Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions
Abstract
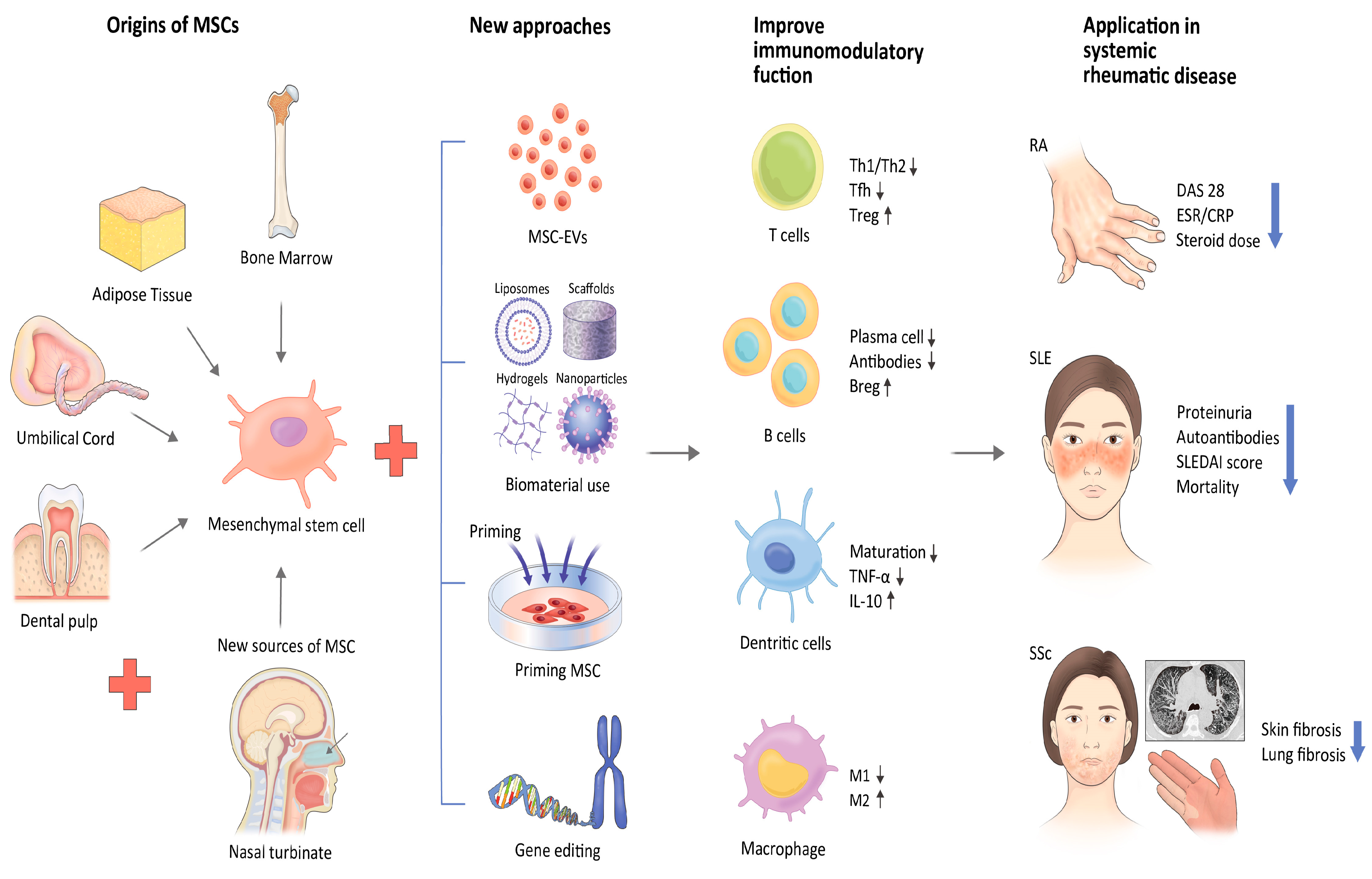
:1. Introduction
2. Characteristics of MSCs
3. Immunomodulatory Properties of MSCs
4. Rheumatoid Arthritis
4.1. MSC-Based Therapy and Mode of Action in RA
4.2. MSC-Based Therapy and Recent Clinical Applications in RA
5. Systemic Lupus Erythematosus
5.1. MSC-Based Therapy and Mode of Action in SLE
5.2. MSC-Based Therapy and Recent Clinical Applications in SLE
6. Systemic Sclerosis
6.1. MSC-Based Therapy and Mode of Action in SSc
6.2. MSC-Based Therapy and Recent Clinical Applications in SSc
| Published Year | MSCs Source | Sample Size and Target | Administration Routes | Outcome | Serious Adverse Events | Ref |
|---|---|---|---|---|---|---|
| 2011 | Allogenic BM | 5, Diffuse type SSc | IV, single, 1 × 106 cells/kg | Slight improvement of MRSS. | - | [75] |
| 2017 | SVF | 12, SSc, Hand dysfunction | SC | Improvement of hand pain, finger edema, and Raynaud’s phenomenon | - | [70] |
| 2017 | Autologous AT with PRP | 7, SSc, Oro-facial fibrosis | SC | Improvement of perioral fibrosis. | - | [76] |
| 2017 | Allogenic UC | 14, Diffuse type SSc (3 with ILD) | IV, single, 1 × 106 cells/kg (Combined with plasma exchange) | Improvement of MRSS and ILD. ↓: anti-Scl70 antibody, TGF-β, VEGF | - | [74] |
| 2019 | Autologous AT | 62, SSc, Oro-facial fibrosis | SC | Improvement of perioral fibrosis. | 1 case of wound infection | [77] |
| 2019 | Autologous AT | 38 SSc, Digital ulcer (25 Treatment vs. 13 Placebo) | SC | Improvement of ischemic digital ulcers in all of treatment group. ↓: Pain ↑: Finger capillary. | - | [78] |
| 2020 | SVF | 18, SSc, Hand dysfunction | SC, single, 3.61 × 106 cells (average) | Improvement of skin fibrosis, hand edema, and active ulcers. | - | [79] |
| 2022 | SVF | 40 SSc, Hand dysfunction (20 Treatment vs. 20 Placebo) | SC | No difference between two groups. | - | [71] |
| 2022 | Autologous AT | 88 SSc, Hand dysfunction (48 Treatment vs. 40 Placebo) | SC | No difference between two groups. | 1 case of aspiration pneumonia in treatment group | [80] |
7. MSC-Based Therapy: Current Challenges and Limitations
8. MSC-Based Therapy: Novel Approaches to Overcoming Challenges
8.1. Extracellular Vesicles from MSCs
8.2. Biomaterial Strategies Applied to MSC-Based Therapy
8.3. Preconditioning/Priming and Genetic Modification of MSCs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Harna, B.; Kalra, P.; Arya, S.; Jeyaraman, N.; Nallakumarasamy, A.; Jeyaraman, M.; Rajendran, R.L.; Oh, E.J.; Khanna, M.; Rajendran, U.M.; et al. Mesenchymal stromal cell therapy for patients with rheumatoid arthritis. Exp. Cell. Res. 2023, 423, 113468. [Google Scholar] [CrossRef] [PubMed]
- Lisnevskaia, L.; Murphy, G.; Isenberg, D. Systemic lupus erythematosus. Lancet 2014, 384, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, J.; Tian, Y.; Li, H.; Zhang, L.; Yang, J.; Wang, J.; Zhang, J.; Yan, S.; Xu, D. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 714832. [Google Scholar] [CrossRef]
- Barsotti, S.; Orlandi, M.; Codullo, V.; Di Battista, M.; Lepri, G.; Della Rossa, A.; Guiducci, S. One year in review 2019: Systemic sclerosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 119), 3–14. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell. Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Costa, L.; Esparza, P.; Landin, M.; Diaz-Rodriguez, P.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cells in Homeostasis and Systemic Diseases: Hypothesis, Evidences, and Therapeutic Opportunities. Int. J. Mol. Sci. 2019, 20, 3738. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Kwok, S.-K. Recent Advances in Cell Therapeutics for Systemic Autoimmune Diseases. Immune Netw. 2022, 22, e10. [Google Scholar] [CrossRef]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal Stem or Stromal Cells: Toward a Better Understanding of Their Biology? Transfus. Med. Hemother 2010, 37, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Del Papa, B.; Sportoletti, P.; Cecchini, D.; Rosati, E.; Balucani, C.; Baldoni, S.; Fettucciari, K.; Marconi, P.; Martelli, M.F.; Falzetti, F.; et al. Notch1 modulates mesenchymal stem cells mediated regulatory T-cell induction. Eur. J. Immunol. 2013, 43, 182–187. [Google Scholar] [CrossRef]
