Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus
Abstract
:1. Introduction
2. Results
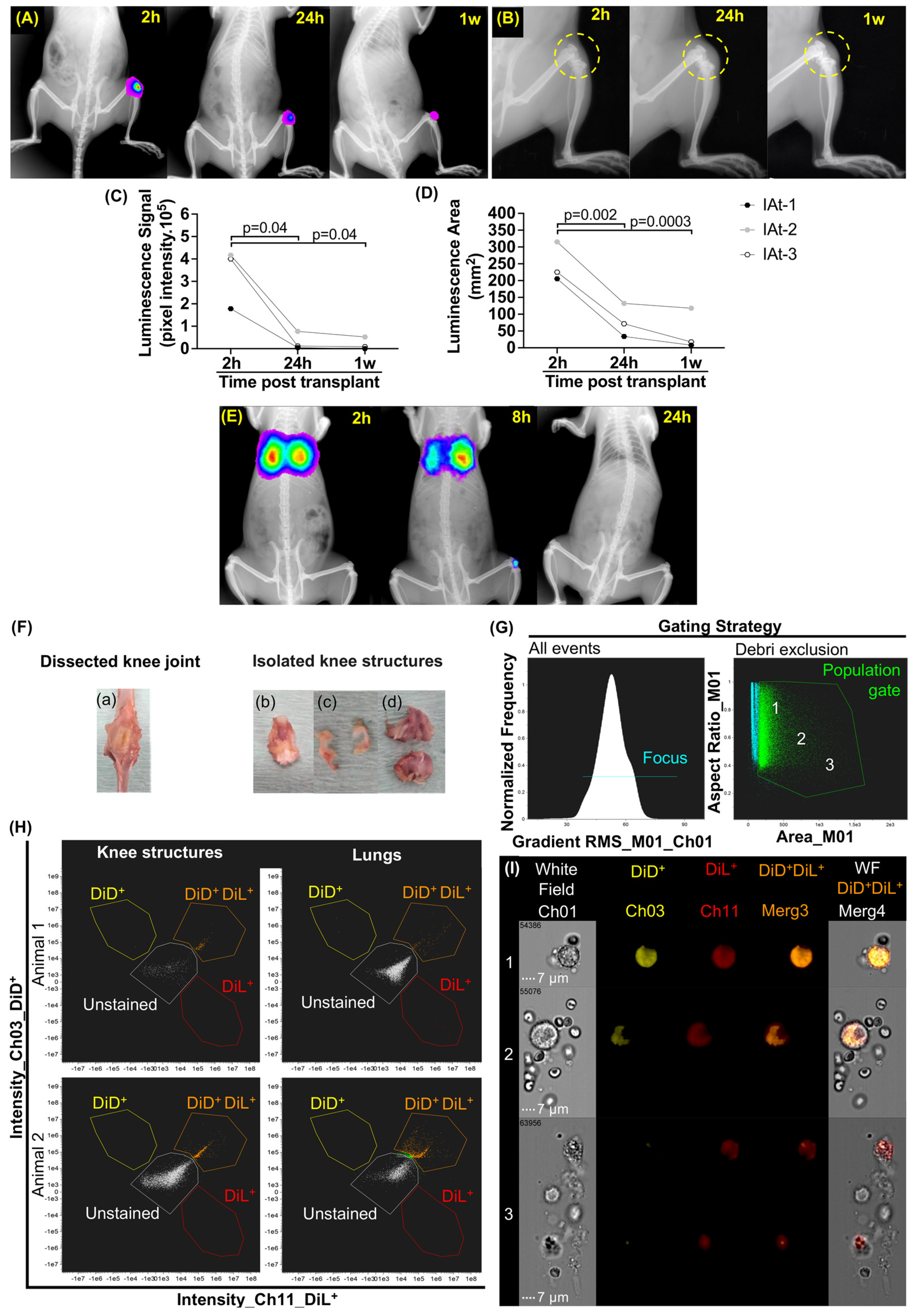
2.1. Localization of MSCs in DMM–OA-Afflicted Knees When Delivered by IAt or IVt
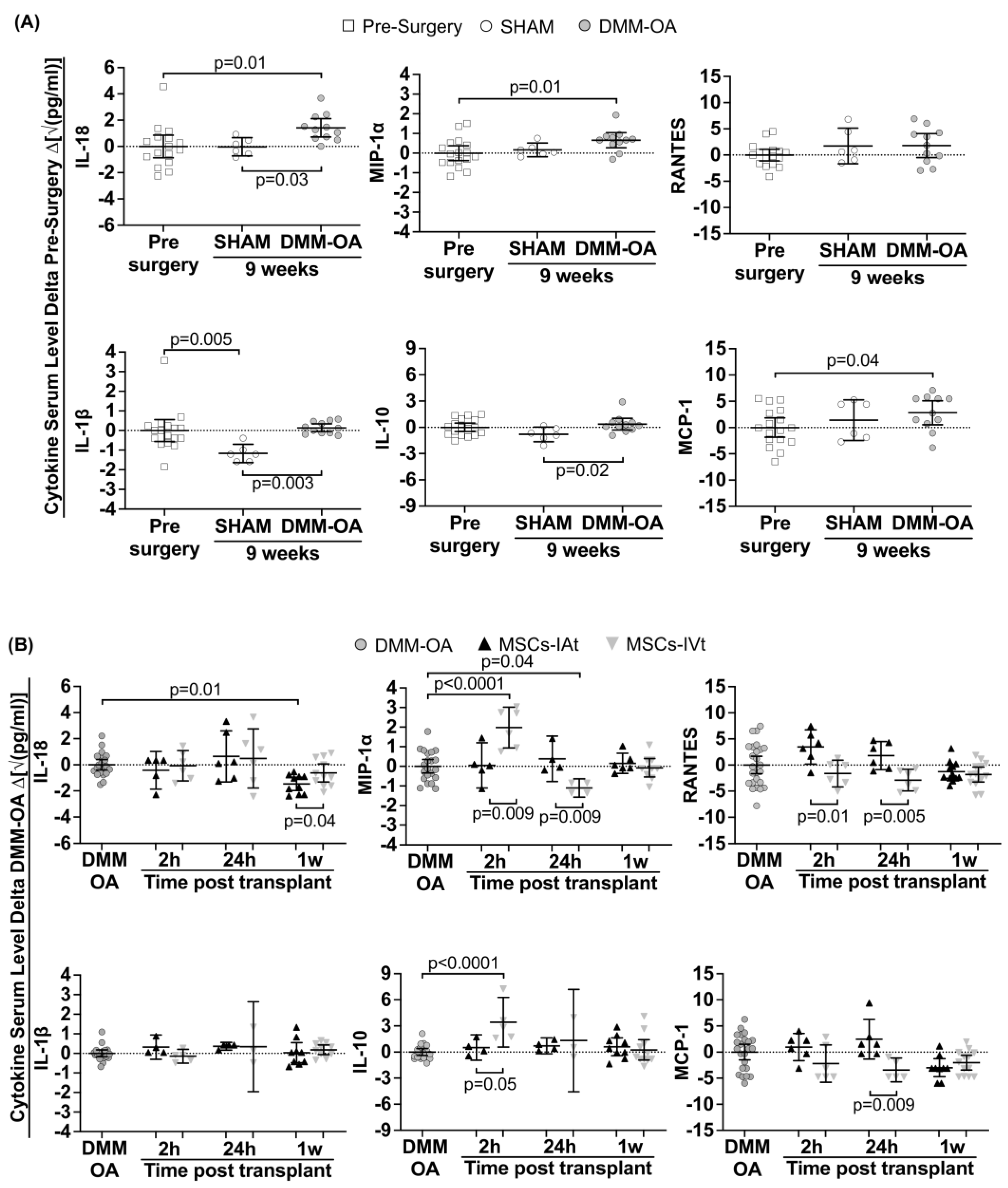
2.2. Induction of Systemic Cytokines/Chemokines in DMM–OA-Afflicted Rats
2.3. Short-Term Regulation of Systemic Cytokines/Chemokines by Transplanted MSCs
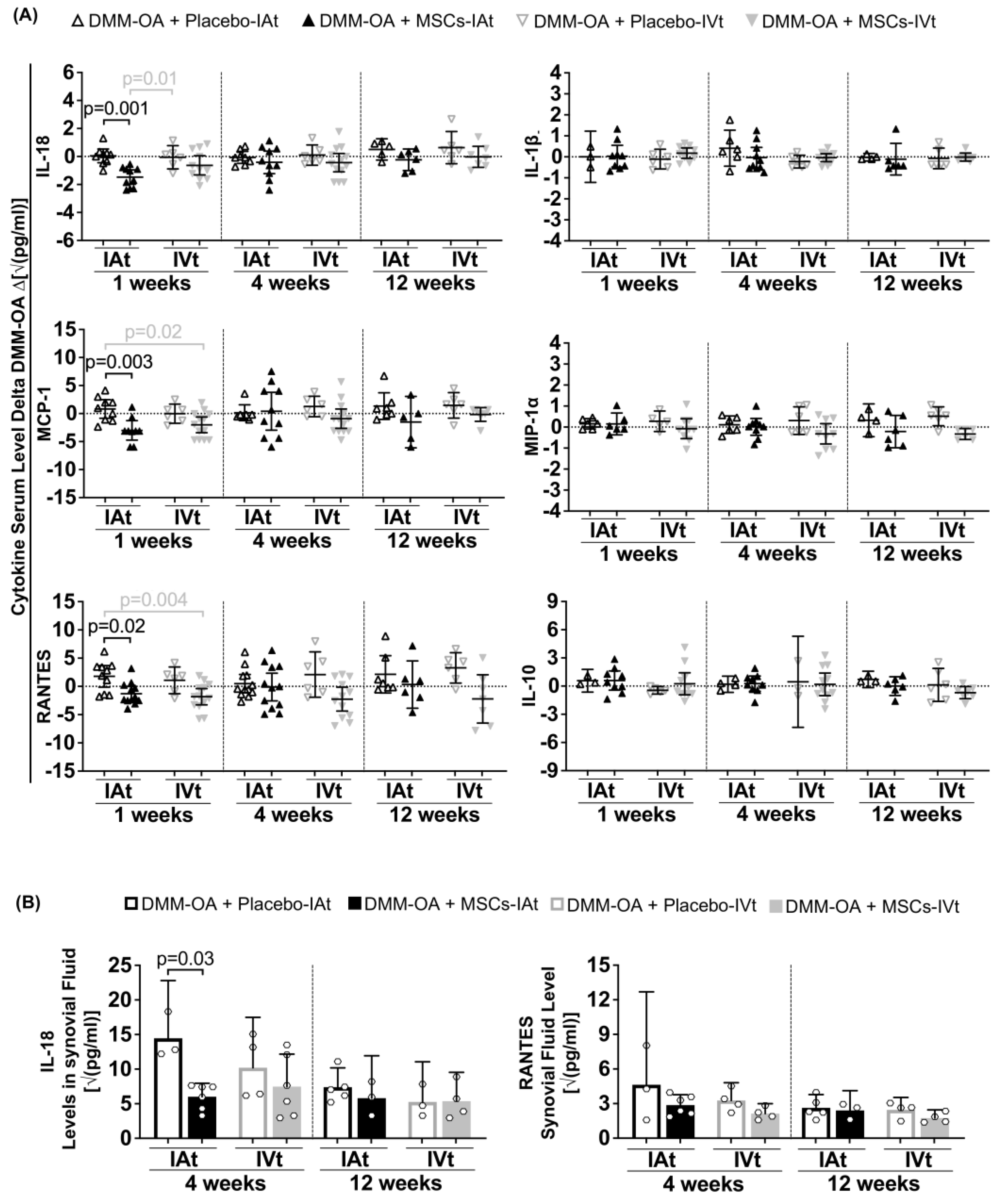
2.4. Long-Term Systemic and Local Regulation of Cytokines by Transplanted MSCs
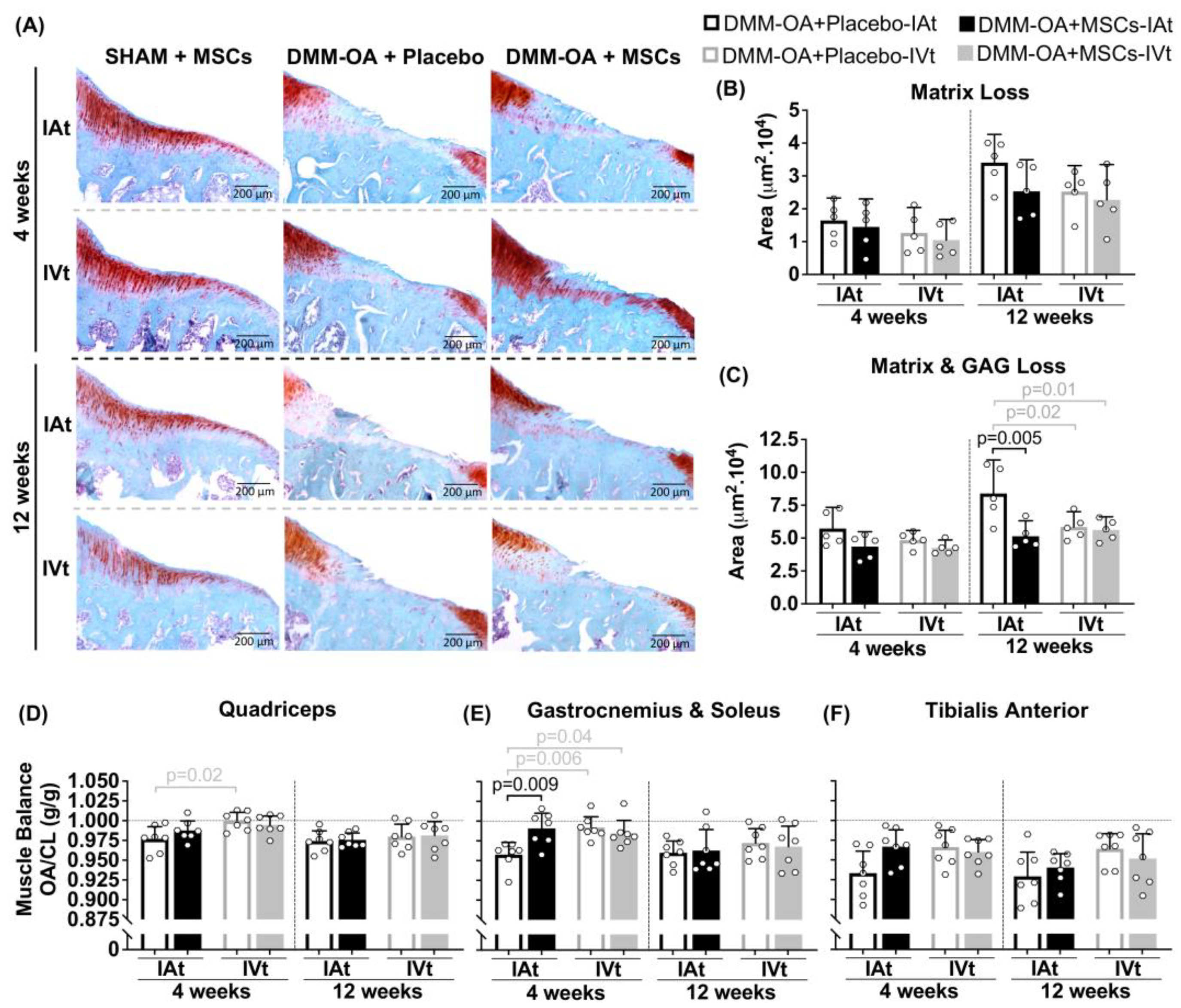
2.5. Changes in Joint Cartilage in Response to MSC Treatment
2.6. Changes in Hindlimbs Muscle Balance Ratio
3. Discussion
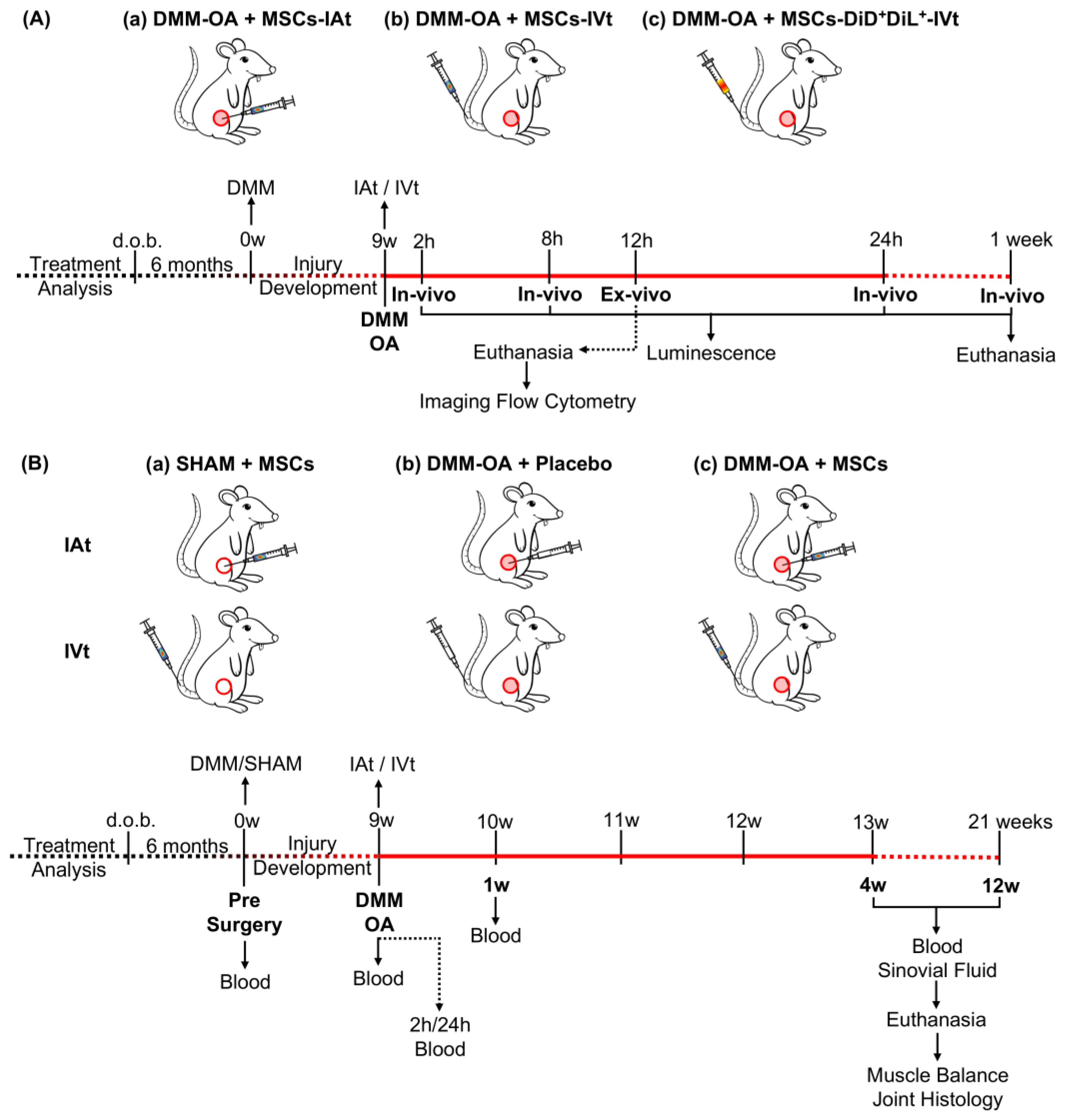
4. Materials and Methods
4.1. Animals
4.2. Animal Model of OA
4.3. MSC Isolation, Characterization and Differentiation
4.4. Luciferase Transgene Expression
4.5. MSC Transplant
4.6. In Vivo Imaging
4.7. Ex Vivo Imaging
4.8. Treatment of Rat Knees Receiving DMM–OA or SHAM Surgery with MSCs or Placebo via IAt or IVt
4.9. Blood Specimen Collection
4.10. Synovial Fluid (SF)
4.11. Cytokine/Chemokine Multiplex Assay
4.12. Histology
4.13. Muscle Weight
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.-Y. Non-Surgical Management of Knee Osteoarthritis: Comparison of ESCEO and OARSI 2019 Guidelines. Nat. Rev. Rheumatol. 2020, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Kwong, J.; Yang, Z.; Hou, Y.; Zhang, W.; Ma, B.; Lin, J. Intra-Articular Injection of Mesenchymal Stem Cells in Treating Knee Osteoarthritis: A Systematic Review of Animal Studies. Osteoarthr. Cartil. 2018, 26, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Isho, T.; Kuroki, H.; Takahashi, M.; Aoyama, T. Effectiveness of Mesenchymal Stem Cells for Treating Patients with Knee Osteoarthritis: A Meta-Analysis toward the Establishment of Effective Regenerative Rehabilitation. NPJ Regen. Med. 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef]
