The Parallel Structure–Activity Relationship Screening of Three Compounds Identifies the Common Agonist Pharmacophore of Pyrrolidine Bis-Cyclic Guanidine Melanocortin-3 Receptor (MC3R) Small-Molecule Ligands
Abstract
1. Introduction
2. Results
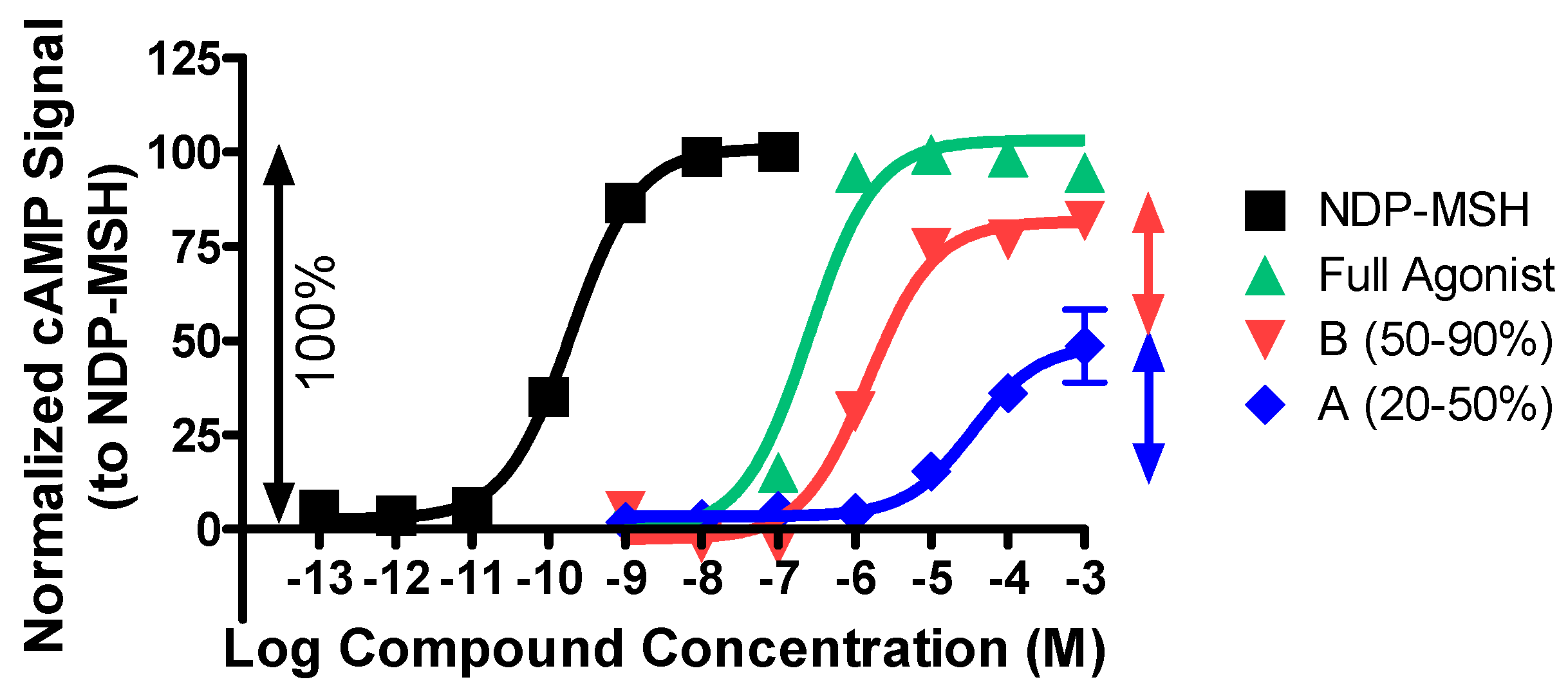
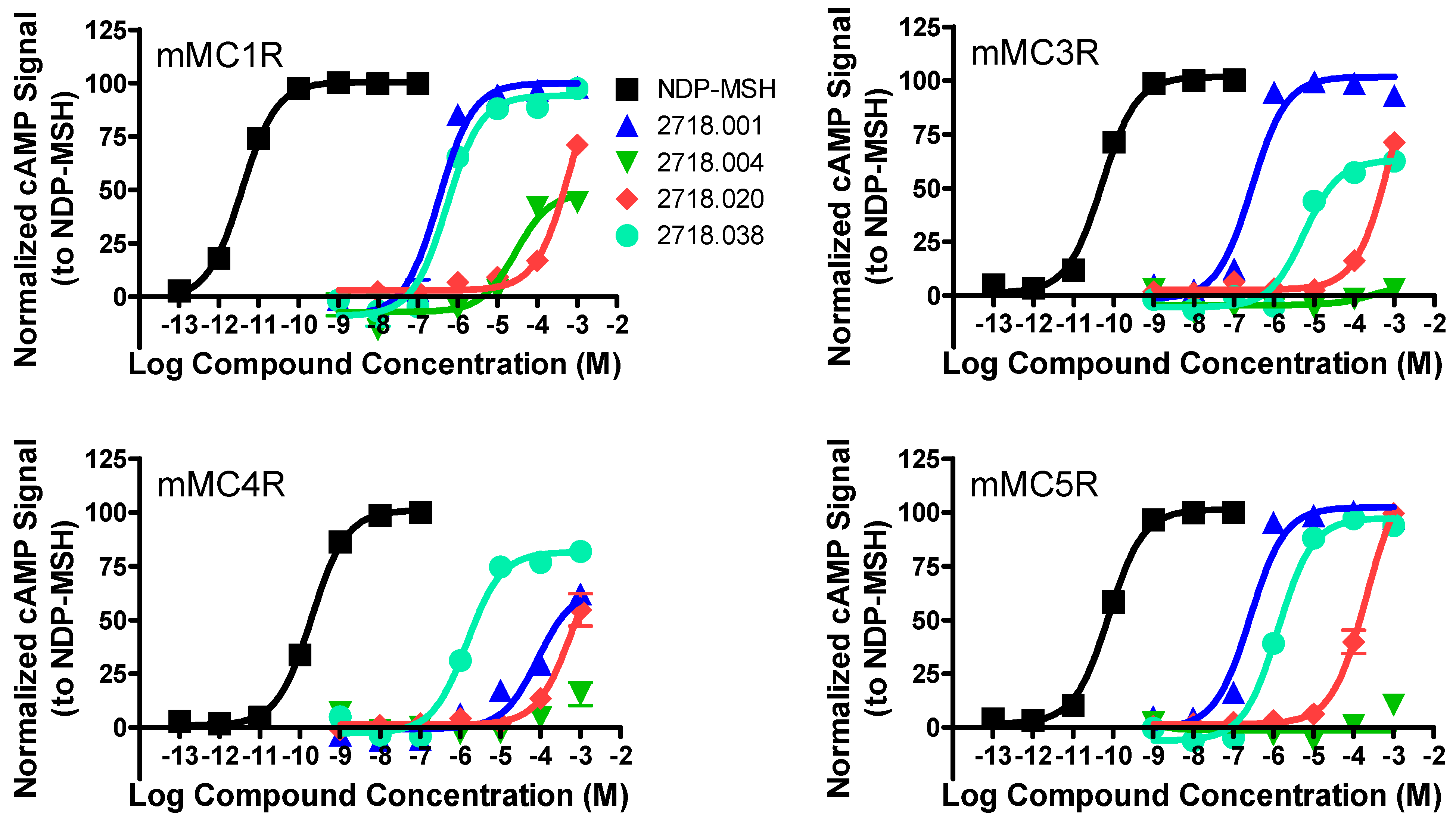
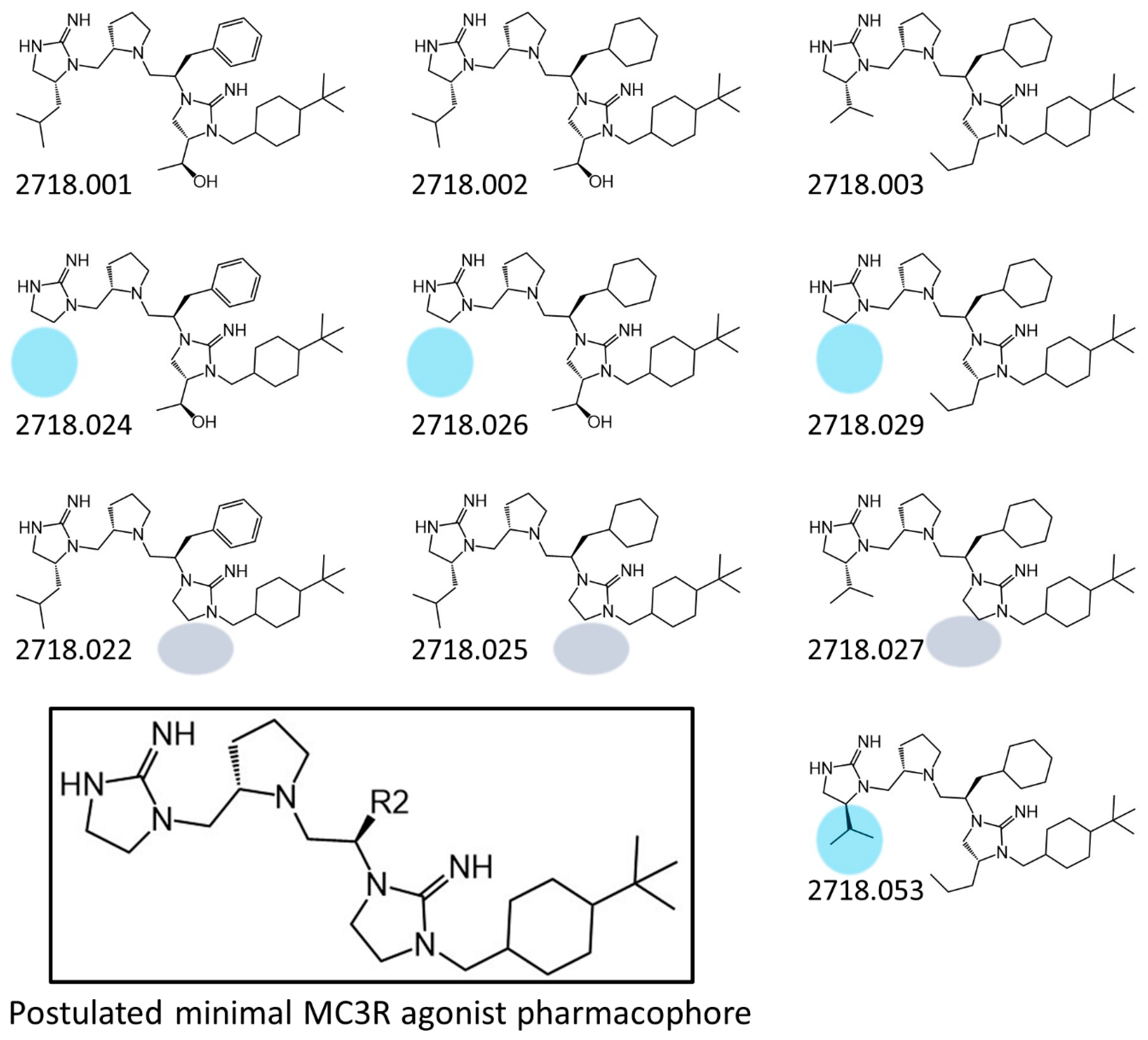
2.1. Structure–Activity Relationship Studies of 2718.001
| Compound ID | Chemical Structure | Functionalities | mMC1R | mMC3R | mMC4R | mMC5R | |
|---|---|---|---|---|---|---|---|
| EC50 (nM) | EC50 (nM) | EC50 (nM) | pA2 | EC50 (nM) | |||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.055 ± 0.008 | 0.069 ± 0.009 | 0.31 ± 0.04 | 0.10 ± 0.01 | ||
| 2718.001 | 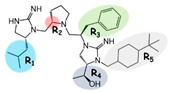 | R1: R-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 320 ± 30 | 270 ± 20 | 60% @ 1 mM | 6.0 ± 0.1 | 260 ± 30 |
| Functionality Truncation Analogs | |||||||
| 2718.024 | 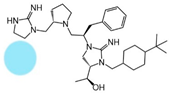 | R1: hydrogen R2: S-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 390 ± 50 | 3000 ± 700 | 50% @ 1 mM | 6.3 ± 0.1 | 480 ± 50 |
| 2718.023 | 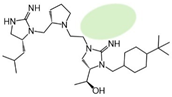 | R1: R-isobutyl R2: S-pyrrolidine R3: hydrogen R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 85% @ 1 mM | 30% @ 1 mM | 40,000 ± 20,000 (A) | 80% @ 1 mM | |
| 2718.022 | 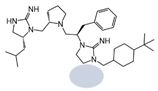 | R1: R-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: hydrogen R5: 4-tbutyl-cyclohexyl-methyl | 400 ± 40 | 380 ± 70 | 50% @ 1 mM | 6.1 ± 0.1 | 320 ± 10 |
| 2718.004 |  | R1: R-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: ethyl | 20,600 ± 600 (A) | >1,000,000 | >1,000,000 | >1,000,000 | |
| 2718.006 |  | R1: R-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: hydrogen R5: hydrogen | 40% @ 1 mM | >1,000,000 | 20% @ 1 mM | 30% @ 1 mM | |
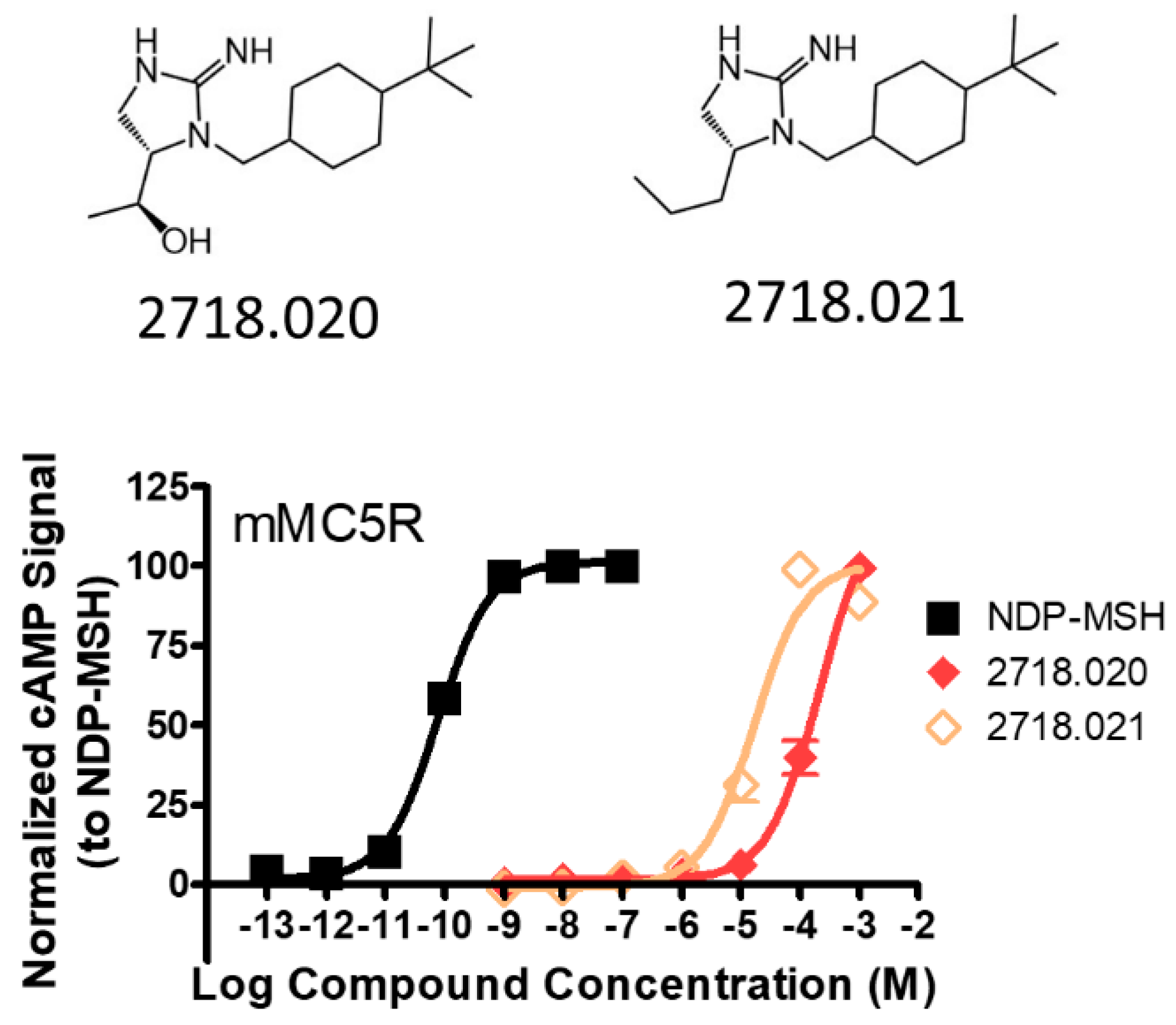
| 2718.020 | 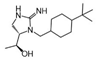 | R1: R2: R3: R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 70% @ 1 mM | 70% @ 1 mM | 55% @ 1 mM | 140,000 ± 30,000 | |
| Single Residue Stereocenter Inversion Analogs | |||||||
| 2718.033 |  | R1: S-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 380 ± 40 | 3800 ± 600 (B) | 60% @ 1 mM | 900 ± 300 | |
| 2718.032 |  | R1: R-isobutyl R2: R-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 400 ± 60 | 1500 ± 400 (B) | 55% @ 1 mM | 600 ± 200 | |
| 2718.031 |  | R1: R-isobutyl R2: S-pyrrolidine R3: S-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 2300 ± 900 | 55% @ 1 mM | 55% @ 1 mM | 70% @ 1 mM | |
| 2718.030 | 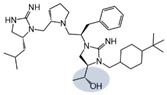 | R1: R-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 4000 ± 2000 (B) | 50% @ 1 mM | 60% @ 1 mM | 5000 ± 2000 (B) | |
| Double Residue Stereocenter Inversion Analogs | |||||||
| 2718.040 |  | R1: S-isobutyl R2: R-pyrrolidine R3: R-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 900 ± 300 | 3700 ± 400 (B) | 60% @ 1 mM | 500 ± 30 | |