- Usha Shalini, P.; Vidyasagar, J.V.; Kona, L.K.; Ponnana, M.; Chelluri, L.K. In vitro allogeneic immune cell response to mesenchymal stromal cells derived from human adipose in patients with rheumatoid arthritis. Cell. Immunol. 2017, 314, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, Q.; Chen, X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front. Immunol. 2020, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2, 3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 11592. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.Y.; Zhou, P.J.; He, Z.; Yan, H.Z.; Xu, D.D.; Wang, Y.; Fu, W.Y.; Ruan, B.B.; Wang, S.; et al. Use of immune modulation by human adipose-derived mesenchymal stem cells to treat experimental arthritis in mice. Am. J. Transl. Res. 2017, 9, 2595–2607. [Google Scholar]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Santalla, M.; Mancheño-Corvo, P.; Menta, R.; Lopez-Belmonte, J.; DelaRosa, O.; Bueren, J.A.; Dalemans, W.; Lombardo, E.; Garin, M.I. Human Adipose-Derived Mesenchymal Stem Cells Modulate Experimental Autoimmune Arthritis by Modifying Early Adaptive T Cell Responses. STEM CELLS 2015, 33, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Zhang, Z.; Zhou, M.; Sun, Y.; Su, D.; Feng, X.; Gao, X.; Shi, S.; Chen, W.; et al. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Sci. Rep. 2015, 5, 12777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garimella, M.G.; Kour, S.; Piprode, V.; Mittal, M.; Kumar, A.; Rani, L.; Pote, S.T.; Mishra, G.C.; Chattopadhyay, N.; Wani, M.R. Adipose-Derived Mesenchymal Stem Cells Prevent Systemic Bone Loss in Collagen-Induced Arthritis. J. Immunol. 2015, 195, 5136–5148. [Google Scholar] [CrossRef] [Green Version]
- Kehoe, O.; Cartwright, A.; Askari, A.; El Haj, A.J.; Middleton, J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J. Transl. Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadmanfar, S.; Labibzadeh, N.; Emadedin, M.; Jaroughi, N.; Azimian, V.; Mardpour, S.; Kakroodi, F.A.; Bolurieh, T.; Hosseini, S.E.; Chehrazi, M.; et al. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 499–506. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Cong, X.; Liu, G.; Zhou, J.; Bai, B.; Li, Y.; Bai, W.; Li, M.; Ji, H.; et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev. 2013, 22, 3192–3202. [Google Scholar] [CrossRef] [Green Version]
- Ghoryani, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Ghasemi, A.; Poursamimi, J.; Mohammadi, M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed. Pharmacother. 2019, 109, 1834–1840. [Google Scholar] [CrossRef]
- Wang, L.; Huang, S.; Li, S.; Li, M.; Shi, J.; Bai, W.; Wang, Q.; Zheng, L.; Liu, Y. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug. Des. Devel Ther. 2019, 13, 4331–4340. [Google Scholar] [CrossRef] [Green Version]
- Álvaro-Gracia, J.M.; Jover, J.A.; García-Vicuña, R.; Carreño, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martínez-Taboada, V.M.; Taylor, P.; Martín-Martín, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Zhao, R.; Guo, W.; Zhu, M.; Xing, W.; Jiang, D.; Liu, C.; Xu, X. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J. Transl. Med. 2018, 16, 165. [Google Scholar] [CrossRef] [Green Version]