- Lopez-Santalla, M.; Bueren, J.A.; Garin, M.I. Mesenchymal Stem/Stromal Cell-Based Therapy for the Treatment of Rheumatoid Arthritis: An Update on Preclinical Studies. EBioMedicine 2021, 69, 103427. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Johnson, V.; Webb, T.; Santangelo, K.S.; Dow, S.; Duerr, F.M. Evaluation of Intravenously Delivered Allogeneic Mesenchymal Stem Cells for Treatment of Elbow Osteoarthritis in Dogs: A Pilot Study. Vet. Comp. Orthop. Traumatol. 2019, 32, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pernas, P.; Rodríguez-Lesende, I.; de la Fuente, A.; Mateos, J.; Fuentes, I.; de Toro, J.; Blanco, F.J.; Arufe, M.C. CD105+-Mesenchymal Stem Cells Migrate into Osteoarthritis Joint: An Animal Model. PLoS ONE 2017, 12, e0188072. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.H.; Na, H.S.; Kwon, J.Y.; Kim, G.-Y.; Jung, K.; Cho, K.-H.; Kim, S.A.; Go, E.J.; Park, M.-J.; et al. The Therapeutic Effect of STAT3 Signaling-Suppressed MSC on Pain and Articular Cartilage Damage in a Rat Model of Monosodium Iodoacetate-Induced Osteoarthritis. Front. Immunol. 2018, 9, 2881. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yao, Y.; Zhao, D.; Zou, H.; Lai, C.; Xiang, G.; Wang, G.; Luo, L.; Shi, Y.; Li, Y.; et al. LncRNA H19 Secreted by Umbilical Cord Blood Mesenchymal Stem Cells through MicroRNA-29a-3p/FOS Axis for Central Sensitization of Pain in Advanced Osteoarthritis. Am. J. Transl. Res. 2021, 13, 1245. [Google Scholar]
- Mostafa, A.; Korayem, H.E.; Fekry, E.; Hosny, S. The Effect of Intra-Articular versus Intravenous Injection of Mesenchymal Stem Cells on Experimentally-Induced Knee Joint Osteoarthritis. J. Microsc. Ultrastruct. 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, H.; Wang, L.; Zhu, B.; Ma, L.; Du, F.; Li, L.; Qiu, L. Ultrasound Combined with SDF-1α Chemotactic Microbubbles Promotes Stem Cell Homing in an Osteoarthritis Model. J. Cell Mol. Med. 2020, 24, 10816. [Google Scholar] [CrossRef] [PubMed]