| 2718.039 |  | R1: S-isobutyl R2: S-pyrrolidine R3: S-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 4000 ± 2000 (B) | 50% @ 1 mM | 55% @ 1 mM | 45,000 ± 5000 (B) | |
| 2718.037 |  | R1: S-isobutyl R2: S-pyrrolidine R3: R-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 900 ± 200 (B) | 50% @ 1 mM | 55% @ 1 mM | 7000 ± 1000 (B) | |
| 2718.038 | 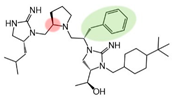 | R1: R-isobutyl R2: R-pyrrolidine R3: S-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 600 ± 100 | 5900 ± 900 (B) | 1600 ± 300 (B) | 1400 ± 300 | |
| 2718.036 | 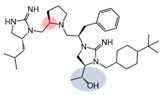 | R1: R-isobutyl R2: R-pyrrolidine R3: R-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 900 ± 300 | 50% @ 1 mM | 55% @ 1 mM | 5800 ± 900 (B) | |
| 2718.035 | 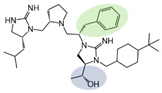 | R1: R-isobutyl R2: S-pyrrolidine R3: S-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 3700 ± 700 | 55% @ 1 mM | 55% @ 1 mM | 23,000 ± 9000 (B) | |
| Triple Residue Stereocenter Inversion Analogs | |||||||
| 2718.044 |  | R1: S-isobutyl R2: R-pyrrolidine R3: S-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 7000 ± 2000 (B) | 5600 ± 800 (A) | 50% @ 1 mM | 2900 ± 900 (B) | |
| 2718.043 |  | R1: S-isobutyl R2: R-pyrrolidine R3: R-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 3500 ± 200 (B) | 55% @ 1 mM | 55% @ 1 mM | 8000 ± 2000 (B) | |
| 2718.042 | 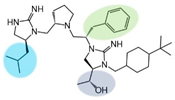 | R1: S-isobutyl R2: S-pyrrolidine R3: S-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 2900 ± 200 | 65% @ 1 mM | 65% @ 1 mM | 21,300 ± 700 (B) | |
| 2718.041 |  | R1: R-isobutyl R2: R-pyrrolidine R3: S-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 40,000 ± 10,000 | 60% @ 1 mM | 60% @ 1 mM | 8000 ± 1000 (B) | |
| Quadruple Residue Stereocenter Inversion Analogs | |||||||
| 2718.034 | 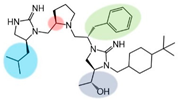 | R1: S-isobutyl R2: R-pyrrolidine R3: S-benzyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 75% @ 1 mM | 50% @ 1 mM | 55% @ 1 mM | 3000 ± 1000 | |
2.2. Structure–Activity Relationship Studies of 2718.002
| Compound ID | Chemical Structure | Functionalities | mMC1R | mMC3R | mMC4R | mMC5R | |
|---|---|---|---|---|---|---|---|
| EC50 (nM) | EC50 (nM) | EC50 (nM) | pA2 | EC50 (nM) | |||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.055 ± 0.008 | 0.069 ± 0.009 | 0.31 ± 0.04 | 0.10 ± 0.01 | ||
| 2718.002 | 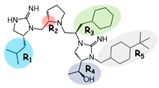 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 270 ± 20 | 240 ± 8 | 1200 ± 300 (A) | 6.2 ± 0.1 | 290 ± 10 |
| Functionality Truncation Analogs | |||||||
| 2718.026 | 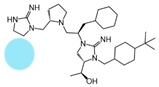 | R1: hydrogen R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 460 ± 30 | 2600 ± 200 | 1800 ± 500 (A) | 6.2 ± 0.1 | 300 ± 10 |
| 2718.025 | 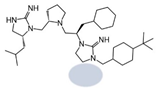 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: hydrogen R5: 4-tbutyl-cyclohexyl-methyl | 510 ± 90 | 1000 ± 300 | 60% @ 1 mM | 6.1 ± 0.1 | 600 ± 100 |
