfredi, C.; Torremadé, J.; Ibarra, F.P.; Geli, J.S.; Romero-Otero, J.; García-Baquero, R.; Poblador, A.F.; Barbará, M.R.; Campos-Juanatey, F.; et al. Multicenter prospective study of grafting with collagen fleece TachoSil in patients with Peyronie’s disease. J. Sex. Med. 2020, 17, 2279–2286. [Google Scholar] [CrossRef]
- Gelbard, M.K.; Rosenbloom, J. Fibroproliferative disorders and diabetes: Understanding the pathophysiologic relationship between Peyronie’s disease, Dupuytren disease and diabetes. Endocrinol. Diabetes. Metab. 2020, 4, e00195. [Google Scholar] [CrossRef]
- Segundo, A.; Glina, S. Prevalence, risk factors, and erectile dysfunction associated with Peyronie’s disease among men seeking urological care. Sex. Med. 2020, 8, 230–236. [Google Scholar] [CrossRef]
- Herati, A.S.; Pastuszak, A.W. The genetic basis of Peyronie disease: A review. Sex. Med. Rev. 2016, 4, 85–94. [Google Scholar] [CrossRef]
- Milenkovic, U.; Ilg, M.M.; Cellek, S.; Albersen, M. Pathophysiology and future therapeutic perspectives for resolving fibrosis in Peyronie’s disease. Sex. Med. Rev. 2019, 7, 679–689. [Google Scholar] [CrossRef]
- Krakhotkin, D.V.; Chernylovskyi, V.A.; Mottrie, A.; Greco, F.; Bugaev, R.A. New insights into the pathogenesis of Peyronie’s disease: A narrative review. Chronic. Dis. Transl. Med. 2020, 6, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, E.L.; Teloken, C.; Ting, H.Y.; Lucas, M.L.; Teodosio da Ros, C.; Ary Vargas Souto, C. Prevalence of Peyronie’s disease in men over 50 years old in southern Brazil. Int. J. Impot. Res. 2001, 13, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Heck, M.; Teloken, P.; Siegrist, T.; Nelson, C.J.; Mulhall, J.P. Peyronie’s disease following radical prostatectomy: Incidence and predictors. J. Sex. Med. 2010, 7, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Shimabukuro, T.; Matsuyama, H. The prevalence of Peyronie’s disease in Japan: A study in men undergoing maintenance hemodialysis and routine health checks. J. Sex. Med. 2012, 9, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Ralph, D.; Kagioglu, A.; Garaffa, G.; Shamsodini, A.; Bivalacqua, T.; Glina, S.; Hakim, L.; Sadeghi-Nejad, H.; Broderick, G. Evidence-based management guidelines on Peyronie’s disease. J. Sex. Med. 2016, 13, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Capoccia, E.; Levine, L.A. Contemporary review of Peyronie’s disease treatment. Curr. Urol. Rep. 2018, 19, 51. [Google Scholar] [CrossRef]
- Cocci, A.; Russo, G.I.; Briganti, A.; Salonia, A.; Cacciamani, G.; Capece, M.; Falcone, M.; Timpano, M.; Cito, G.; Verze, P.; et al. Predictors of treatment success after collagenase Clostridium histolyticum injection for Peyronie’s disease: Development of a nomogram from a multicentre single-arm, non-placebo controlled clinical study. BJU Int. 2018, 122, 680–687. [Google Scholar] [CrossRef]
- Devine, C.J., Jr.; Somers, K.D.; Jordan, S.G.; Schlossberg, S.M. Proposal: Trauma as the cause of the Peyronie’s lesion. J. Urol. 1997, 157, 285–290. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Q.; Che, X.; Zhu, L.; Zhang, Z.; Chen, Y.; Dai, Y. Repeated micro-trauma of the penile tunica albuginea: A new animal model of Peyronie’s disease. Urol. Int. 2018, 100, 228–239. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Novo, E.; Cannito, S.; Paternostro, C.; Bocca, C.; Miglietta, A.; Parola, M. Cellular and molecular mechanisms in liver fibrogenesis. Arch. Biochem. Biophys. 2014, 548, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, U.; Ilg, M.; Cellek, S.; Albersen, M.E. What role do pharmaceuticals play in the treatment of Peyronie’s disease and is there a need for new emerging drugs? Expert Opin. Emerg. Drugs 2019, 24, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Dwyer, D.N.; Ashley, S.L.; Moore, B.B. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L590–L601. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Shi, J.; Chen, L.; Lv, Z.; Chen, X.; Cao, H.; Xiang, Z.; Han, X. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun. Signal. 2018, 16, 89. [Google Scholar] [CrossRef]
- Wu, L.; Huang, K.; Li, Q.; Wu, H.; Gao, Y.; Xu, X.; Liu, X.; Han, L. Crosstalk between myofibroblasts and macrophages: A regulative factor of valvular fibrosis in calcific aortic valve disease. Cell Biol. Int. 2023, 47, 754–767. [Google Scholar] [CrossRef]
- Lis-López, L.; Bauset, C.; Seco-Cervera, M.; Cosín-Roger, J. Is the macrophage phenotype determinant for fibrosis development? Biomedicines 2021, 9, 1747. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Györfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Van de Water, L. Mechanisms by which fibrin and fibronectin appear in healing wounds: Implications for Peyronie’s disease. J. Urol. 1997, 157, 306–310. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Jiang, Y.; Beller, D.I.; Frendl, G.; Graves, D.T. Monocyte chemoattractant protein 1 regulates adhesion molecule expression and cytokine production in human monocytes. J. Immunol. 1992, 148, 2423–2438. [Google Scholar] [CrossRef]
- Young, L.R.; Gulleman, P.M.; Short, C.W.; Tanjore, H.; Sherrill, T.; Qi, A.; McBride, A.P.; Zaynagetdinov, R.; Benjamin, J.T.; Lawson, W.E.; et al. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. JCI Insight 2016, 1, e88947. [Google Scholar] [CrossRef]
- Magee, T.R.; Qian, A.; Rajfer, J.; Sander, F.C.; Levine, L.A.; Gonzalez-Cadavid, N.F. Gene expression profiles in the Peyronie’s disease plaque. Urology 2002, 59, 451–457. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, G.; Wang, Z.; Maddah, S.A.; Lue, T.F. Upregulation of monocyte chemoattractant protein 1 and effects of transforming growth factor-beta 1 in Peyronie’s disease. Biochem. Biophys. Res. Commun. 2002, 295, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 373, 96. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. AMJ. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Zielinski, C.E. T helper cell subsets: Diversification of the field. Eur. J. Immunol. 2023, 15, e22. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.F.C.; Cardoso, F.G.R.; Ferreira, N.S.; Corazza, B.J.M.; Valera, M.M.C.; Nascimento, G.G.; Martinho, F.C. Effects of calcium hydroxide intracanal medications on T helper (Th1, Th2, Th9, Th17, and Tfh) and regulatory T (Treg) cell cytokines in apical periodontitis: A CONSORT RCT. J. Endod. 2022, 48, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing macrophages in vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Todd, N.W.; Scheraga, R.G.; Galvin, J.R.; Iacono, A.T.; Britt, E.J.; Luzina, I.G.; Burke, A.P.; Atamas, S.P. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J. Inflamm. Res. 2013, 6, 63–70. [Google Scholar] [CrossRef]