- Afzali, M.F.; Pannone, S.C.; Martinez, R.B.; Campbell, M.A.; Sanford, J.L.; Pezzanite, L.M.; Kurihara, J.; Johnson, V.; Dow, S.W.; Santangelo, K.S. Intravenous Injection of Adipose-Derived Mesenchymal Stromal Cells Benefits Gait and Inflammation in a Spontaneous Osteoarthritis Model. J. Orthopeadic Res. 2022, 41, 902–912. [Google Scholar] [CrossRef]
- ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; de Munter, W.; Grevers, L.C.; Jeanson, J.; Noël, D.; Casteilla, L.; Jorgensen, C.; et al. Antiinflammatory and Chondroprotective Effects of Intraarticular Injection of Adipose-Derived Stem Cells in Experimental Osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef]
- Enomoto, T.; Akagi, R.; Ogawa, Y.; Yamaguchi, S.; Hoshi, H.; Sasaki, T.; Sato, Y.; Nakagawa, R.; Kimura, S.; Ohtori, S.; et al. Timing of Intra-Articular Injection of Synovial Mesenchymal Stem Cells Affects Cartilage Restoration in a Partial Thickness Cartilage Defect Model in Rats. Cartilage 2020, 11, 122–129. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; van Dalen, S.; ter Huurne, M.; Roth, J.; Vogl, T.; Noël, D.; Jorgensen, C.; van den Berg, W.B.; van de Loo, F.A.; Blom, A.B.; et al. Treatment Efficacy of Adipose-Derived Stem Cells in Experimental Osteoarthritis Is Driven by High Synovial Activation and Reflected by S100A8/A9 Serum Levels. Osteoarthr. Cartil. 2014, 22, 1158–1166. [Google Scholar] [CrossRef]