| 2718.010 | 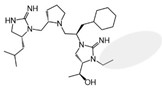 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: ethyl | 5000 ± 1000 (B) | >1,000,000 | 20% @ 1 mM | 45,5000 ± 35,000 (A) | |
| 2718.011 | 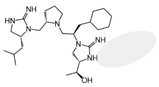 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: hydrogen | 15,000 ± 4000 (B) | >1,000,000 | >1,000,000 | 40% @ 1 mM | |
| 2718.012 |  | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: hydrogen R5: hydrogen | 15,000 ± 4000 (B) | >1,000,000 | >1,000,000 | 25% @ 1 mM | |
| Single Residue Stereocenter Inversion Analogs | |||||||
| 2718.048 | 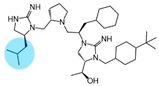 | R1: S-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 390 ± 30 | 2900 ± 200 (B) | 65% @ 1 mM | 650 ± 80 | |
| 2718.047 | 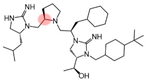 | R1: R-isobutyl R2: R-pyrrolidine R3: R-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 870 ± 70 | 85% @ 10 µM | 3100 ± 200 (B) | 450 ± 30 | |
| 2718.046 | 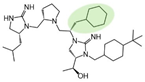 | R1: R-isobutyl R2: S-pyrrolidine R3: S-cyclohexyl-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 1800 ± 200 | 9000 ± 1000 (B) | 55% @ 1 mM | 5000 ± 1000 | |
| 2718.045 | 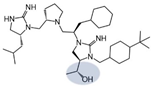 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 1000 ± 100 | 3500 ± 200 (B) | 50% @ 1 mM | 2200 ± 700 | |
| Quadruple Residue Stereocenter Inversion Analogs | |||||||
| 2718.049 |  | R1: S-isobutyl R2: R-pyrrolidine R3: S-cyclohexyl-methyl R4: (R,R)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 8000 ± 2000 (B) | 65% @ 1 mM | 65% @ 1 mM | 2800 ± 100 | |
2.3. Structure–Activity Relationship Studies at the R3 Position of 2718.001 and 2718.002
| Compound ID | Chemical Structure | Functionalities | mMC1R | mMC3R | mMC4R | mMC5R |
|---|---|---|---|---|---|---|
| EC50 (nM) | EC50 (nM) | EC50 (nM) | EC50 (nM) | |||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.055 ± 0.008 | 0.069 ± 0.009 | 0.31 ± 0.04 | 0.10 ± 0.01 | |
| R3 Substitution Analogs | ||||||
| 2718.061 | 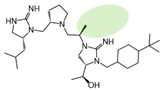 | R1: R-isobutyl R2: S-pyrrolidine R3: R-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 65% @ 1 mM | 20% @ 1 mM | >1,000,000 | 65% @ 1 mM |
| 2718.060 | 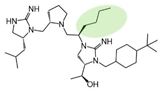 | R1: R-isobutyl R2: S-pyrrolidine R3: R-butyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 2200 ± 700 (B) | 2200 ± 200 (B) | 45% @ 1 mM | 1100 ± 300 |
| 2718.057 |  | R1: R-isobutyl R2: S-pyrrolidine R3: R-4-hydroxy-benzyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 500 ± 100 | 11,000 ± 2000 (B) | 50% @ 1 mM | 6000 ± 2000 (B) |
| 2718.055 | 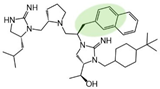 | R1: R-isobutyl R2: S-pyrrolidine R3: R-2-methyl-naphthalene R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 1000 ± 200 | 7000 ± 3000 (B) | 70% @ 1 mM | 8000 ± 2000 |
| 2718.056 |  | R1: R-isobutyl R2: S-pyrrolidine R3: R-(1H-indol-3-yl)-methyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 6000 ± 3000 (B) | 70% @ 1 mM | 75% @ 1 mM | 14,000 ± 2000 |