- Bosello, S.; Angelucci, C.; Lama, G.; Alivernini, S.; Proietti, G.; Tolusso, B.; Sica, G.; Gremese, E.; Ferraccioli, G. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res. Ther. 2018, 20, 75. [Google Scholar] [CrossRef]
- Beesley, C.F.; Goldman, N.R.; Taher, T.E.; Denton, C.P.; Abraham, D.J.; Mageed, R.A.; Ong, V.H. Dysregulated B cell function and disease pathogenesis in systemic sclerosis. Front. Immunol. 2023, 13, 999008. [Google Scholar] [CrossRef]
- Taher, T.E.; Ong, V.H.; Bystrom, J.; Hillion, S.; Simon, Q.; Denton, C.P.; Pers, J.O.; Abraham, D.J.; Mageed, R.A. Association of defective regulation of autoreactive interleukin-6-producing transitional B lymphocytes with disease in patients with systemic sclerosis. Arthritis. Rheumatol. 2018, 70, 450–461. [Google Scholar] [CrossRef]
- Forestier, A.; Guerrier, T.; Jouvray, M.; Giovannelli, J.; Lefèvre, G.; Sobanski, V.; Hauspie, C.; Hachulla, E.; Hatron, P.Y.; Zéphir, H.; et al. Altered B lymphocyte homeostasis and functions in systemic sclerosis. Autoimmun. Rev. 2018, 17, 244–255. [Google Scholar] [CrossRef]
- Ucero, A.C.; Bakiri, L.; Roediger, B.; Suzuki, M.; Jimenez, M.; Mandal, P.; Braghetta, P.; Bonaldo, P.; Paz-Ares, L.; Fustero-Torre, C.; et al. Fra-2-expressing macrophages promote lung fibrosis in mice. J. Clin. Invest. 2019, 129, 3293–3309. [Google Scholar] [CrossRef] [PubMed]
- Heidt, T.; Courties, G.; Dutta, P.; Sager, H.B.; Sebas, M.; Iwamoto, Y.; Sun, Y.; Da Silva, N.; Panizzi, P.; van der Laan, A.M.; et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 2014, 115, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Karim, M.R.; Izawa, T.; Kuwamura, M.; Yamate, J. Immunophenotypical characterization of M1/M2 macrophages and lymphocytes in cisplatin-induced rat progressive renal fibrosis. Cells 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Atar, A.; Kural, A.; Yenice, G.; Comez, I.; Tugcu, V.K. Role of interleukin-6 and pentraxin 3 as an early marker in Peyronie’s disease. Kaohsiung J. Med. Sci. 2017, 33, 195–200. [Google Scholar] [CrossRef]
- Zimmermann, R.P.; Feil, G.; Bock, C.; Hoeltl, L.; Stenzl, A. Significant alterations of serum cytokine levels in patient with Peyronie’s disease. Int. Braz. J. Urol. 2008, 34, 457–466; discussion 466. [Google Scholar] [CrossRef][Green Version]
- Cui, Y.; Chen, L.; Wang, X.; Yu, L.; Wu, J. Identifying hub genes, key pathways and key immune-related genes in Peyronie’s disease by integrated bioinformatic analysis. Front. Pharmacol. 2022, 13, 1019358. [Google Scholar] [CrossRef]
- Hamid, T.; Xu, Y.; Ismahil, M.A.; Rokosh, G.; Jinno, M.; Zhou, G.; Wang, Q.; Prabhu, S.D. Cardiac mesenchymal stem cells promote fibrosis and remodeling in heart failure: Role of PDGF signaling. JACC Basic. Transl. Sci. 2022, 7, 465–483. [Google Scholar] [CrossRef]
- Lucattelli, M.; Lunghi, B.; Fineschi, S.; Mirone, V.; d’Emmanuele di Villa Bianca, R.; Longo, N.I.C.; De Palma, R.; Sorrentino, R.; Lungarella, G.; Cirino, G.I. A new mouse model of Peyronie’s disease: An increased expression of hypoxia-inducible factor-1 target genes during the development of penile changes. Int. J. Biochem. Cell Biol. 2008, 40, 2638–2648. [Google Scholar] [CrossRef]
- Layton, T.B.; Williams, L.; Yang, N.; Zhang, M.; Lee, C.; Feldmann, M.; Trujillo, G.; Furniss, D.; Nanchahal, J. A vasculature niche orchestrates stromal cell phenotype through PDGF signaling: Importance in human fibrotic disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2120336119. [Google Scholar] [CrossRef]
- Xiao, L.; Dudley, A.C. Fine-tuning vascular fate during endothelial-mesenchymal transition. J. Pathol. 2017, 241, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakim, M.; Lin, X.; Ogawa, R. The Japanese experience with basic fibroblast growth factor in cutaneous wound management and scar prevention: A systematic review of clinical and biological Aspects. Dermatol. Ther. (Heidelb.) 2020, 10, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, J.P.; Thom, J.; Lubrano, T.; Shankey, T.V. Basic fibroblast growth factor expression in Peyronie’s disease. Basic fibroblast growth factor expression in Peyronie’s disease. J. Urol. 2001, 165, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Cohn, R.D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef]
- Lim, S.; McMahon, C.D.; Matthews, K.G.; Devlin, G.P.; Elston, M.S.; Conaglen, J.V. Absence of myostatin improves cardiac function following myocardial infarction. Heart Lung Circ. 2018, 27, 693–701. [Google Scholar] [CrossRef]
- Cantini, L.P.; Ferrini, M.G.; Vernet, D.; Magee, T.R.; Qian, A.; Gelfand, R.A.; Rajfer, J.; Gonzalez-Cadavid, N.F. Profibrotic role of myostatin in Peyronie’s disease. J. Sex. Med. 2008, 5, 1607–1622. [Google Scholar] [CrossRef]
- Covarrubias, A.E.; Lecarpentier, E.; Lo, A.; Salahuddin, S.; Gray, K.J.; Karumanchi, S.A.; Zsengellér, Z.K. AP39, a modulator of mitochondrial bioenergetics, reduces antiangiogenic response and oxidative stress in hypoxia-exposed trophoblasts: Relevance for preeclampsia pathogenesis. Am. J. Pathol. 2019, 189, 104–114. [Google Scholar] [CrossRef]