- Gowhari Shabgah, A.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Ghoryani, M.; Mohammadi, M. Possible Anti-inflammatory Effects of Mesenchymal Stem Cells Transplantation via Changes in CXCL8 Levels in Patients with Refractory Rheumatoid Arthritis. Int. J. Mol. Cell. Med. 2019, 8, 191–199. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Yao, M.; Yang, L.; Ao, L.; Hu, X.; Li, Z.; Wu, X.; Tan, Y.; Xing, W.; et al. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-γ treatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 1298–1304. [Google Scholar] [CrossRef]
- Ghoryani, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. The Sufficient Immunoregulatory Effect of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation on Regulatory T Cells in Patients with Refractory Rheumatoid Arthritis. J. Immunol. Res. 2020, 2020, 3562753. [Google Scholar] [CrossRef]
- Gowhari Shabgah, A.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Ghasemi, A.; Ghoryani, M.; Mohammadi, M. A significant decrease of BAFF, APRIL, and BAFF receptors following mesenchymal stem cell transplantation in patients with refractory rheumatoid arthritis. Gene 2020, 732, 144336. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Stebbings, K.A.; Kim, H.; Park, H.; Chang, D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: A phase I/IIa, open-label, non-randomized pilot trial. Stem Cell. Res. Ther. 2022, 13, 88. [Google Scholar] [CrossRef]
- Kronbichler, A.; Brezina, B.; Gauckler, P.; Quintana, L.F.; Jayne, D.R.W. Refractory lupus nephritis: When, why and how to treat. Autoimmun. Rev. 2019, 18, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekvig, O.P. Systemic Lupus Erythematosus: Definitions, Contexts, Conflicts, Enigmas. Front. Immunol. 2018, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.; Xu, Z.; Xu, A.; Liu, Y.; Fu, Q.; Wang, J.; Huang, F.; Zheng, Y.; Qi, G.; Sun, B.; et al. Human gingiva-derived mesenchymal stem cells are therapeutic in lupus nephritis through targeting of CD39(-)CD73 signaling pathway. J. Autoimmun. 2020, 113, 102491. [Google Scholar] [CrossRef]
- Schena, F.; Gambini, C.; Gregorio, A.; Mosconi, M.; Reverberi, D.; Gattorno, M.; Casazza, S.; Uccelli, A.; Moretta, L.; Martini, A.; et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2776–2786. [Google Scholar] [CrossRef]
- Park, M.J.; Kwok, S.K.; Lee, S.H.; Kim, E.K.; Park, S.H.; Cho, M.L. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell. Transplant. 2015, 24, 2367–2377. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Feng, X.; Sun, L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2017, 14, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.; Jeong, M.; Kim, S.; Jang, K.; Kang, B.K.; Lee, D.Y.; Bae, S.C.; Kim, K.S.; Youn, J. Infusion of Human Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Autoimmune Nephritis in a Lupus Model by Suppressing Follicular Helper T-Cell Development. Cell. Transplant. 2016, 25, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, J.S.; Lee, H.K.; Park, E.J.; Jeon, H.W.; Kang, Y.J.; Lee, T.Y.; Kim, K.S.; Bae, S.C.; Park, J.H.; et al. Mesenchymal Stem Cells Ameliorate Renal Inflammation in Adriamycin-induced Nephropathy. Immune Netw. 2019, 19, e36. [Google Scholar] [CrossRef] [PubMed]
- Carrion, F.; Nova, E.; Ruiz, C.; Diaz, F.; Inostroza, C.; Rojo, D.; Mönckeberg, G.; Figueroa, F.E. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus 2010, 19, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wang, D.; Liang, J.; Zhang, H.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Ye, S.; Hu, X.; et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Barbado, J.; Tabera, S.; Sánchez, A.; García-Sancho, J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus 2018, 27, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Hassanzadeh, H.; Jahandoust, F.; Miri, R.; Bidkhori, H.R.; Monzavi, S.M.; Sanjar-Moussavi, N.; Matin, M.M.; Shariati-Sarabi, Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: Results of a phase I clinical trial. Curr. Res. Transl. Med. 2022, 70, 103324. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Liang, J.; Li, X.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Lu, L.; Gilkeson, G.S.; et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell. Transplant. 2013, 22, 2267–2277. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhang, Y.; Zhang, M.; Chen, J.; Li, X.; Hu, X.; Jiang, S.; Shi, S.; Sun, L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res. Ther. 2014, 16, R79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Wang, D.; Zhang, H.; Feng, X.; Gilkeson, G.S.; Shi, S.; Sun, L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin. Rheumatol. 2014, 33, 1611–1619. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.; Feng, X.; Yuan, X.; Zhao, S.; Zhang, H.; Liang, J.; Zhao, C.; Wang, H.; Hua, B.; et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: A 6-year follow-up study. Clin. Exp. Med. 2017, 17, 333–340. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, P.; Guo, Y.; Lim, T.O. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann. Rheum. Dis. 2017, 76, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Choi, C.B.; Kim, M.S.; Nam, J.Y.; Lee, T.Y.; Lee, Y.T.; Kim, S.; Han, S.B.; Bae, S.C. Safety and tolerability of bone marrow-derived mesenchymal stem cells in lupus animal models and a phase I clinical trial in humans. Lupus 2022, 31, 1245–1253. [Google Scholar] [CrossRef]
- Kamen, D.L.; Wallace, C.; Li, Z.; Wyatt, M.; Paulos, C.; Wei, C.; Wang, H.; Wolf, B.J.; Nietert, P.J.; Gilkeson, G. Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE. Lupus Sci. Med. 2022, 9, e000704. [Google Scholar] [CrossRef]
- Elhai, M.; Meune, C.; Boubaya, M.; Avouac, J.; Hachulla, E.; Balbir-Gurman, A.; Riemekasten, G.; Airò, P.; Joven, B.; Vettori, S.; et al. Mapping and predicting mortality from systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1897–1905. [Google Scholar] [CrossRef]
- Bussone, G.; Mouthon, L. Interstitial lung disease in systemic sclerosis. Autoimmun. Rev. 2011, 10, 248–255. [Google Scholar] [CrossRef]
- Varga, J. Systemic sclerosis: An update. Bull. NYU Hosp. Jt. Dis. 2008, 66, 198–202. [Google Scholar]
- Benfaremo, D.; Svegliati, S.; Paolini, C.; Agarbati, S.; Moroncini, G. Systemic Sclerosis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2022, 10, 163. [Google Scholar] [CrossRef]
- Watt, S.M.; Gullo, F.; van der Garde, M.; Markeson, D.; Camicia, R.; Khoo, C.P.; Zwaginga, J.J. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 2013, 108, 25–53. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [Green Version]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhu, S.; Li, Y.; Lu, Q.; Zhang, Q.; Su, L.; Zhang, Q.; Zhao, Y.; Luo, Y.; Liu, Y. Human umbilical cord mesenchymal stem cells ameliorate skin fibrosis development in a mouse model of bleomycin-induced systemic sclerosis. Exp. Ther. Med. 2020, 20, 257. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Hou, J.; Zheng, K.; Wei, D.; Zhang, A.; Wang, S.; Mei, H.; Li, C.; Cheng, L.; Sun, X. Umbilical Cord Mesenchymal Stem Cells for Inhibiting the Fibrosis and Autoimmune Development in HOCl-Induced Systemic Scleroderma Mouse Model. Int. J. Stem Cells 2021, 14, 262–274. [Google Scholar] [CrossRef]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.-R.; Nguyen, P.-S.; Chabannon, C.; Colavolpe, N.; Reynier, J.-C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daumas, A.; Magalon, J.; Jouve, E.; Truillet, R.; Casanova, D.; Giraudo, L.; Veran, J.; Benyamine, A.; Dignat-George, F.; Magalon, G.; et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Curr. Res. Transl. Med. 2017, 65, 40–43. [Google Scholar] [CrossRef]