| 2718.059 | 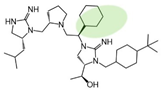 | R1: R-isobutyl R2: S-pyrrolidine R3: R-cyclohexyl R4: (S,S)-1-hydroxyethyl R5: 4-tbutyl-cyclohexyl-methyl | 70% @ 1 mM | 11,000 ± 4000 (A) | 55% @ 1 mM | 6000 ± 1000 |
2.4. Structure–Activity Relationship Studies of 2718.003
| Compound ID | Chemical Structure | Functionalities | mMC1R | mMC3R | mMC4R | mMC5R | |
|---|---|---|---|---|---|---|---|
| EC50 (nM) | EC50 (nM) | EC50 (nM) | pA2 | EC50 (nM) | |||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.055 ± 0.008 | 0.069 ± 0.009 | 0.31 ± 0.04 | 0.10 ± 0.01 | ||
| 2718.003 | 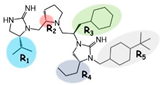 | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 640 ± 30 | 2000 ± 300 | 2500 ± 600 (A) | 5.9 ± 0.1 | 1200 ± 200 |
| Functionality Truncation Analogs | |||||||
| 2718.029 | 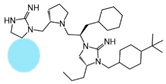 | R1: hydrogen R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 1100 ± 300 | 3300 ± 100 | 2900 ± 400 (B) | 5.8 ± 0.1 | 1300 ± 100 |
| 2718.028 |  | R1: R-isopropyl R2: S-pyrrolidine R3: hydrogen R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 10,000 ± 6000 (B) | 35% @ 1 mM | 35% @ 1 mM | 19,000 ± 5000 (B) | |
| 2718.027 |  | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: hydrogen R5: 4-tbutyl-cyclohexyl-methyl | 340 ± 30 | 2100 ± 200 | 45% @ 1 mM | 6.2 ± 0.1 | 510 ± 70 |
| 2718.014 | 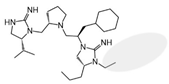 | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: ethyl | 9000 ± 3000 (B) | 20% @ 1 mM | >1,000,000 | 50% @ 1 mM | |
| 2718.015 | 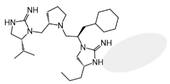 | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: hydrogen | 11,000 ± 4000 (B) | >1,000,000 | >1,000,000 | 35% @ 1 mM | |
| 2718.016 | 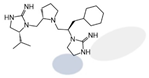 | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: hydrogen R5: hydrogen | 30,000 ± 10,000 (B) | >1,000,000 | 25% @ 1 mM | 55% @ 1 mM | |
| 2718.021 | 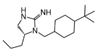 | R1: R2: R3: R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 80% @ 100 µM | 50% @ 100 µM | 50% @ 100 µM | 17,000 ± 4000 | |
| Single Residue Stereocenter Inversion Analogs | |||||||
| 2718.053 | 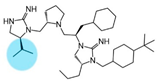 | R1: S-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 1000 ± 300 | 2400 ± 200 | 60% @ 1 mM | 5.9 ± 0.1 | 2400 ± 300 |
| 2718.052 | 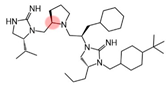 | R1: R-isopropyl R2: R-pyrrolidine R3: R-cyclohexyl-methyl R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 2500 ± 300 | 2600 ± 100 (B) | 12,000 ± 2000 (B) | 2920 ± 70 | |
| 2718.051 |  | R1: R-isopropyl R2: S-pyrrolidine R3: S-cyclohexyl-methyl R4: R-propyl R5: 4-tbutyl-cyclohexyl-methyl | 6000 ± 2000 | 65% @ 1 mM | 50,000 ± 10,000 (B) | 11,000 ± 3000 | |
| 2718.050 |  | R1: R-isopropyl R2: S-pyrrolidine R3: R-cyclohexyl-methyl R4: S-propyl R5: 4-tbutyl-cyclohexyl-methyl | 3200 ± 500 | 27,000 ± 8000 (B) | 39,000 ± 6000 (B) | 4200 ± 500 | |
| Quadruple Residue Stereocenter Inversion Analogs | |||||||