- Li, X.; Xing, J.; Wang, H.; Yu, E. The SLC34A2-ROS-HIF-1-induced up-regulation of EZH2 expression promotes proliferation and chemo-resistance to apoptosis in colorectal cancer. Biosci. Rep. 2019, 39, BSR20180268. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Kim, S.M.; Yang, C.E.; Song, S.Y.; Lee, W.J.; Lee, J.H. Hypoxia-induced epithelial-to-mesenchymal transition mediates fibroblast abnormalities via ERK activation in cutaneous wound healing. Int. J. Mol. Sci. 2019, 20, 2546. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Men, C.; Wu, J.; Liu, L. Bioinformatics-based identification of potential hypoxia-related genes associated with Peyronie’s disease. Am. J. Mens Health 2022, 16, 15579883221111720. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix. Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Randles, M.; Lennon, R. Applying proteomics to investigate extracellular matrix in health and disease. Curr. Top. Membr. 2015, 76, 171e196. [Google Scholar]
- Paulis, G.; De Giorgio, G.; Paulis, L. Role of oxidative stress in Peyronie’s disease: Biochemical evidence and experiences of treatment with antioxidants. Int. J. Mol. Sci. 2022, 23, 15969. [Google Scholar] [CrossRef]
- Del Carlo, M.; Cole, A.A.; Levine, L.A. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1beta and transforming growth factor-beta in Peyronie’s plaque fibroblasts. J. Urol. 2008, 179, 2447–2455. [Google Scholar] [CrossRef]
- Watanabe, M.S.; Theodoro, T.R.; Coelho, N.L.; Mendes, A.; Leonel, M.L.P.; Mader, A.M.; Nader, H.B.; Glina, S.; Pinhal, M.A.S. Extracellular matrix alterations in the Peyronie’s disease. J. Adv. Res. 2017, 8, 455–461. [Google Scholar] [CrossRef]
- Flevaris, P.; Vaughan, D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin. Thromb. Hemost. 2017, 43, 169–177. [Google Scholar] [CrossRef]
- Davila, H.H.; Magee, T.R.; Zuniga, F.I.; Rajfer, J.; Gonzalez-Cadavid, N.F. Peyronie’s disease associated with increase in plasminogen activator inhibitor in fibrotic plaque. Urology 2005, 65, 645–648. [Google Scholar] [CrossRef]
- Micallef, L.; Vedrenne, N.; Billet, F.; Coulomb, B.; Darby, I.A.; Desmoulière, A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair 2012, 5 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Lokmic, Z.; Musyoka, J.; Hewitson, T.D.; Darby, I.A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 2012, 296, 139–185. [Google Scholar]
- Szardening-Kirchner, C.; Konrad, L.; Hauck, E.W.; Haag, S.M.; Eickelberg, O.; Weidner, W. Upregulation of mRNA expression of MCP-1 by TGF-beta1 in fibroblast cells from Peyronie’s disease. World. J. Urol. 2009, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Eardley, I.; Giuliano, F.; Hatzichristou, D.; Moncada, I.; Salonia, A.; Vardi, Y.; Wespes, E.; European Association of Urology. EAU guidelines on penile curvature. Eur. Urol. 2012, 62, 543–552. [Google Scholar] [CrossRef]
- Hao, M.; Han, X.; Yao, Z.; Zhang, H.; Zhao, M.; Peng, M.; Wang, K.; Shan, Q.; Sang, X.; Wu, X.; et al. The pathogenesis of organ fibrosis: Focus on necroptosis. Br. J. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zorba, O.U.; Sirma, S.; Ozgon, G.; Salabas, E.; Ozbek, U.; Kadioglu, A. Comparison of apoptotic gene expression profiles between Peyronie’s disease plaque and tunica albuginea. Clin. Exp. Med. 2012, 21, 607–614. [Google Scholar]
- Loreto, C.; La Rocca, G.; Anzalone, R.; Caltabiano, R.; Vespasiani, G.; Castorina, S.; Ralph, D.J.; Cellek, S.; Musumeci, G.; Giunta, S.; et al. The role of intrinsic pathway in apoptosis activation and progression in Peyronie’s disease. Biomed. Res. Int. 2014, 616149. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Barbagli, G.; Djinovic, R.; Vespasiani, G.; Carnazza, M.L.; Miano, R.; Musumeci, G.; Sansalone, S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and its death receptor (DR5) in Peyronie’s disease. A biomolecular study of apoptosis activation. J. Sex. Med. 2011, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Lagares, D.N. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Garaffa, G.; Trost, L.W.; Serefoglu, E.C.; Ralph, D.; Hellstrom, W.J. Understanding the course of Peyronie’s disease. Int. J. Clin. Pract. 2013, 67, 781–788. [Google Scholar] [CrossRef]
- Milenkovic, U.; Boeckx, B.; Lambrechts, D.; Janky, R.; Hatzichristodoulou, G.; van Renterghem, K.; Gevaert, T.; Cellek, S.; Bivalacqua, T.J.; De Ridder, D.; et al. Single-cell transcriptomics uncover a novel role of myeloid cells and T-lymphocytes in the fibrotic microenvironment in Peyronie’s disease. Eur. Urol. Focus 2022, 8, 814–828. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor-β in myocardial disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef]
- Gundogdu, G.; Nguyen, T.; Namasivayam, A.; Starek, S.; Gelman, J.; Mauney, J.R. Characterization of a novel rabbit model of Peyronie’s disease. Int. J. Impot. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Yuan, J.H. Animal models of Peyronie’s disease: An update. Zhonghua Nan Ke Xue 2016, 22, 446–449. [Google Scholar] [PubMed]
- Distler, J.H.; Feghali-Bostwick, C.; Soare, A.; Asano, Y.; Distler, O.; Abraham, D.J. Review: Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis Rheumatol. 2017, 69, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Park, J.; Yu, M.R.; Kim, Y.S.; Ha, H.; Lee, H.B. Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-beta1 during renal fibrosis in diabetes. Am. J. Nephrol. 2009, 30, 481–490. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Misra, D.P.; Prasad, N.; Rastogi, K.; Singh, H.; Rai, M.K.; Agarwal, V. Serotonin 5HT2A receptor antagonism mediated anti-inflammatory and anti-fibrotic effect in adriamycin-induced CKD in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1269–1279. [Google Scholar]
- Chaturvedi, S.; Misra, D.P.; Prasad, N.; Rastogi, K.; Singh, H.; Rai, M.K.; Agarwal, V.I. 5-HT2 and 5-HT2B antagonists attenuate pro-fibrotic phenotype in human adult dermal fibroblasts by blocking TGF-β1 induced non-canonical signaling pathways including STAT3: Implications for fibrotic diseases like scleroderma. Int. J. Rheum. Dis. 2018, 21, 2128–2138. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. αv integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Chen, J.; Xu, Y.J. Thrombospondin-1: A key protein that induces fibrosis in diabetic complications. Diabetes Res. 2020, 2020, 8043135. [Google Scholar] [CrossRef]