- van Spil, W.E.; Szilagyi, I.A. Osteoarthritis Year in Review 2019: Biomarkers (Biochemical Markers). Osteoarthr. Cartil. 2020, 28, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Bapat, S.; Hubbard, D.; Munjal, A.; Hunter, M.; Fulzele, S. Pros and Cons of Mouse Models for Studying Osteoarthritis. Clin. Transl. Med. 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of Osteoarthritis: The Good, the Bad and the Promising. Osteoarthr. Cartil. 2019, 27, 230. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The Surgical Destabilization of the Medial Meniscus (DMM) Model of Osteoarthritis in the 129/SvEv Mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.B.D.; Antonioli, E.; Souza, J.G.; Dias, O.F.M.; Ferretti, M. Erratum to ‘Mesenchymal Stromal Cells Tracking and Cytokines Kinetics Following Intra-Articular and Intravenous Transplantation 27 (2019) S203–S204’. Osteoarthr. Cartil. 2021, 29, 294–296. [Google Scholar] [CrossRef]
- Loeser, R.F.; Olex, A.L.; McNulty, M.A.; Carlson, C.S.; Callahan, M.; Ferguson, C.; Fetrow, J.S. Disease Progression and Phasic Changes in Gene Expression in a Mouse Model of Osteoarthritis. PLoS ONE 2013, 8, e54633. [Google Scholar] [CrossRef]
- David, M.A.; Smith, M.K.; Pilachowski, R.N.; White, A.T.; Locke, R.C.; Price, C. Early, Focal Changes in Cartilage Cellularity and Structure Following Surgically Induced Meniscal Destabilization in the Mouse. J. Orthop. Res. 2017, 35, 537–547. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal Stem Cells for Treatment of Steroid-Resistant, Severe, Acute Graft-versus-Host Disease: A Phase II Study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Shim, G.; Lee, S.; Han, J.; Kim, G.; Jin, H.; Miao, W.; Yi, T.-G.; Cho, Y.K.; Song, S.U.; Oh, Y.-K. Pharmacokinetics and In Vivo Fate of Intra-Articularly Transplanted Human Bone Marrow-Derived Clonal Mesenchymal Stem Cells. Stem Cells Dev. 2015, 24, 1124–1132. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not Single but Periodic Injections of Synovial Mesenchymal Stem Cells Maintain Viable Cells in Knees and Inhibit Osteoarthritis Progression in Rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Schüler, C.; Ginner, N.; Erben, R.G. Intra-Articularly Injected Mesenchymal Stem Cells Promote Cartilage Regeneration, but Do Not Permanently Engraft in Distant Organs. Sci. Rep. 2019, 9, 10153. [Google Scholar] [CrossRef] [PubMed]
- Maerz, Y.Z.T.; Fleischer, M.; Newton, M.D.; Davidson, A.; Salisbury, M.; Altman, P.; Kurdziel, Y.Z.M.D.; Anderson, Z.K.; Bedi, A.; Baker, K.C. Acute Mobilization and Migration of Bone Marrow-Derived Stem Cells Following Anterior Cruciate Ligament Rupture. Osteoarthr. Cartil. 2017, 25, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, B.A.; Anderson, M.J.; Lee, C.A.; Williams, J.C.; Yik, J.H.N.; Haudenschild, D.R. Musculoskeletal Changes Following Non-Invasive Knee Injury Using a Novel Mouse Model of Post-Traumatic Osteoarthritis. Osteoarthr. Cartil. 2012, 20, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.; Patruno, M.; Zayed, M.; Beerts, C. Homing of Radiolabelled Xenogeneic Equine Peripheral Blood-Derived MSCs towards a Joint Lesion in a Dog. Front. Vet. Sci. 2022, 9, 1035175. [Google Scholar]
- Wang, Y.; Xu, D.; Long, L.; Deng, X.; Tao, R.; Huang, G. Correlation between Plasma, Synovial Fluid and Articular Cartilage Interleukin-18 with Radiographic Severity in 33 Patients with Osteoarthritis of the Knee. Clin. Exp. Med. 2014, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Zhang, Y.; Peng, X.; Li, J. Correlation between Osteoarthritis and Monocyte Chemotactic Protein-1 Expression: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Yang, Z.B.; Zhang, Z.J.; Zhang, Z.Q.; Kang, Y.; Huang, G.X.; Wang, S.W.; Huang, H.; Liao, W.M. CCL3 Serves as a Potential Plasma Biomarker in Knee Degeneration (Osteoarthritis). Osteoarthr. Cartil. 2015, 23, 1405–1411. [Google Scholar] [CrossRef]