| 2718.054 | 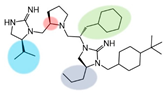 | R1: S-isopropyl R2: R-pyrrolidine R3: S-cyclohexyl-methyl R4: S-propyl R5: 4-tbutyl-cyclohexyl-methyl | 65% @ 1 mM | 55% @ 1 mM | 60% @ 1 mM | 3500 ± 300 | |
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Compound Synthesis
4.3. Compound Purification
4.4. AlphaScreen Assay
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhajlani, V.; Muceniece, R.; Wikberg, J.E. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 1993, 195, 866–873. [Google Scholar] [CrossRef]
- Chhajlani, V.; Wikberg, J.E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992, 309, 417–420. [Google Scholar] [CrossRef]
- Gantz, I.; Konda, Y.; Tashiro, T.; Shimoto, Y.; Miwa, H.; Munzert, G.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268, 8246–8250. [Google Scholar] [CrossRef]
- Gantz, I.; Miwa, H.; Konda, Y.; Shimoto, Y.; Tashiro, T.; Watson, S.J.; DelValle, J.; Yamada, T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268, 15174–15179. [Google Scholar] [CrossRef]
- Gantz, I.; Shimoto, Y.; Konda, Y.; Miwa, H.; Dickinson, C.J.; Yamada, T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1214–1220. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Cone, R.D. The cloning of a family of genes that encode the melanocortin receptors. Science 1992, 257, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Roselli-Rehfuss, L.; Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Low, M.J.; Tatro, J.B.; Entwistle, M.L.; Simerly, R.B.; Cone, R.D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. USA 1993, 90, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- Griffon, N.; Mignon, V.; Facchinetti, P.; Diaz, J.; Schwartz, J.C.; Sokoloff, P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200, 1007–1014. [Google Scholar] [CrossRef]
- Nakanishi, S.; Inoue, A.; Kita, T.; Nakamura, M.; Chang, A.C.; Cohen, S.N.; Numa, S. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature 1979, 278, 423–427. [Google Scholar] [CrossRef]
- Bultman, S.J.; Michaud, E.J.; Woychik, R.P. Molecular characterization of the mouse agouti locus. Cell 1992, 71, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Duhl, D.M.; Vrieling, H.; Cordes, S.P.; Ollmann, M.M.; Winkes, B.M.; Barsh, G.S. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993, 7, 454–467. [Google Scholar] [CrossRef]
- Lu, D.; Willard, D.; Patel, I.R.; Kadwell, S.; Overton, L.; Kost, T.; Luther, M.; Chen, W.; Woychik, R.P.; Wilkison, W.O.; et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 1994, 371, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.K.; Kerns, J.A.; Chen, Y.R.; Gantz, I.; Barsh, G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.M.; Mao, C.; MacNeil, T.; Kalyani, R.; Smith, T.; Weinberg, D.; Tota, M.R.; Van der Ploeg, L.H.T. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem. Biophys. Res. Commun. 1997, 237, 629–631. [Google Scholar] [CrossRef]
- Shutter, J.R.; Graham, M.; Kinsey, A.C.; Scully, S.; Luthy, R.; Stark, K.L. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997, 11, 593–602. [Google Scholar] [CrossRef]