- Yi, W.; Chen, F.; Zhang, H.; Tang, P.; Yuan, M.; Wen, J.; Wang, S.; Cai, Z. Role of angiotensin II in aging. Front. Aging Neurosci. 2022, 14, 1002138. [Google Scholar] [CrossRef] [PubMed]
- Canguven, O.; Lagoda, G.; Sezen, S.F.; Burnett, A.L. Losartan preserves erectile function after bilateral cavernous nerve injury via antifibrotic mechanisms in male rats. J. Urol. 2009, 181, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
- Gur, S.; Kadowitz, P.J.; Hellstrom, W.J. Drugs of the future for Peyronie’s disease. Med. Hypotheses 2012, 78, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bragina, M.E.; Costa-Fraga, F.; Sturny, M.; Ebadi, B.; Ruoccolo, R.T.; Santos, R.A.S.; Fraga-Silva, R.A.; Stergiopulos, N. Characterization of the renin-angiotensin system in aged cavernosal tissue and its role in penile fibrosis. J. Sex. Med. 2020, 17, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.M.; Hauck, E.W.; Szardening-Kirchner, C.; Diemer, T.; Cha, E.S.; Weidner, W.; Eickelberg, O. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie’s disease. Eur. Urol. 2007, 51, 255–261. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck van Capelle, C.; Spit, M.; Ten Dijke, P. Current perspectives on inhibitory SMAD7 in health and disease. Current perspectives on inhibitory SMAD7 in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 691–715. [Google Scholar] [CrossRef]
- Choi, M.J.; Song, K.M.; Park, J.M.; Kwon, M.H.; Kwon, K.D.; Park, S.H.; Ryu, D.S.; Ryu, J.K.; Suh, J.K. Effect of SMAD7 gene overexpression on TGF-β1-induced profibrotic responses in fibroblasts derived from Peyronie’s plaque. Asian J. Androl. 2015, 17, 487–492. [Google Scholar]
- Wang, W.; Ding, W.; Zhang, X.; Wu, S.; Yu, T.; Cui, X.; Xie, Y.; Yang, D.; Lin, C. Intratunical injection of rat-derived bone marrow mesenchymal stem cells prevents fibrosis and is associated with increased Smad7 expression in a rat model of Peyronie’s disease. Stem. Cell. Res. Ther. 2022, 13, 390. [Google Scholar] [CrossRef]
- Wahab, N.A.; Weston, B.S.; Mason, R.M. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J. Am. Soc. Nephrol. 2005, 16, 340–351. [Google Scholar] [CrossRef]
- Yu, X.Y.; Sun, Q.; Zhang, Y.M.; Zou, L.; Zhao, Y.Y. TGF-β/Smad Signaling Pathway in Tubulointerstitial Fibrosis. Front. Pharmacol. 2022, 13, 860588. [Google Scholar] [CrossRef]
- Pavone, C.; Napoli, G.; Caruana, G.; Alonge, V.; Usala, M.; Abbadessa, D. Safety and tolerability of local treatment with iloprost, a prostacyclin analogue, in patients with Peyronie’s disease: A phase I study. BJU Int. 2012, 110, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sarenac, T.; Trapecar, M.; Gradisnik, L.; Rupnik, M.S.; Pahor, D. Single-cell analysis reveals IGF-1 potentiation of inhibition of the TGF-β/Smad pathway of fibrosis in human keratocytes in vitro. Sci. Rep. 2016, 6, 34373. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.G.; Psarros, C.; Gekas, A.; Vandoros, G.P.; Philippou, A.; Koutsilieris, M. Alternative splicing of IGF1 gene as a potential factor in the pathogenesis of Peyronie’s disease. In Vivo 2016, 30, 251–256. [Google Scholar]
- Chatzifrangkeskou, M.; Le Dour, C.; Wu, W.; Morrow, J.P.; Joseph, L.C.; Beuvin, M.; Sera, F.; Homma, S.; Vignier, N.; Mougenot, N.; et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet. 2016, 25, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK signaling pathway in renal fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef]
- Liu, N.; Feng, J.; Lu, X.; Yao, Z.; Liu, Q.; Lv, Y.; Han, Y.; Deng, J.; Zhou, Y. Isorhamnetin inhibits liver fibrosis by reducing autophagy and inhibiting extracellular matrix formation via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediat. Inflamm. 2019, 2019, 6175091. [Google Scholar] [CrossRef] [PubMed]
- Toque, H.A.; Nunes, K.P.; Yao, L.; Liao, J.K.; Webb, R.C.; Caldwell, R.B.; Caldwell, R.W. Activated Rho kinase mediates diabetes-induced elevation of vascular arginase activation and contributes to impaired corpora cavernosa relaxation: Possible involvement of p38 MAPK activation. J. Sex. Med. 2013, 10, 1502–1515. [Google Scholar] [CrossRef]
- Song, W.H.; Son, H.; Kim, S.W.; Paick, J.S.; Cho, M.C. Role of Jun amino-terminal kinase (JNK) in apoptosis of cavernosal tissue during acute phase after cavernosal nerve injury. Asian J. Androl. 2018, 20, 50–55. [Google Scholar]
- Mercer, P.F.; Woodcock, H.V.; Eley, J.D.; Platé, M.; Sulikowski, M.G.; Durrenberger, P.F.; Franklin, L.; Nanthakumar, C.B.; Man, Y.; Genovese, F.; et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 2016, 71, 701–711. [Google Scholar] [CrossRef]
- Tang, Q.; Markby, G.R.; MacNair, A.J.; Tang, K.; Tkacz, M.; Parys, M.; Phadwal, K.; MacRae, V.E.; Corcoran, B.M. TGF-β-induced PI3K/AKT/mTOR pathway controls myofibroblast differentiation and secretory phenotype of valvular interstitial cells through the modulation of cellular senescence in a naturally occurring in vitro canine model of myxomatous mitral valve disease. Cell Prolif. 2023, e13435. [Google Scholar] [CrossRef]
- Palumbo-Zerr, K.; Zerr, P.; Distler, A.; Fliehr, J.; Mancuso, R.; Huang, J.; Mielenz, D.; Tomcik, M.; Fürnrohr, B.G.; Scholtysek, C.; et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat. Med. 2015, 21, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Ryu, Y.L.; Lee, H.S.; Lee, H.; Son, M.K.; Yan, H.H.; Hong, S.W.; Ryu, J.K.; Hong, S.; Suh, J.K.; et al. A novel PI3K inhibitor alleviates fibrotic responses in fibroblasts derived from Peyronie’s plaques. Int. J. Oncol. 2013, 42, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Duspara, K.; Bojanic, K.; Pejic, J.I.; Kuna, L.; Kolaric, T.O.; Nincevic, V.; Smolic, R.; Vcev, A.; Glasnovic, M.; Curcic, I.B.; et al. Targeting the Wnt signaling pathway in liver fibrosis for rug options: An update. J. Clin. Transl. Hepatol. 2021, 9, 960–971. [Google Scholar]