- Eseonu, O.I.; de Bari, C. Homing of Mesenchymal Stem Cells: Mechanistic or Stochastic? Implications for Targeted Delivery in Arthritis. Rheumatology 2015, 54, 210–218. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. CCL3/CCR1 Mediates CD14+CD16− Circulating Monocyte Recruitment in Knee Osteoarthritis Progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Vithran, D.T.A.; Kwabena, B.R.; Xiao, W.; Li, Y. CC Chemokines and Receptors in Osteoarthritis: New Insights and Potential Targets. Arthritis Res. Ther. 2023, 25, 113. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Obeidat, A.M.; Wokosin, D.L.; Ren, D.; Miller, R.J.; Malfait, A.M.; Miller, R.E. The Role of Intra-Articular Neuronal CCR2 Receptors in Knee Joint Pain Associated with Experimental Osteoarthritis in Mice. Arthritis Res. Ther. 2021, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Nenasheva, T.; Nikolaev, A.; Diykanov, D.; Sukhanova, A.; Tcyganov, E.; Panteleev, A.; Bocharova, I.; Serdyuk, Y.; Nezlin, L.; Radaeva, T.; et al. The Introduction of Mesenchymal Stromal Cells Induces Different Immunological Responses in the Lungs of Healthy and M. Tuberculosis Infected Mice. PLoS ONE 2017, 12, e0178983. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Rhijn, M.R.; Engela, A.U.; Korevaar, S.S.; Mensah, F.K.F.; Franquesa, M.; de Bruin, R.W.F.; Betjes, M.G.H.; Weimar, W.; Baan, C.C. Mesenchymal Stem Cells Induce an Inflammatory Response After Intravenous Infusion. Stem Cells Dev. 2013, 22, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Rasmussen, G.L. Circulating IL-10 Is Compromised in Patients Predisposed to Developing and in Patients with Severe Knee Osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Rowland-Jones, S.L. RANTES: A Versatile and Controversial Chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of Mesenchymal Stem Cell Therapy for the Treatment of Osteoarthritis of the Knee: A Concise Review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- Dai, S.