- Chen, W.; Kelly, M.A.; Opitz-Araya, X.; Thomas, R.E.; Low, M.J.; Cone, R.D. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Vaisse, C.; Clement, K.; Guy-Grand, B.; Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 1998, 20, 113–114. [Google Scholar] [CrossRef]
- Yeo, G.S.; Farooqi, I.S.; Aminian, S.; Halsall, D.J.; Stanhope, R.G.; O’Rahilly, S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998, 20, 111–112. [Google Scholar] [CrossRef]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Butler, A.A.; Kesterson, R.A.; Khong, K.; Cullen, M.J.; Pelleymounter, M.A.; Dekoning, J.; Baetscher, M.; Cone, R.D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 2000, 141, 3518–3521. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.H.; et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.Y.H.; Williamson, A.; Finer, S.; Day, F.R.; Tadross, J.A.; Goncalves Soares, A.; Wade, K.; Sweeney, P.; Bedenbaugh, M.N.; Porter, D.T.; et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature 2021, 599, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Bedenbaugh, M.N.; Maldonado, J.; Pan, P.; Fowler, K.; Williams, S.Y.; Gimenez, L.E.; Ghamari-Langroudi, M.; Downing, G.; Gui, Y.; et al. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med. 2021, 13, eabd6434. [Google Scholar] [CrossRef] [PubMed]
- Doering, S.R.; Freeman, K.T.; Schnell, S.M.; Haslach, E.M.; Dirain, M.; Debevec, G.; Geer, P.; Santos, R.G.; Giulianotti, M.A.; Pinilla, C.; et al. Discovery of mixed pharmacology melanocortin-3 agonists and melanocortin-4 receptor tetrapeptide antagonist compounds (TACOs) based on the sequence Ac-Xaa1-Arg-(pI)DPhe-Xaa4-NH2. J. Med. Chem. 2017, 60, 4342–4357. [Google Scholar] [CrossRef]
- Fan, W.; Boston, B.A.; Kesterson, R.A.; Hruby, V.J.; Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997, 385, 165–168. [Google Scholar] [CrossRef]
- Adank, D.N.; Lunzer, M.M.; Ericson, M.D.; Koeperich, Z.M.; Wilber, S.L.; Fleming, K.A.; Haskell-Luevano, C. Comparative intracerebroventricular and intrathecal administration of a nanomolar macrocyclic melanocortin receptor agonist MDE6-5-2c (c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro]) decreases food intake in mice. ACS Chem. Neurosci. 2020, 11, 3051–3063. [Google Scholar] [CrossRef]
- Adank, D.N.; Lunzer, M.M.; Lensing, C.J.; Wilber, S.L.; Gancarz, A.M.; Haskell-Luevano, C. Comparative in vivo investigation of intrathecal and intracerebroventricular administration with melanocortin ligands MTII and AGRP into mice. ACS Chem. Neurosci. 2018, 9, 320–327. [Google Scholar] [CrossRef]
- Irani, B.G.; Xiang, Z.M.; Yarandi, H.N.; Holder, J.R.; Moore, M.C.; Bauzo, R.M.; Proneth, B.; Shaw, A.M.; Millard, W.J.; Chambers, J.B.; et al. Implication of the melanocortin-3 receptor in the regulation of food intake. Eur. J. Pharmacol. 2011, 660, 80–87. [Google Scholar] [CrossRef]