- Tu, M.; Wei, T.; Jia, Y.; Wang, Y.; Wu, J. Molecular mechanisms of alveolar epithelial cell senescence and idiopathic pulmonary fibrosis: A narrative review. J. Thorac. Dis. 2023, 15, 186–203. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; He, W. Emerging therapeutic strategies for attenuating tubular EMT and kidney fibrosis by targeting Wnt/β-catenin signaling. Front. Pharmacol. 2022, 12, 830340. [Google Scholar] [CrossRef] [PubMed]
- De Young, L.X.; Bella, A.J.; O’Gorman, D.B.; Gan, B.S.; Lim, K.B.; Brock, G.B. Protein biomarker analysis of primary Peyronie’s disease cells. J. Sex. Med. 2010, 7, 99–106. [Google Scholar] [CrossRef]
- Ten Dam, E.P.M.; van Driel, M.F.; de Jong, I.J.; Werker, P.M.N.; Bank, R.A. Glimpses into the molecular pathogenesis of Peyronie’s disease. Aging Male 2020, 23, 962–970. [Google Scholar] [CrossRef]
- Sato, M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm. Venereol. 2006, 86, 300–307. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, W.L.; Sider, K.L.; Yip, C.Y.; Simmons, C.A. β-catenin mediates mechanically regulated, transforming growth factor-β1induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 590–597. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef]
- Liang, R.; Šumová, B.; Cordazzo, C.; Mallano, T.; Zhang, Y.; Wohlfahrt, T.; Dees, C.; Ramming, A.; Krasowska, D.; Michalska-Jakubus, M.; et al. The transcription factor GLI2 as a downstream mediator of transforming growth factor-β-induced fibroblast activation in SSc. Ann. Rheum. Dis. 2017, 76, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, J.; Wu, Z.; Liu, T.; Ullenbruch, M.R.; Ding, L.; Henke, C.A.; Bitterman, P.B.; Phan, S.H. Reemergence of hedgehog mediates epithelial-mesenchymal crosstalk in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 52, 418–428. [Google Scholar] [CrossRef] [PubMed]
- El-Agroudy, N.N.; El-Naga, R.N.; El-Razeq, R.A.; El-Demerdash, E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br. J. Pharmacol. 2016, 173, 3248–3260. [Google Scholar] [CrossRef]
- Horn, A.; Palumbo, K.; Cordazzo, C.; Dees, C.; Akhmetshina, A.; Tomcik, M.; Zerr, P.; Avouac, J.; Gusinde, J.; Zwerina, J.; et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012, 64, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, X.; Lu, H.; Chen, B.; Bai, Y. An overview of hedgehog signaling in fibrosis. Mol. Pharmacol. 2015, 87, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Petzold, J.; Gentleman, E. Intrinsic mechanical cues and their impact on stem cells and embryogenesis. Front. Cell Dev. Biol. 2021, 9, 761871. [Google Scholar] [CrossRef]
- Mia, M.M.; Singh, M.K. New insights into Hippo/YAP signaling in fbrotic diseases. Cells 2022, 11, 2065. [Google Scholar] [CrossRef]
- Dou, C.; Liu, Z.; Tu, K.; Zhang, H.; Chen, C.; Yaqoob, U.; Wang, Y.; Wen, J.; van Deursen, J.; Sicard, D.; et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 2018, 154, 2209–2221. [Google Scholar] [CrossRef]
- Qian, A.; Meals, R.A.; Rajfer, J.; Gonzalez-Cadavid, N.F. Comparison of gene expression profiles between Peyronie’s disease and Dupuytren’s contracture. Urology 2004, 64, 399–404. [Google Scholar] [CrossRef]
- Milenkovic, U.; Ilg, M.M.; Zuccato, C.; Ramazani, Y.; De Ridder, D.; Albersen, M. Simvastatin and the Rho-kinase inhibitor Y-27632 prevent myofibroblast transformation in Peyronie’s disease-derived fibroblasts via inhibition of YAP/TAZ nuclear translocation. BJU Int. 2019, 123, 703–715. [Google Scholar] [CrossRef]
- Kim, C.L.; Choi, S.H.; Mo, J.S. Role of the Hippo pathway in fibrosis and cancer. Cells 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Petrouj, I.G.; Nikou, S.; Madduri, S.; Nifora, M.; Bravou, V.; Kalbermatten, D.F. The role of Hippo signaling pathway and ILK in the pathophysiology of human hypertrophic scars and keloids: An immunohistochemical investigation. Cells 2022, 11, 3426. [Google Scholar]
- Hao, Y.C.; Yu, L.P.; Li, Q.; Zhang, X.W.; Zhao, Y.P.; He, P.Y.; Xu, T.; Wang, X.F. Effects of integrin-linked kinase on human corpus cavernosum smooth muscle cell cytoskeletal organisation. Andrologia 2013, 45, 78–85. [Google Scholar] [CrossRef]
- Nakamura, R.; Hiwatashi, N.; Bing, R.; Doyle, C.P.; Branski, R.C. Concurrent YAP/TAZ and SMAD signaling mediate vocal fold fibrosis. Sci. Rep. 2021, 11, 13484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Zhu, L.L.; Zhang, Z.; Chen, H.; Chen, Y.; Dai, Y.T. Estradiol attenuates the TGF-β1-induced conversion of primary TAFs into myofibroblasts and inhibits collagen production and myofibroblast contraction by modulating the Smad and Rho/ROCK signaling pathways. Int. J. Mol. Med. 2015, 36, 801–807. [Google Scholar] [CrossRef]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. Neuroinflammation 2022, 19, 223. [Google Scholar] [CrossRef]
- Sing, C.F.; Haviland, M.B.; Reilly, S.L. Genetic architecture of common multifactorial diseases. Ciba. Found Symp. 1996, 197, 211–229. [Google Scholar]
- Somers, K.D.; Winters, B.A.; Dawson, D.M.; Leffell, M.S.; Wright, G.L., Jr.; Devine, C.J., Jr.; Gilbert, D.A.; Horton, C.E. Chromosome abnormalities in Peyronie’s disease. J. Urol. 1987, 137, 672–675. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Nicholson, B.; Pierpaoli, S.; Lubrano, T.; Shankey, T.V. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie’s disease. Int. J. Impot. Res. 2004, 16, 288–293. [Google Scholar] [CrossRef]
- Hauck, E.W.; Hauptmann, A.; Schmelz, H.U.; Bein, G.; Weidner, W.; Hackstein, H. Prospective analysis of single nucleotide polymorphisms of the transforming growth factor beta-1 gene in Peyronie’s disease. J. Urol. 2003, 169, 369–372. [Google Scholar] [CrossRef]
- Dolmans, G.H.; Werker, P.M.; de Jong, I.J.; Nijman, R.J.; LifeLines Cohort, S.; Wijmenga, C.; Ophoff, R.A. WNT2 locus is involved in genetic susceptibility of Peyronie’s disease. J. Sex. Med. 2012, 9, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Dullea, A.; Efimenko, I.; Firdaus, F.; Griswold, A.; Arora, H.; Masterson, T.; Ramasamy, R. Whole-genome sequencing identifies novel heterozygous mutation in ALMS1 in three men with both Peyronie’s and Dupuytren’s disease. Urology 2022, 166, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Satta, M.; Lago-Docampo, M.; Bea-Mascato, B.; Solarat, C.; Castro-Sánchez, S.; Christensen, S.T.; Valverde, D. ALMS1 regulates TGF-β signaling and morphology of primary cilia. Front. Cell Dev. Biol. 2021, 9, 623829. [Google Scholar] [CrossRef] [PubMed]