-M.; Shan, Z.-Z.; Nishioka, K.; Yudoh, K. Implication of Interleukin 18 in Production of Matrix Metalloproteinases in Articular Chondrocytes in Arthritis: Direct Effect on Chondrocytes May Not Be Pivotal. Ann. Rheum. Dis. 2005, 64, 735–742. [Google Scholar] [CrossRef]
- Toupet, K.; Maumus, M.; Peyrafitte, J.-A.; Bourin, P.; van Lent, P.L.E.M.; Ferreira, R.; Orsetti, B.; Pirot, N.; Casteilla, L.; Jorgensen, C.; et al. Long-Term Detection of Human Adipose-Derived Mesenchymal Stem Cells After Intraarticular Injection in SCID Mice. Arthritis Rheum. 2013, 65, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Shi, H.; Wang, L.; Yan, C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med. Sci. Monit. 2016, 22, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; She, G.; Gui, T.; Hou, H.; Li, J.; Chen, Y.; Zha, Z. Knee Muscle Atrophy Is a Risk Factor for Development of Knee Osteoarthritis in a Rat Model. J. Orthop. Transl. 2020, 22, 67. [Google Scholar] [CrossRef]
- Fazeli, M.S.; McIntyre, L.; Huang, Y.; Chevalier, X. Intra-Articular Placebo Effect in the Treatment of Knee Osteoarthritis: A Survey of the Current Clinical Evidence. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, K.; Uysal, M.; Şahiner, G.G.; Danışman, M.; Baş, O.; Takır, S.; Coşkun, Z.Ü.; Akdeniz, E.; Esenyel, C.Z. Evaluation of Different Intraarticular Injection Volumes to Assess Optimum Efficient Amount; an Experimental Study in Rat Knee Joints. J. Pharmacol. Toxicol. Methods 2020, 101, 106658. [Google Scholar] [CrossRef] [PubMed]