- Doering, S.R.; Freeman, K.; Debevec, G.; Geer, P.; Santos, R.G.; Lavoi, T.M.; Giulianotti, M.A.; Pinilla, C.; Appel, J.R.; Houghten, R.A.; et al. Discovery of nanomolar melanocortin-3 receptor (MC3R)-selective small molecule pyrrolidine bis-cyclic guanidine agonist compounds via a high-throughput “unbiased” screening campaign. J. Med. Chem. 2021, 64, 5577–5592. [Google Scholar] [CrossRef]
- Haslach, E.M.; Huang, H.; Dirain, M.; Debevec, G.; Geer, P.; Santos, R.G.; Giulianotti, M.A.; Pinilla, C.; Appel, J.R.; Doering, S.R.; et al. Identification of tetrapeptides from a mixture based positional scanning library that can restore nM full agonist function of the L106P, I69T, I102S, A219V, C271Y, and C271R human melanocortin-4 polymorphic receptors (hMC4Rs). J. Med. Chem. 2014, 57, 4615–4628. [Google Scholar] [CrossRef]
- Fleming, K.A.; Freeman, K.T.; Powers, M.D.; Santos, R.G.; Debevec, G.; Giulianotti, M.A.; Houghten, R.A.; Doering, S.R.; Pinilla, C.; Haskell-Luevano, C. Discovery of polypharmacological melanocortin-3 and -4 receptor probes and identification of a 100-fold selective nM MC3R agonist versus a µM MC4R partial agonist. J. Med. Chem. 2019, 62, 2738–2749. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.D.; Doering, S.R.; Larson, C.M.; Freeman, K.T.; LaVoi, T.M.; Donow, H.M.; Santos, R.G.; Cho, R.H.; Koerperich, Z.M.; Giulianotti, M.A.; et al. Functional mixture-based positional scan identifies a library of antagonist tetrapeptide sequences (LAtTeS) with nanomolar potency for the melanocortin-4 receptor and equipotent with the endogenous AGRP(86-132) antagonist. J. Med. Chem. 2021, 64, 14860–14875. [Google Scholar] [CrossRef]
- Manku, S.; Laplante, C.; Kopac, D.; Chan, T.; Hall, D.G. A mild and general solid-phase method for the synthesis of chiral polyamines. Solution studies on the cleavage of borane-amine intermediates from the reduction of secondary amides. J. Org. Chem. 2001, 66, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ostresh, J.M.; Schoner, C.C.; Hamashin, V.T.; Nefzi, A.; Meyer, J.P.; Houghten, R.A. Solid-phase synthesis of trisubstituted bicyclic guanidines via cyclization of reduced N-acylated dipeptides. J. Org. Chem. 1998, 63, 8622–8623. [Google Scholar] [CrossRef]
- Houghten, R.A. General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 1985, 82, 5131–5135. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, A.; Ericson, M.D.; Palusak, R.D.; Sorensen, N.B.; Wood, M.S.; Xiang, Z.; Haskell-Luevano, C. Comparative functional alanine positional scanning of the α-melanocyte stimulating hormone and NDP-melanocyte stimulating hormone demonstrates differential structure-activity relationships at the mouse melanocortin receptors. ACS Chem. Neurosci. 2016, 7, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.D.; Schnell, S.M.; Freeman, K.T.; Haskell-Luevano, C. A fragment of the Escherichia coli ClpB heat-shock protein is a micromolar melanocortin 1 receptor agonist. Bioorg. Med. Chem. Lett. 2015, 25, 5306–5308. [Google Scholar] [CrossRef]