- Bea-Mascato, B.; Neira-Goyanes, E.; Iglesias-Rodríguez, A.; Valverde, D. Depletion of ALMS1 affects TGF-β signalling pathway and downstream processes such as cell migration and adhesion capacity. Front. Mol. Biosci. 2022, 9, 992313. [Google Scholar] [CrossRef]
- Jones, D.L.; Haak, A.J.; Caporarello, N.; Choi, K.M.; Ye, Z.; Yan, H.; Varelas, X.; Ordog, T.; Ligresti, G.; Tschumperlin, D.J. TGFβ-induced fibroblast activation requires persistent and targeted HDAC-mediated gene repression. J. Cell. Sci. 2019, 132, jcs233486. [Google Scholar] [CrossRef]
- Kang, D.H.; Yin, G.N.; Choi, M.J.; Song, K.M.; Ghatak, K.; Minh, N.N.; Kwon, M.H.; Seong, D.H.; Ryu, J.K.; Suh, J.K.W. Silencing histone deacetylase 7 alleviates transforming growth factor-β1-induced profibrotic responses in fibroblasts derived from Peyronie’s plaque. World. J. Mens Health 2018, 36, 139–146. [Google Scholar] [CrossRef]
- Wang, M.; Huo, Z.; Wu, L.; Liu, F.; Liang, J.; He, X.; Yang, D. The role of miR-29 in the mechanism of fibrosis. Mini. Rev. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Dos Santos, V.G.; Dos Santos, G.A.; Neto, C.B.; Viana, N.I.; Pimenta, R.; Guimarães, V.R.; Candido, P.; Romão, P.; de Camargo, J.A.; Leite, K.R.M.; et al. Downregulation of miR-29b is associated with Peyronie’s disease. Urologia 2022, 89, 451–455. [Google Scholar] [CrossRef]
- Hauck, E.W.; Hauptmann, A.; Haag, S.M.; Bohnert, A.; Weidner, W.; Bein, G.; Hackstein, H. Alpha-1-antitrypsin levels and genetic variation of the alpha-1-antitrypsin gene in Peyronie’s disease. Eur. Urol. 2004, 46, 623–628. [Google Scholar] [CrossRef]
- Schwarzer, U.; Sommer, F.; Klotz, T.; Braun, M.; Reifenrath, B.; Engelmann, U. The prevalence of Peyronie’s disease: Results of a large survey. BJU Int. 2001, 88, 727–730. [Google Scholar] [CrossRef]
- Perimenis, P.; Athanasopoulos, A.; Gyftopoulos, K.; Katsenis, G.; Barbalias, G. Peyronie’s disease: Epidemiology and clinical presentation of 134 cases. Int. Urol. Nephrol. 2001, 32, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.A.; Morgentaler, A. Testosterone deficiency and Peyronie’s disease: Pilot data suggesting a significant relationship. J. Sex. Med. 2009, 6, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Park, H.J.; Park, N.C. Does testosterone deficiency exaggerate the clinical symptoms of Peyronie’s disease? Int. J. Urol. 2011, 18, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, G.; Biagiotti, G.; Lo Giudice, C. Association between Peyronie disease and low serum testosterone levels: Detection and therapeutic considerations. J. Androl. 2012, 33, 381–388. [Google Scholar] [CrossRef]
- Mitsui, Y.; Yamabe, F.; Hori, S.; Uetani, M.; Aoki, H.; Sakurabayashi, K.; Okawa, M.; Kobayashi, H.; Nakajima, K.; Nagao, K. Significant inverse association of testosterone level with penile deformity severity in Japanese males with Peyronie’s disease. Int. J. Urol. 2023, 30, 36–42. [Google Scholar] [CrossRef]
- Mishra, S.; Deng, J.J.; Gowda, P.S.; Rao, M.K.; Lin, C.L.; Chen, C.L.; Huang, T.; Sun, L.Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014, 33, 4097–4106. [Google Scholar] [CrossRef]
- Mitsui, Y.; Kobayashi, H.; Yamabe, F.; Nakajima, K.; Nagao, K. ABO blood type and risk of Peyronie’s disease in Japanese males. World J. Mens Health 2022, 40, 509–516. [Google Scholar] [CrossRef]
- Alkout, A.M.; Blackwell, C.C.; Weir, D.M. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J. Infect. Dis. 2000, 181, 1364–1369. [Google Scholar] [CrossRef]
- Bei, J.X.; Li, Y.; Jia, W.H.; Feng, B.J.; Zhou, G.; Chen, L.Z.; Feng, Q.S.; Low, H.Q.; Zhang, H.; He, F.; et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat. Genet. 2010, 42, 599–603. [Google Scholar] [CrossRef]
- Liu, W.C.; Chuang, H.C.; Chou, C.L.; Lee, Y.H.; Chiu, Y.J.; Wang, Y.L.; Chiu, H.W. Cigarette smoke exposure increases glucose-6-phosphate dehydrogenase, autophagy, fibrosis, and senescence in kidney cells in vitro and in vivo. Oxid. Med. Cell. Longev. 2022, 2022, 5696686. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Massion, P.P.; Gonzalez, A.L.; Lu, P.; Datta, P.K. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol. Cancer Ther. 2012, 11, 2362–2372. [Google Scholar] [CrossRef]
- Wu, Y.; Song, P.; Yuan, X.; Li, D. Exploring the effect of dapagliflozin on alcoholic kidney injury and renal interstitial fibrosis in rats based on TIMP-1/MMP-24 pathway. Evid. Based Complement. Alternat. Med. 2021, 2021, 6538189. [Google Scholar] [CrossRef]
- Zdanowicz, K.; Kowalczuk-Kryston, M.; Olanski, W.; Werpachowska, I.; Mielech, W.; Lebensztejn, D.M. Increase in serum MMP-9 and TIMP-1 concentrations during alcohol intoxication in adolescents-a preliminary study. Biomolecules 2022, 12, 710. [Google Scholar] [CrossRef]
- Paulis, G.; Paulis, A.; Perletti, G.A. Congenital penile curvature as a possible risk factor for the onset of Peyronie’s disease, and psychological consequences of penile curvature. Arch. Ital. Urol. Androl. 2023, 95, 11238. [Google Scholar] [CrossRef] [PubMed]
- Bias, W.B.; Nyberg, L.M., Jr.; Hochberg, M.C.; Walsh, P.C. Peyronie’s disease: A newly recognized autosomal-dominant trait. Am. J. Med. Genet. 1982, 12, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, S.; Dede, E.; Oztunc, F.; Gedikbasi, A.; Yesil, G.; Dedeoglu, R. Mutation identification and prediction for severe cardiomyopathy in Alström syndrome, and review of the literature for cardiomyopathy. Orphanet. J. Rare Dis. 2022, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Zulato, E.; Favaretto, F.; Veronese, C.; Campanaro, S.; Marshall, J.D.; Romano, S.; Cabrelle, A.; Collin, G.B.; Zavan, B.; Belloni, A.S.; et al. ALMS1-deficient fibroblasts over-express extra-cellular matrix components, display cell cycle delay and are resistant to apoptosis. PLoS ONE 2011, 6, e19081. [Google Scholar] [CrossRef]