- Daumas, A.; Magalon, J.; Jouve, E.; Casanova, D.; Philandrianos, C.; Abellan Lopez, M.; Mallet, S.; Veran, J.; Auquit-Auckbur, I.; Farge, D.; et al. Adipose tissue-derived stromal vascular fraction for treating hands of patients with systemic sclerosis: A multicentre randomized trial Autologous AD-SVF versus placebo in systemic sclerosis. Rheumatology 2022, 61, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Schendel, M.; Föll, J.; Müller, L.P.; Keysser, G.; Behre, G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia 2008, 22, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Loisel, S.; Resche-Rigon, M.; Lansiaux, P.; Colmegna, I.; Langlais, D.; Charles, C.; Pugnet, G.; Maria, A.T.J.; Chatelus, E.; et al. Safety and preliminary efficacy of allogeneic bone marrow-derived multipotent mesenchymal stromal cells for systemic sclerosis: A single-centre, open-label, dose-escalation, proof-of-concept, phase 1/2 study. Lancet Rheumatol. 2022, 4, e91–e104. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, J.; Tang, X.; Wang, D.; Feng, X.; Wang, F.; Hua, B.; Wang, H.; Sun, L. Sustained benefit from combined plasmapheresis and allogeneic mesenchymal stem cells transplantation therapy in systemic sclerosis. Arthritis Res. Ther. 2017, 19, 165. [Google Scholar] [CrossRef]
- Keyszer, G.; Christopeit, M.; Fick, S.; Schendel, M.; Taute, B.M.; Behre, G.; Müller, L.P.; Schmoll, H.J. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: Report of five cases. Arthritis Rheum. 2011, 63, 2540–2542. [Google Scholar] [CrossRef]
- Blezien, O.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Effects of Fat Grafting Containing Stem Cells in Microstomia and Microcheilia Derived from Systemic Sclerosis. Aesthetic Plast. Surg. 2017, 41, 839–844. [Google Scholar] [CrossRef]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Del Papa, N.; Di Luca, G.; Andracco, R.; Zaccara, E.; Maglione, W.; Pignataro, F.; Minniti, A.; Vitali, C. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: Results of a monocentric randomized controlled study. Arthritis Res. Ther. 2019, 21, 7. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Lee, Y.J.; Koh, J.H.; Lee, J.; Min, H.K.; Kim, M.Y.; Kim, K.J.; Lee, S.J.; Rhie, J.W.; Kim, W.U.; et al. Clinical Efficacy and Safety of Injection of Stromal Vascular Fraction Derived from Autologous Adipose Tissues in Systemic Sclerosis Patients with Hand Disability: A Proof-Of-Concept Trial. J. Clin. Med. 2020, 9, 3023. [Google Scholar] [CrossRef]
- Khanna, D.; Caldron, P.; Martin, R.W.; Kafaja, S.; Spiera, R.; Shahouri, S.; Shah, A.; Hsu, V.; Ervin, J.; Simms, R.; et al. Adipose-Derived Regenerative Cell Transplantation for the Treatment of Hand Dysfunction in Systemic Sclerosis: A Randomized Clinical Trial. Arthritis Rheumatol. 2022, 74, 1399–1408. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Shin, S.; Jeong, S.Y.; Lim, S.U.; Lee, D.W.; Kwon, Y.K.; Kang, J.; Kim, S.W.; Jung, C.K.; Lee, C.; et al. Nasal Turbinate Mesenchymal Stromal Cells Preserve Characteristics of Their Neural Crest Origin and Exert Distinct Paracrine Activity. J. Clin. Med. 2021, 10, 1792. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Gosálvez, J.; Roy, R.; Zaragoza, P.; Martín-Burriel, I.; et al. Priming Equine Bone Marrow-Derived Mesenchymal Stem Cells with Proinflammatory Cytokines: Implications in Immunomodulation-Immunogenicity Balance, Cell Viability, and Differentiation Potential. Stem Cells Dev. 2017, 26, 15–24. [Google Scholar] [CrossRef]