- Galbán, C.J.; Ling, S.M.; Galbán, C.J.; Taub, D.D.; Gurkan, I.; Fishbein, K.W.; Spencer, R.G. Effects of Knee Injection on Skeletal Muscle Metabolism and Contractile Force in Rats. Osteoarthr. Cartil. 2007, 15, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.V.; De Castro, S.O.; De Albuquerque, C.Z.; De Moura Mattaraia, V.G.; Santoro, M.L. The Gingival Vein as a Minimally Traumatic Site for Multiple Blood Sampling in Guinea Pigs & Hamsters. PLoS ONE 2017, 12, e0177967. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Santocildes, G.; Merino, M.; Fabiani, F.; Pagès, T.; Marotta, M.; Viscor, G.; Torrella, J.R. Histomorphological and Functional Contralateral Symmetry in the Gastrocnemius Muscles of the Laboratory Rat. J. Anat. 2022, 241, 692–701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias de Oliveira, F.B.; Antonioli, E.; Dias, O.F.M.; de Souza, J.G.; Agarwal, S.; Chudzinski-Tavassi, A.M.; Ferretti, M. Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus. Int. J. Mol. Sci. 2023, 24, 15543. https://doi.org/10.3390/ijms242115543
Dias de Oliveira FB, Antonioli E, Dias OFM, de Souza JG, Agarwal S, Chudzinski-Tavassi AM, Ferretti M. Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus. International Journal of Molecular Sciences. 2023; 24(21):15543. https://doi.org/10.3390/ijms242115543
Chicago/Turabian StyleDias de Oliveira, Felipe Bruno, Eliane Antonioli, Olívia Furiama Metropolo Dias, Jean Gabriel de Souza, Sudha Agarwal, Ana Marisa Chudzinski-Tavassi, and Mario Ferretti. 2023. "Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus" International Journal of Molecular Sciences 24, no. 21: 15543. https://doi.org/10.3390/ijms242115543
APA StyleDias de Oliveira, F. B., Antonioli, E., Dias, O. F. M., de Souza, J. G., Agarwal, S., Chudzinski-Tavassi, A. M., & Ferretti, M. (2023). Comparative Effects of Intra-Articular versus Intravenous Mesenchymal Stromal Cells Therapy in a Rat Model of Osteoarthritis by Destabilization of Medial Meniscus. International Journal of Molecular Sciences, 24(21), 15543. https://doi.org/10.3390/ijms242115543