- Altorok, N.; Tsou, P.S.; Coit, P.; Khanna, D.; Sawalha, A.H. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann. Rheum. Dis. 2015, 74, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Christensen, M.B.; Hotaling, J.M.; Pastuszak, A.W. A review of inflammation and fibrosis: Implications for the pathogenesis of Peyronie’s disease. World J. Urol. 2020, 38, 253–261. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Shen, Z.J.; Zhou, X.L.; Lu, Y.L.; Chen, Z.D. Effect of androgen deprivation on penile ultrastructure. Asian J. Androl. 2003, 5, 33–36. [Google Scholar] [PubMed]
- Karavitakis, M.; Komninos, C.; Simaioforidis, V.; Kontos, S.; Lefakis, G.; Politis, V.; Koritsiadis, G.; Konstantellou, K.; Doumanis, G. The relationship between androgens, regulators of collagen metabolism, and Peyronie’s disease: A case control study. J. Sex. Med. 2010, 7, 4011–4017. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.W.; Verges, D.; Matthews, J.; Carson, C.C.; Coward, R.M. Low testosterone has a similar prevalence among men with sexual dysfunction due to either Peyronie’s disease or erectile dysfunction and does not correlate with Peyronie’s disease severity. J. Sex. Med. 2015, 12, 690–696. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Matsushita, K.; Nelson, C.J. Testosterone levels are not associated with magnitude of deformity in men with Peyronie’s disease. J. Sex. Med. 2019, 16, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Boeri, L.; Capogrosso, P.; Oreggia, D.; Cazzaniga, W.; Pozzi, E.; Belladelli, F.; Baudo, A.; Abbate, C.; Montorsi, F.; et al. Serum testosterone levels are not associated with the severity of penile curvature in men with Peyronie’s disease-findings from a cross-sectional study. Int. J. Impot. Res. 2020, 33, 832–838. [Google Scholar] [CrossRef]
- Khanna, S.; Shankar Raman, V.; Badwal, S.; Vinu Balraam, K.V. Quantification of the androgen and estrogen receptors in the penile tissues of hypospadias in comparison with normal children. Fetal. Pediatr. Pathol. 2023, 42, 175–186. [Google Scholar] [CrossRef]
- Bjekic, M.D.; Vlajinac, H.D.; Sipetic, S.B.; Marinkovic, J.M. Risk factors for Peyronie’s disease: A case-control study. BJU Int. 2006, 97, 570–574. [Google Scholar] [CrossRef]
- La Pera, G.; Pescatori, E.S.; Calabrese, M.; Boffini, A.; Colombo, F.; Andriani, E.; Natali, A.; Vaggi, L.; Catuogno, C.; Giustini, M.; et al. Peyronie’s disease: Prevalence and association with cigarette smoking. a multicenter population-based study in men aged 50–69 years. Eur. Urol. 2001, 40, 525–530. [Google Scholar] [CrossRef]
- El-Sakka, A.I. Prevalence of Peyronie’s disease among patients with erectile dysfunction. Eur. Urol. 2006, 49, 564–569. [Google Scholar] [CrossRef]
- Langton, A.K.; Tsoureli-Nikita, E.; Merrick, H.; Zhao, X.; Antoniou, C.; Stratigos, A.; Akhtar, R.; Derby, B.; Sherratt, M.J.; Watson, R.E.; et al. The systemic influence of chronic smoking on skin structure and mechanical function. J. Pathol. 2020, 251, 420–428. [Google Scholar] [CrossRef]
- Chilton, C.P.; Castle, W.M.; Westwood, C.A.; Pryor, J.P. Factors associated in the aetiology of peyronie’s disease. Br. J. Urol. 1982, 54, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Kitamori, K.; Naito, H.; Tamada, H.; Kobayashi, M.; Miyazawa, D.; Yasui, Y.; Sonoda, K.; Tsuchikura, S.; Yasui, N.; Ikeda, K.; et al. Development of novel rat model for high-fat and high-cholesterol diet-induced steatohepatitis and severe fibrosis progression in SHRSP5/Dmcr. Environ. Health. Prev. Med. 2012, 17, 173–182. [Google Scholar] [CrossRef]
- Yuan, Y.; Naito, H.; Jia, X.; Kitamori, K.; Nakajima, T. Combination of hypertension along with a high fat and cholesterol diet induces severe hepatic inflammation in rats via a signaling network comprising NF-κB, MAPK, and Nrf2 pathways. Nutrients 2017, 9, 1018. [Google Scholar] [CrossRef]
- Jorgenson, E.; Matharu, N.; Palmer, M.R.; Yin, J.; Shan, J.; Hoffmann, T.J.; Thai, K.K.; Zhou, X.; Hotaling, J.M.; Jarvik, G.P.; et al. Genetic variation in the SIM1 locus is associated with erectile dysfunction. Proc. Natl. Acad. Sci. USA 2018, 115, 11018–11023. [Google Scholar] [CrossRef] [PubMed]
- Bovijn, J.; Jackson, L.; Censin, J.; Chen, C.Y.; Laisk, T.; Laber, S.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Smoller, J.W.; et al. GWAS identifies risk locus for erectile dysfunction and implicates hypothalamic neurobiology and diabetes in etiology. Am. J. Hum. Genet. 2019, 104, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Christensen, M.B.; Hotaling, J.M.; Pastuszak, A.W. Erectile dysfunction and Peyronie’s disease: Genetic diseases? Eur. Urol. Focus 2020, 6, 572–574. [Google Scholar] [CrossRef]
- Sasaki, N.; Uesato, R.; Yamauchi, T.; Ishibashi, Y.; Nakaji, S. Epidemiology of Dupuytren’s disease in Japanese general population. J. Hand. Surg. Asian Pac. Vol. 2021, 26, 229–234. [Google Scholar] [CrossRef]
- Dolmans, G.H.; Werker, P.M.; Hennies, H.C.; Furniss, D.; Festen, E.A.; Franke, L.; Becker, K.; van der Vlies, P.; Wolffenbuttel, B.H.; Tinschert, S.; et al. Wnt signaling and Dupuytren’s disease. N. Engl. J. Med. 2011, 365, 307–317. [Google Scholar] [CrossRef]
- Ten Dam, E.J.; van Beuge, M.M.; Bank, R.A.; Werker, P.M.J. Further evidence of the involvement of the Wnt signaling pathway in Dupuytren’s disease. J. Cell Commun. Signal. 2016, 10, 33–40. [Google Scholar] [CrossRef]
- Ng, M.; Thakkar, D.; Southam, L.; Werker, P.; Ophoff, R.; Becker, K.; Nothnagel, M.; Franke, A.; Nürnberg, P.; Espirito-Santo, A.I.; et al. A genome-wide association study of Dupuytren Disease reveals 17 additional variants implicated in fibrosis. Am. J. Hum. Genet. 2017, 101, 417–427. [Google Scholar] [CrossRef]

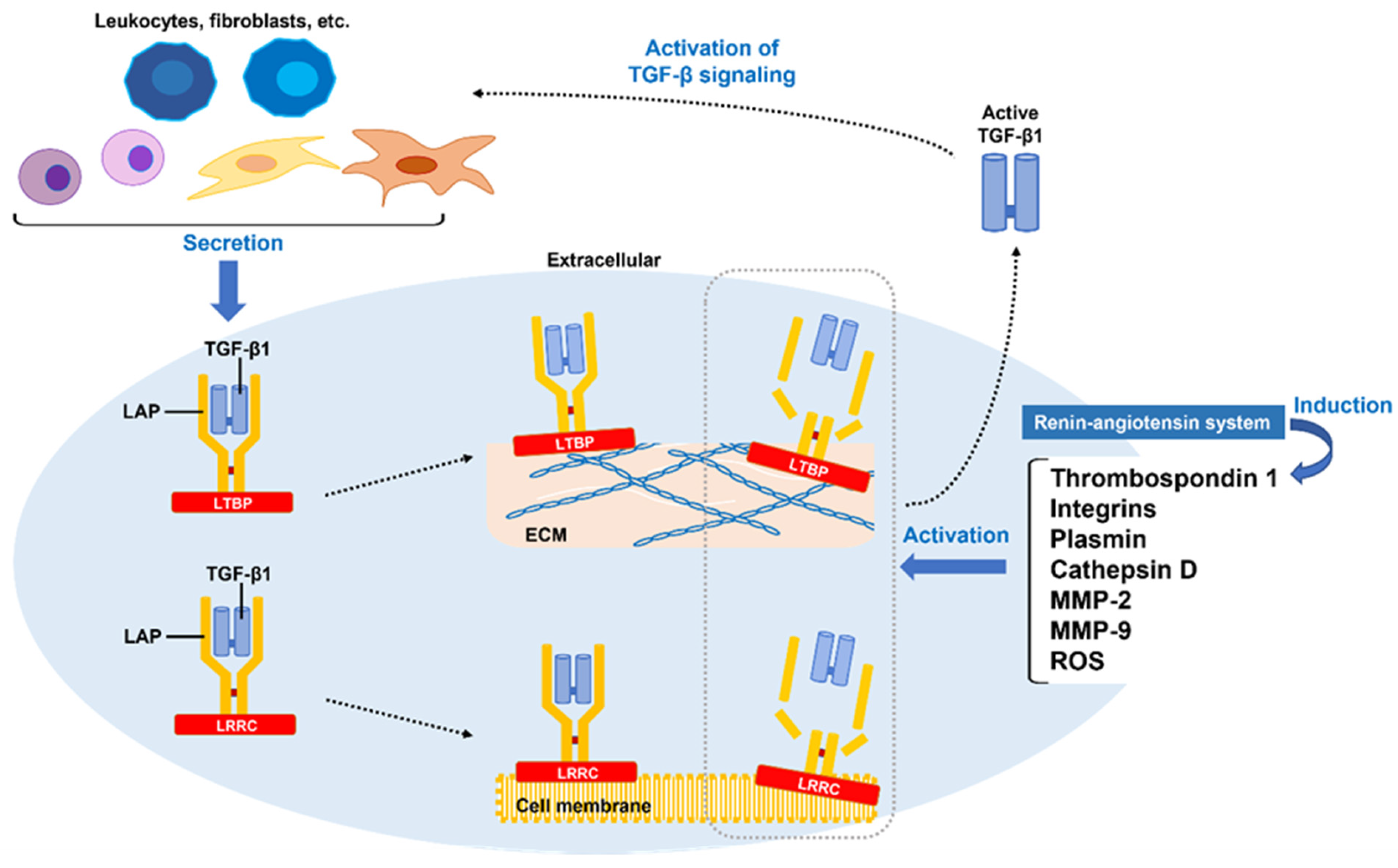
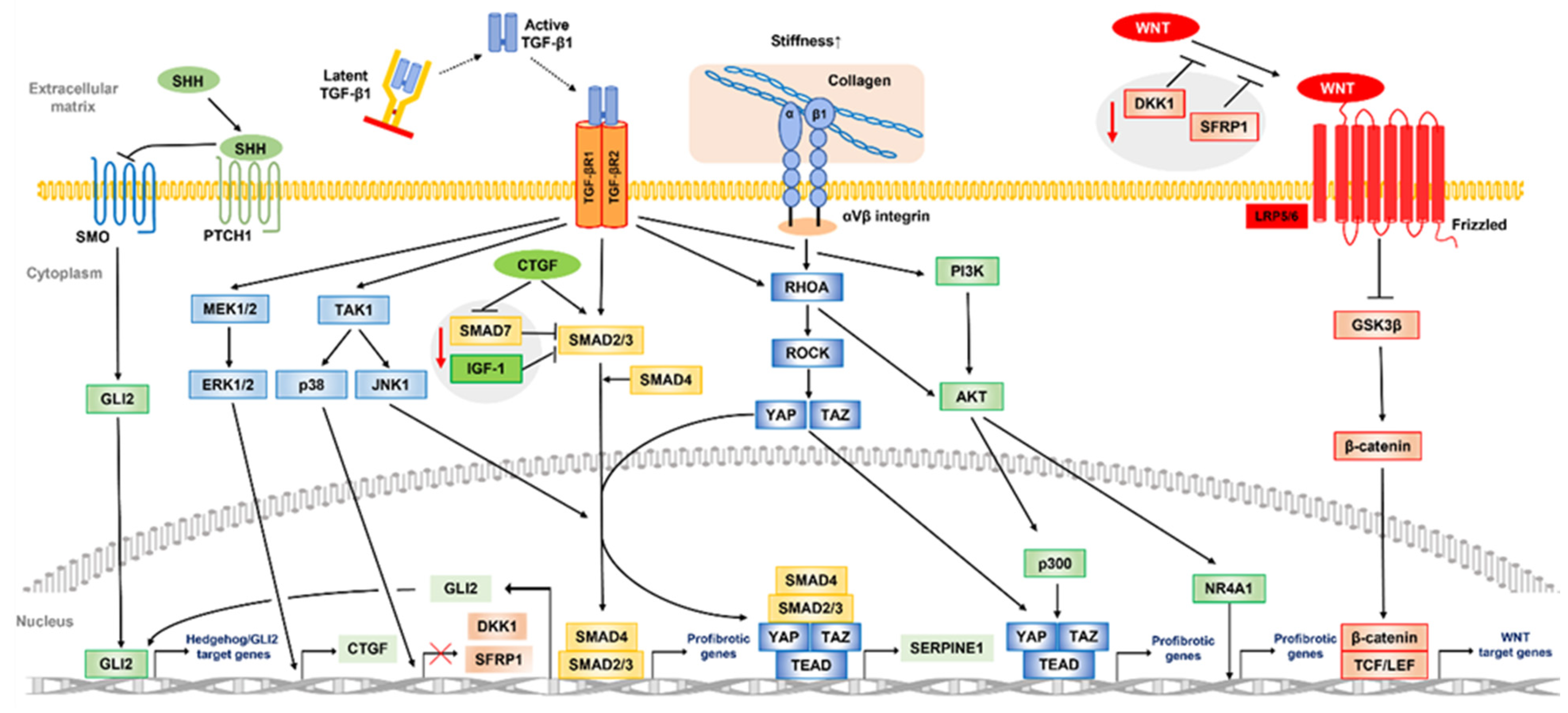
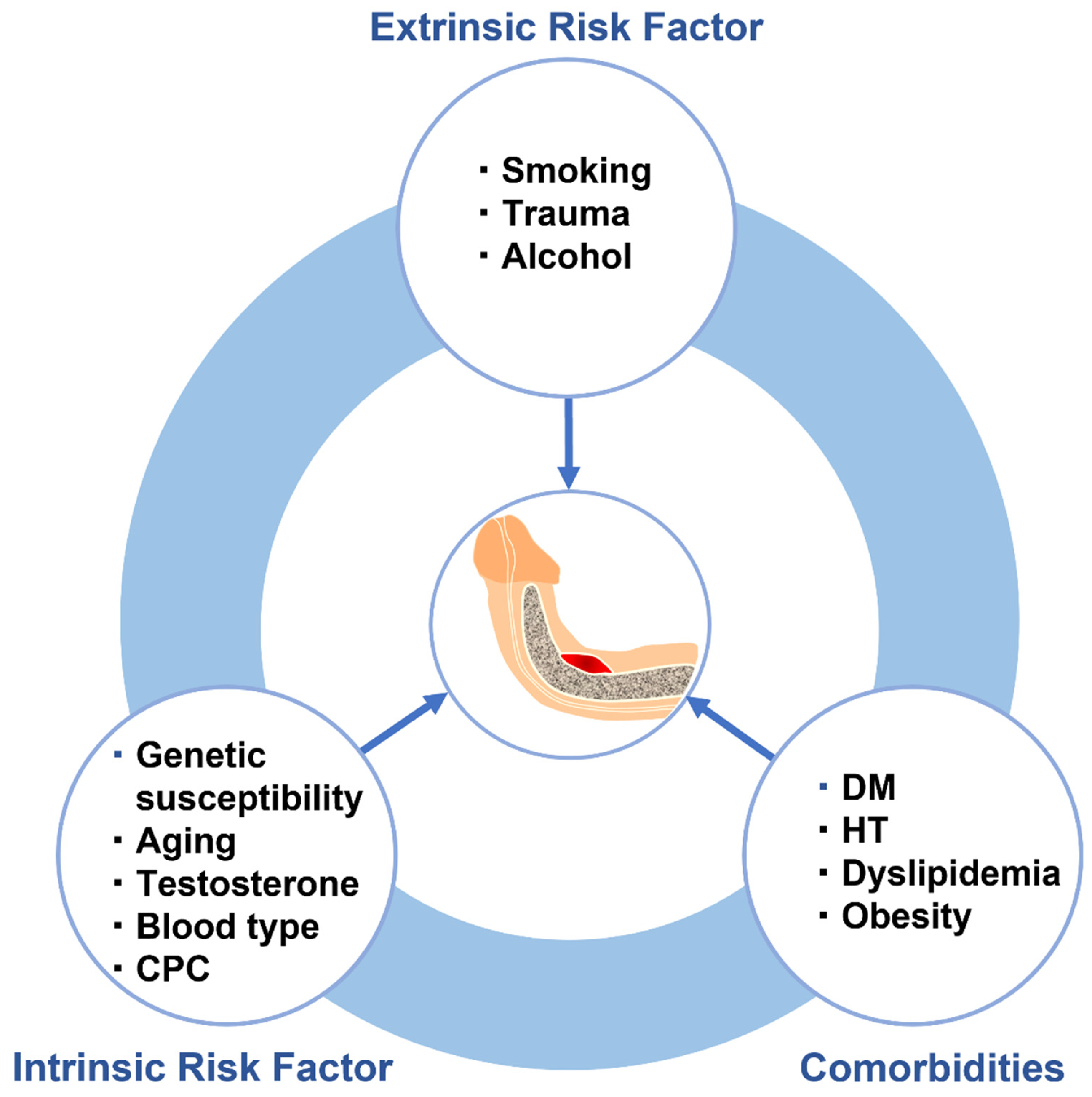
| Associated Molecules | Production Sources | Upregulators/Activators | Physiological Activities |
|---|---|---|---|