- Huang, X.P.; Sun, Z.; Miyagi, Y.; McDonald Kinkaid, H.; Zhang, L.; Weisel, R.D.; Li, R.K. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef] [Green Version]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R., 3rd; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell. Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Bird, A.K.; Meednu, N.; Dauenhauer, K.; Liesveld, J.; Anolik, J.; Looney, R.J. Bone Marrow-Derived Mesenchymal Stem Cells From Patients With Systemic Lupus Erythematosus Have a Senescence-Associated Secretory Phenotype Mediated by a Mitochondrial Antiviral Signaling Protein-Interferon-β Feedback Loop. Arthritis Rheumatol. 2017, 69, 1623–1635. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.J.; Xiong, A.J.; Li, Y.H.; Pan, S.Y.; Zhang, Q.P.; Zhao, Y.; Liu, Y.; Marion, T.N. Mesenchymal Stem Cells: Allogeneic MSC May Be Immunosuppressive but Autologous MSC Are Dysfunctional in Lupus Patients. Front. Cell. Dev. Biol. 2019, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Lyamina, S.; Baranovskii, D.; Kozhevnikova, E.; Ivanova, T.; Kalish, S.; Sadekov, T.; Klabukov, I.; Maev, I.; Govorun, V. Mesenchymal Stromal Cells as a Driver of Inflammaging. Int. J. Mol. Sci. 2023, 24, 6372. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Yu, K.R. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020, 53, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galipeau, J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical "Mesenchymal Stem Cells" at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell. Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar]
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.P.; Lacerda, S.M.S.N.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H.; et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytom. Part. A 2014, 85, 43–77. [Google Scholar] [CrossRef] [Green Version]
- de Witte, S.F.H.; Lambert, E.E.; Merino, A.; Strini, T.; Douben, H.; O’Flynn, L.; Elliman, S.J.; de Klein, A.; Newsome, P.N.; Baan, C.C.; et al. Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy 2017, 19, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Choi, B.; Lee, C.J.; Moon, J.H.; Kim, M.K.; Chung, E.; Song, S.U. Culturing at Low Cell Density Delays Cellular Senescence of Human Bone Marrow-Derived Mesenchymal Stem Cells in Long-Term Cultures. Int. J. Stem Cells 2021, 14, 103–111. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, H.-c.; Chen, N.; Ren, Y.; Gao, R.; Li, Q.; Deng, Y.; Han, X.; Zhang, X.; Xiang, A.P.; et al. Unveiling the improved targeting migration of mesenchymal stem cells with CXC chemokine receptor 3-modification using intravital NIR-II photoacoustic imaging. J. Nanobiotechnology 2022, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mikrani, R.; Zubair, H.M.; Taleb, A.; Naveed, M.; Baig, M.; Zhang, Q.; Li, C.; Habib, M.; Cui, X.; et al. Systemic and local delivery of mesenchymal stem cells for heart renovation: Challenges and innovations. Eur. J. Pharm. Pharmacol. 2020, 876, 173049. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, W.; Chen, X.; Lian, Y.; Wang, J.; Cai, C.; Huang, L.; Wang, T.; Ren, J.; Xiang, A.P. CXCR5-Overexpressing Mesenchymal Stromal Cells Exhibit Enhanced Homing and Can Decrease Contact Hypersensitivity. Mol. Ther. 2017, 25, 1434–1447. [Google Scholar] [CrossRef] [Green Version]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell. Investig. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell. Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Liu, X.L.; Sun, J.M.; Yang, J.H.; Xu, D.H.; Yan, S.S. Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: A systematic review. World J. Stem Cells 2020, 12, 879–896. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.J.; Bae, E.H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M.; et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Sun, W.; Yan, S.; Yang, C.; Yang, J.; Wang, H.; Li, C.; Zhang, L.; Zhao, L.; Zhang, J.; Cheng, M.; et al. Mesenchymal Stem Cells-derived Exosomes Ameliorate Lupus by Inducing M2 Macrophage Polarization and Regulatory T Cell Expansion in MRL/lpr Mice. Immunol. Investig. 