- Lensing, C.J.; Freeman, K.T.; Schnell, S.M.; Speth, R.C.; Zarth, A.T.; Haskell-Luevano, C. Developing a biased unmatched bivalent ligand (BUmBL) design strategy to target the GPCR homodimer allosteric signaling (cAMP over β-arrestin 2 recruitment) within the melanocortin receptors. J. Med. Chem. 2019, 62, 144–158. [Google Scholar] [CrossRef]
- Lensing, C.J.; Freeman, K.T.; Schnell, S.M.; Adank, D.N.; Speth, R.C.; Haskell-Luevano, C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. J. Med. Chem. 2016, 59, 3112–3128. [Google Scholar] [CrossRef]
- Schioth, H.B.; Chhajlani, V.; Muceniece, R.; Klusa, V.; Wikberg, J.E. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996, 59, 797–801. [Google Scholar] [CrossRef]
- Schild, H.O. pA, a new scale for the measurement of drug antagonism. Br. J. Pharmacol. 1947, 2, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Shields, T.S.; Stork, P.J.S.; Cone, R.D. A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal. Biochem. 1995, 226, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.H.; Anthonavage, M.; Pappas, A.; Rossetti, D.; Cavender, D.; Seiberg, M.; Eisinger, M. Melanocortin-5 receptor and sebogenesis. Eur. J. Pharmacol. 2011, 660, 202–206. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ericson, M.D.; Freeman, K.T.; LaVoi, T.M.; Donow, H.M.; Santos, R.G.; Giulianotti, M.A.; Pinilla, C.; Houghten, R.A.; Haskell-Luevano, C. The Parallel Structure–Activity Relationship Screening of Three Compounds Identifies the Common Agonist Pharmacophore of Pyrrolidine Bis-Cyclic Guanidine Melanocortin-3 Receptor (MC3R) Small-Molecule Ligands. Int. J. Mol. Sci. 2023, 24, 10145. https://doi.org/10.3390/ijms241210145
Ericson MD, Freeman KT, LaVoi TM, Donow HM, Santos RG, Giulianotti MA, Pinilla C, Houghten RA, Haskell-Luevano C. The Parallel Structure–Activity Relationship Screening of Three Compounds Identifies the Common Agonist Pharmacophore of Pyrrolidine Bis-Cyclic Guanidine Melanocortin-3 Receptor (MC3R) Small-Molecule Ligands. International Journal of Molecular Sciences. 2023; 24(12):10145. https://doi.org/10.3390/ijms241210145
Chicago/Turabian StyleEricson, Mark D., Katie T. Freeman, Travis M. LaVoi, Haley M. Donow, Radleigh G. Santos, Marc A. Giulianotti, Clemencia Pinilla, Richard A. Houghten, and Carrie Haskell-Luevano. 2023. "The Parallel Structure–Activity Relationship Screening of Three Compounds Identifies the Common Agonist Pharmacophore of Pyrrolidine Bis-Cyclic Guanidine Melanocortin-3 Receptor (MC3R) Small-Molecule Ligands" International Journal of Molecular Sciences 24, no. 12: 10145. https://doi.org/10.3390/ijms241210145
APA StyleEricson, M. D., Freeman, K. T., LaVoi, T. M., Donow, H. M., Santos, R. G., Giulianotti, M. A., Pinilla, C., Houghten, R. A., & Haskell-Luevano, C. (2023). The Parallel Structure–Activity Relationship Screening of Three Compounds Identifies the Common Agonist Pharmacophore of Pyrrolidine Bis-Cyclic Guanidine Melanocortin-3 Receptor (MC3R) Small-Molecule Ligands. International Journal of Molecular Sciences, 24(12), 10145. https://doi.org/10.3390/ijms241210145