| MCP-1 | Monocytes, macrophages, fibroblasts, | Lipopolysaccharide, | Recruitment and activation of macrophages and monocytes |
| vascular endothelial cells | inflammatory cytokines | ||
| DAMPs | Dead cells, proliferating neutrophils, | Stress, tissue injury, ROS | Recruitment and activation of immune-related cells through toll-like receptors |
| macrophages, lymphocytes, | |||
| natural killer cells, resident cells, | |||
| mesenchymal stem cells | |||
| IFN-γ | Th1 cells | IL-12 | Activation of M1 macrophage polarization |
| TNF-α | Th1 cells, macrophages (M1) | IL-12 | Activation of M1 macrophage polarization, |
| promotion of differentiation of myofibroblasts | |||
| GM-CSF | Th1 cells | IL-12 | Activation of M1 macrophage polarization |
| IL-6 | Macrophages (M1) | IFN-γ, TNF-α, GM-CSF | Promotion of differentiation of myofibroblasts |
| TGF-β1 | Platelets, fibroblasts, Th2 cells, | Th2 cells, PAI-1, integrin, | Promotion of differentiation of myofibroblasts |
| macrophages (M2) | thrombospondin 1, plasmin, | ||
| MMP-2, -9, ROS | |||
| PDGF | Endothelial cells, macrophages (M1), | TGF-β1 | Promotion of differentiation of myofibroblasts |
| platelets | |||
| bFGF | Various cell types | Tissue injury | Promotion of differentiation of myofibroblasts |
| and fibroblast mitogen | |||
| Myostatin | Skeletal muscle | Promotion of differentiation of myofibroblasts | |
| ROS | Macrophages (M1), various cells | Hypoxia, inflammation | Promotion of differentiation of myofibroblasts through |
| ROS-mediated HIF-1α stabilization | |||
| MMPs | Myofobroblasts | TGF-β1, PDGF, TNF-α, bFGF | Extracellular matrix degradation |
| TIMPs | Macrophages, fibroblasts, myofibroblasts | TGF-β1 | Suppression of MMP activity |
| PAI-1 | Various cell types | TGF-β1, collagen | Inhibition of plasmin-mediated MMP activation |
| Pathway | Related Factor/Pathway | Role in Fibrosis |
|---|---|---|
| TGF-β signaling | ||
| Canonical | TGF-β1, TGF-βR, CTGF, SMAD2, 3, 4, | Transcriptional induction of profibrotic genes |
| SMAD7 (downregulation), IGF-1 (downregulation) | (αSMA, Collagen, PAI-1, MMPs, fibronectin, GLI2, etc.) | |
| Non-canonical | ||
| MAPK/ERK signaling | TGF-β1, TGF-βR, MEK, ERK | Transcription induction (CTGF) |
| TGF-β1, TGF-βR, TAK1, p38 | Transcription repression (DKK1, SFRP1) | |
| TGF-β1, TGF-βR, TAK1, JNK1 | Transcriptional induction of profibrotic genes | |
| PI3K/AKT signaling | TGF-β1, TGF-βR, PI3K, AKT, p300, NR4A1 | Transcriptional induction of profibrotic genes |
| WNT/β-catenin signaling | WNT, GSK-3β, β-catenin, TCF, LEF, | Transcriptional induction of WNT target genes |
| DKK1 (downregulation), SFRP1 (downregulation) | ||
| Hedgehog signaling | SHH, PTCH1, SMO, GLI2 | Transcriptional induction of hedgehog/GLI2 target genes |
| TGF-β signaling pathway (canonical) | Stimulation of fibroblast-to-myofibroblast transition | |
| YAP/TAZ signaling | Integrins, RHOA, ROCK, YAP, TAZ | Transcriptional induction of profibrotic genes |
| TGF-β signaling pathway (non-canonical) | Transcription induction (SERPINE1) |
| Risk Factors | Proposed Mechanisms and Pathologic Effects | Molecular Biological Changes | Reference |
|---|---|---|---|
| Intrinsic risk factors | |||
| Genetics | |||
| Chromosomal abnormalities | Familial aggregation | [158,159] | |
| Additions deletions | |||
| Inversions reciprocal translocations | |||
| Single–nucleotide polymorphisms | |||
| TGF-β1 (rs1800471) | Activation of TGF-β pathway | TGF-β1↑ | [160] |
| WNT2 (rs4730775) | Activation of WNT/β-catenin pathway | WNT2↑ | [161] |
| WNT2 (rs4730775) | Activation of WNT/β-catenin pathway | WNT2↑ | [161] |
| ALMS1 (rs45501594, rs34071195, | Evasion of apoptosis | TGF-β1↑ | [162,163,164] |
| rs41291187) | |||
| Epigenetic modifications | |||
| HDACs (up–regulation) | Activation of TGF-β pathway | PPARGC1A↓ | [165,166] |
| MicroRNA-29b (down–regulation) | Regulation of expression of multiple cytokines | ||
| DNA methylation EMT | [167,168] | ||
| Aging | Increase in PD-related comorbidities | α1-antitrypsin↓ ROS↑ | [169] |
| Semi-rigid erection | [170] | ||
| Genetic alterations, epigenetic modifications | [171] | ||
| Low testosterone level | Abnormal collagen metabolism in TA | TGF-βRII↑ | [172,173,174,175,176] |
| ABO blood type (type O) | Promotion of inflammatory reaction | TNF-α↑ IL-6↑ | [177,178,179] |
| Recombination of TGF-β1R1 and ABO gene | |||
| Congenital penile curvature | More penile trauma during intercourse | [177] | |
| Extrinsic risk factors | |||
| Smoking | Induction of profibrotic factor | CTGF↑ PAI-1↑ ROS↑ | [180] |
| Induction of EMT | |||
| Epigenetic modifications | HDACs↑ | [181] | |
| Alcohol | Increase in PD-related comorbidities | ||
| Induction of profibrotic factors | TIMP-1↑ SMAD3↑ MMP-9↑ | [182,183] | |
| Perineal and penile trauma | Promotion of detachment of septal fibers | ||
| Comorbidities | |||
| DM, HT, dyslipidemia, obesity | Vascular endothelial cell damage | TGF-β1↑ MMPs↑ TIMPs↑ROS↑ | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsui, Y.; Yamabe, F.; Hori, S.; Uetani, M.; Kobayashi, H.; Nagao, K.; Nakajima, K. Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease. Int. J. Mol. Sci. 2023, 24, 10133. https://doi.org/10.3390/ijms241210133
Mitsui Y, Yamabe F, Hori S, Uetani M, Kobayashi H, Nagao K, Nakajima K. Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease. International Journal of Molecular Sciences. 2023; 24(12):10133. https://doi.org/10.3390/ijms241210133
Chicago/Turabian StyleMitsui, Yozo, Fumito Yamabe, Shunsuke Hori, Masato Uetani, Hideyuki Kobayashi, Koichi Nagao, and Koichi Nakajima. 2023. "Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease" International Journal of Molecular Sciences 24, no. 12: 10133. https://doi.org/10.3390/ijms241210133
APA StyleMitsui, Y., Yamabe, F., Hori, S., Uetani, M., Kobayashi, H., Nagao, K., & Nakajima, K. (2023). Molecular Mechanisms and Risk Factors Related to the Pathogenesis of Peyronie’s Disease. International Journal of Molecular Sciences, 24(12), 10133. https://doi.org/10.3390/ijms241210133