2022, 51, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Baral, H.; Uchiyama, A.; Yokoyama, Y.; Sekiguchi, A.; Yamazaki, S.; Amalia, S.N.; Inoue, Y.; Ogino, S.; Torii, R.; Hosoi, M.; et al. Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: Possible contribution of miR-196b-5p. J. Dermatol.Sci. 2021, 104, 39–47. [Google Scholar] [CrossRef]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell-Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharm. 2020, 11, 590470. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Sardari, M.; Murke, F.; Skuljec, J.; Pul, R.; Hagemann, N.; Dzyubenko, E.; Dittrich, R.; Gregorius, J.; et al. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke 2020, 51, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Műzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Klimak, M.; Nims, R.J.; Pferdehirt, L.; Collins, K.H.; Harasymowicz, N.S.; Oswald, S.J.; Setton, L.A.; Guilak, F. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 2021, 133, 74–86. [Google Scholar] [CrossRef]
- Leisheng, Z.; Zhihai, H. Mesenchymal Stem/Stromal Cells and Hydrogel Scaffolds for Tissue Engineering. In Hydrogels; Lăcrămioara, P., Mihaela Violeta, G., Cristina-Elena, D.-P., Eds.; IntechOpen: Rijeka, Croatia, 2022; p. 7. [Google Scholar]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Kurzyk, A. Mesenchymal Stem Cell Seeding on 3D Scaffolds. Methods Mol. Biol. 2022, 2429, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ge, H.A.; Wu, G.B.; Cheng, B.; Lu, Y.; Jiang, C. Autophagy Prevents Oxidative Stress-Induced Loss of Self-Renewal Capacity and Stemness in Human Tendon Stem Cells by Reducing ROS Accumulation. Cell. Physiol. Biochem. 2016, 39, 2227–2238. [Google Scholar] [CrossRef]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, C.; Shen, M.; Yang, M.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Chen, H.; Cao, F.; et al. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell. Res. Ther. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.-W.; Choo, K.-B.; Chen, C.-M.; Hung, T.-H.; Chen, Y.-B.; Hsieh, C.-H.; Kuo, H.-P.; Chong, K.-Y. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell. Res. Ther. 2015, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Carvalho AÉ, S.; Sousa, M.R.R.; Alencar-Silva, T.; Carvalho, J.L.; Saldanha-Araujo, F. Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead. Cytokine Growth Factor. Rev. 2019, 47, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell. Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Kim, E.Y.; Kim, H.S.; Park, E.J.; Lee, H.J.; Lee, T.Y.; Kim, K.S.; Bae, S.C.; Hong, J.T.; Kim, Y.; et al. Effect of Human Mesenchymal Stem Cells on Xenogeneic T and B Cells Isolated from Lupus-Prone MRL.Fas (lpr) Mice. Stem Cells Int. 2020, 2020, 5617192. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Simões, S.; Santos, J.M. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.S.; Han, J.H.; Park, Y.J.; Kim, S.W. TGF-β1 overexpressing human MSCs generated using gene editing show robust therapeutic potential for treating collagen-induced arthritis. J. Tissue Eng. Regen. Med. 2021, 15, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.F.; Jin, J.; Wang, H.; Wang, L.S.; Wu, C.T. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J. Cell. Mol. Med. 2022, 26, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
| Published Year | MSCs Source | Patients | Administration Routes and Dose | Outcome | Serious Adverse Events | Ref |
|---|---|---|---|---|---|---|
| 2013 | Allogenic UC | 136 (MSCs) vs. 36 (Control) | IV, single or twice, 4 × 107 cells | (Only in MSCs group) ↓: CRP, RF, DAS28, HAQ TNF-α, IL-6 ↑: Tregs | - | [28] |
| 2017 | Allogenic AT | 46 (MSCs) vs. 7 (Control) | IV, three-times, 1 or 2 or 4 × 106 cells/kg | Just a trend for clinical efficacy in ACR20 response | 1 case of lacunar infarction | [31] |
| 2018 | Autologous BM | 15 (MSCs) vs. 15 (Control) | IA, single, 4 × 107 cells | Improved WOMAC, VAS But, not significantly sustained beyond 12 months. | - | [27] |
| 2018 | Allogenic UC | 52 (MSCs) vs. 53 (Control) | IV, single, 1 × 106 cells/kg | (MSCs responder) Initial IFN-γ↑ -> IL-10↑, Treg/TH17↑ | - | [32] |
| 2019 | Allogenic UC | 64 (MSCs) | IV, single, 4 × 107 cells | (1 year) ↓: ESR, CRP, RF, DAS28, HAQ (3 years) ↓: ESR, CRP, RF, anti-CCP, DAS28, HAQ | - | [30] |
| 2019 | Autologous BM | 9 (MSCs) | IV, single, 1 × 106 cells/kg | ↓: DAS28, VAS ↑: Treg/TH17 | - | [29] |
| 2019 | Autologous BM | 13 (MSCs) | IV, single, 1 × 106 cells/kg | ↓: VAS, CXCL8/12/13 But, not sustained beyond 12 months. | - | [33] |
| 2020 | Allogenic UC | 32 (MSCs) vs. 31 (MSCs+IFN-γ) | IV, single, 1 × 106 cells/kg | (MSCs+ IFN-γ > MSCs) ↓: ESR, CRP, RF ↑: Treg/TH17 | - | [34] |
| 2020 | Autologous BM | 13 (MSCs) | IV, single, 1 × 106 cells/kg | ↑: FOXP3 expression, TGF-β1, IL-10 | - | [35] |
| 2020 | Autologous BM | 13 (MSCs) | IV, single, 1 × 106 cells/kg | ↓: CD19+ B-cell, BAFF, APRIL | - | [36] |
| 2022 | Autologous AT | 15 (MSCs) | IV, single, 2 × 108 cells | ↓: Count of swollen/tender joint | - | [37] |
| Published Year | MSCs Source | Patients | Administration Routes and Dose | Outcome | Serious Adverse Events | Ref |
|---|---|---|---|---|---|---|
| 2010 | Allogenic BM | 15 (MSCs) | IV, single, 1 × 106 cells/kg | ↓: SLEDAI scores, Proteinuria, Anti-dsDNA 2 patients relapse of proteinuria at 1 year. | - | [48] |
| 2010 | Allogenic UC | 16 (MSCs) | IV, single, 1 × 106 cells/kg | (8 months) Improvement of SLEDAI scores and renal function | - | [49] |
| 2013 | Allogenic BM or UC | 87 (MSCs) | IV, single, 1 × 106 cells/kg | (At 4 years) Remission rate: 50% Relapse rate: 23% | - | [52] |
| 2014 | Allogenic UC | 40 (MSCs) | IV, twice, 1 × 106 cells/kg | (12 months) ↓: SLEDAI scores, BILAG index, serum Cr, BUN, Anti-dsDNA ↑: Serum albumin and C3 | - | [53] |
| 2014 | Allogenic BM or UC | 81 (MSCs) | IV, single, 1 × 106 cells/kg | (At 1 year) Remission rate: 60.5% Relapse rate: 22.4% ↓: SLEDAI scores, BILAG index | - | [54] |
| 2017 | Allogenic UC | 9 (MSCs) | IV, twice, 1 × 106 cells/kg | (At 6 years) Long-term good safety. | - | [55] |
| 2017 | Allogenic UC | 12 (MSCs) vs. 6 (Placebo) | IV, twice, 2 × 108 cells | No positive effect. | 1 case of pneumonia | [56] |
| 2018 | Allogenic BM | 3 (MSCs) | IV, single, 1.5 × 106 cells/kg | ↓: SLEDAI scores, Proteinuria | - | [50] |
| 2022 | Allogenic AT | 9 (MSCs) | IV, single, 2 × 106 cells/kg | (At 3 months) Complete response: 33.3% Partial response: 44.4% (At 6 months) ↓: SLEDAI scores (Slightly increased at 12 months) | - | [51] |
| 2022 | Allogenic BM | 6 (MSCs) | IV, twice, 2~3 × 106 cells/kg | Maximum tolerate dose: 3 × 106 cells/kg | - | [57] |
| 2022 | Allogenic UC | 6 (MSCs) | IV, single, 1 × 106 cells/kg | (At 1 year) SRI-4: 83.3% ↓: CD27IgD double negative B cells, Anti-dsDNA ↑: GARP-TGFβ | - | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-W.; Kwok, S.-K. Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions. Int. J. Mol. Sci. 2023, 24, 10161. https://doi.org/10.3390/ijms241210161
Lee B-W, Kwok S-K. Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions. International Journal of Molecular Sciences. 2023; 24(12):10161. https://doi.org/10.3390/ijms241210161
Chicago/Turabian StyleLee, Bong-Woo, and Seung-Ki Kwok. 2023. "Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions" International Journal of Molecular Sciences 24, no. 12: 10161. https://doi.org/10.3390/ijms241210161
APA StyleLee, B.-W., & Kwok, S.-K. (2023). Mesenchymal Stem/Stromal Cell-Based Therapies in Systemic Rheumatic Disease: From Challenges to New Approaches for Overcoming Restrictions. International Journal of Molecular Sciences, 24(12), 10161. https://doi.org/10.3390/ijms241210161






