Chronic Heat Stress Affects Bile Acid Profile and Gut Microbiota in Broilers
Abstract
:1. Introduction
2. Results
2.1. Chronic HS Inhibits Growth Performance and Leads to Hepatic Lipid Deposition in Broilers
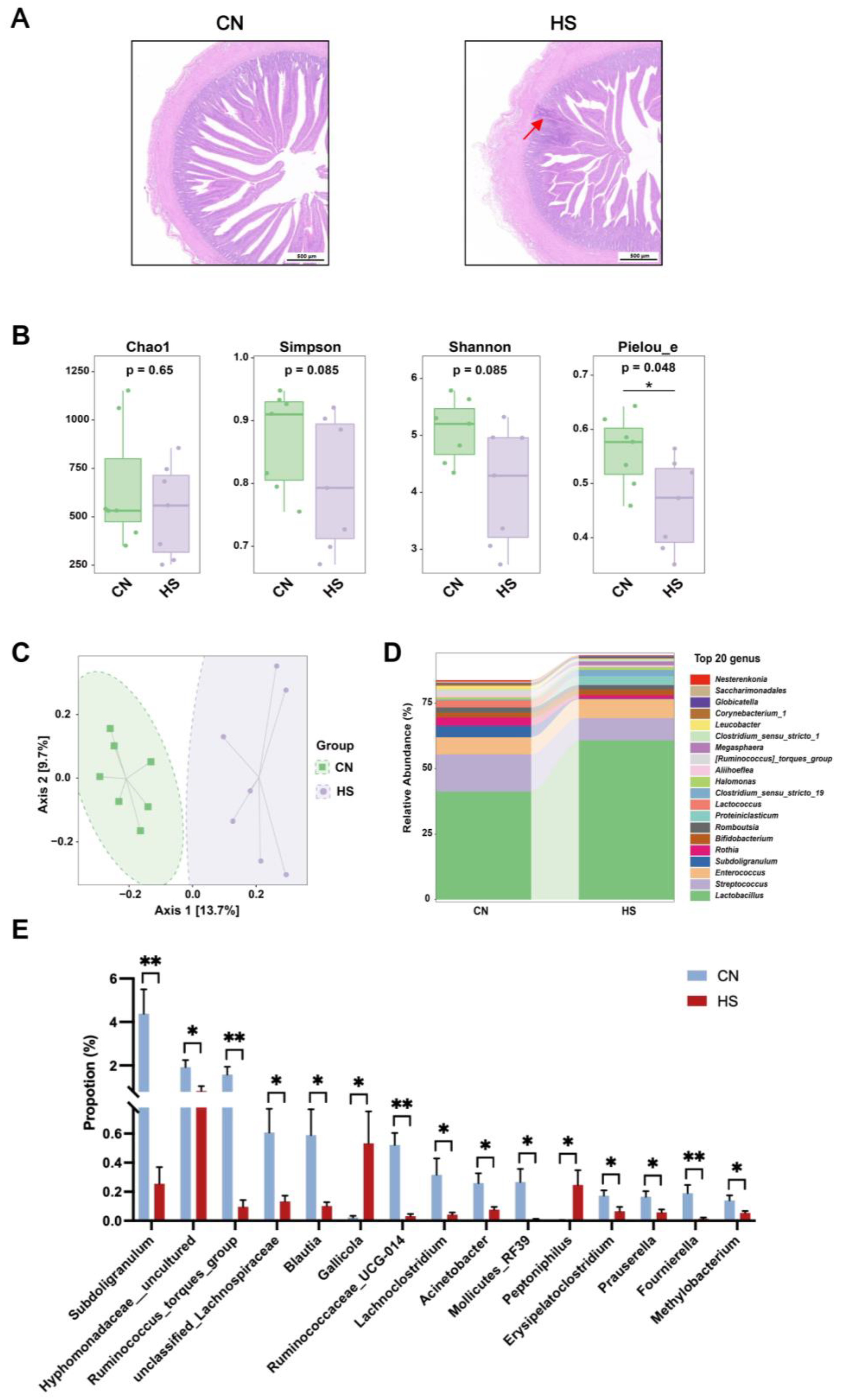
2.2. Chronic HS Led to Intestinal Inflammation and Disrupted Gut Microbiota Composition
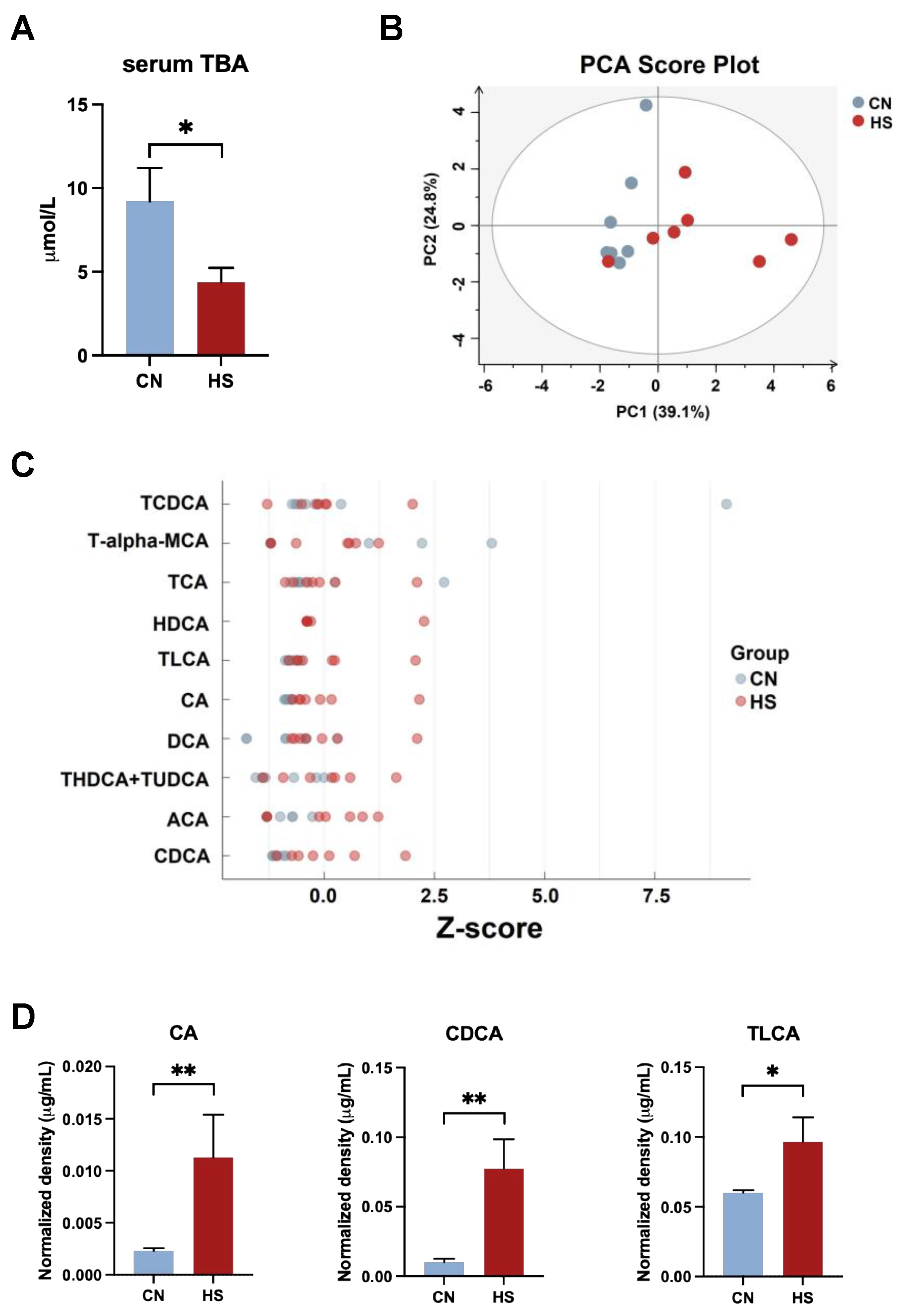
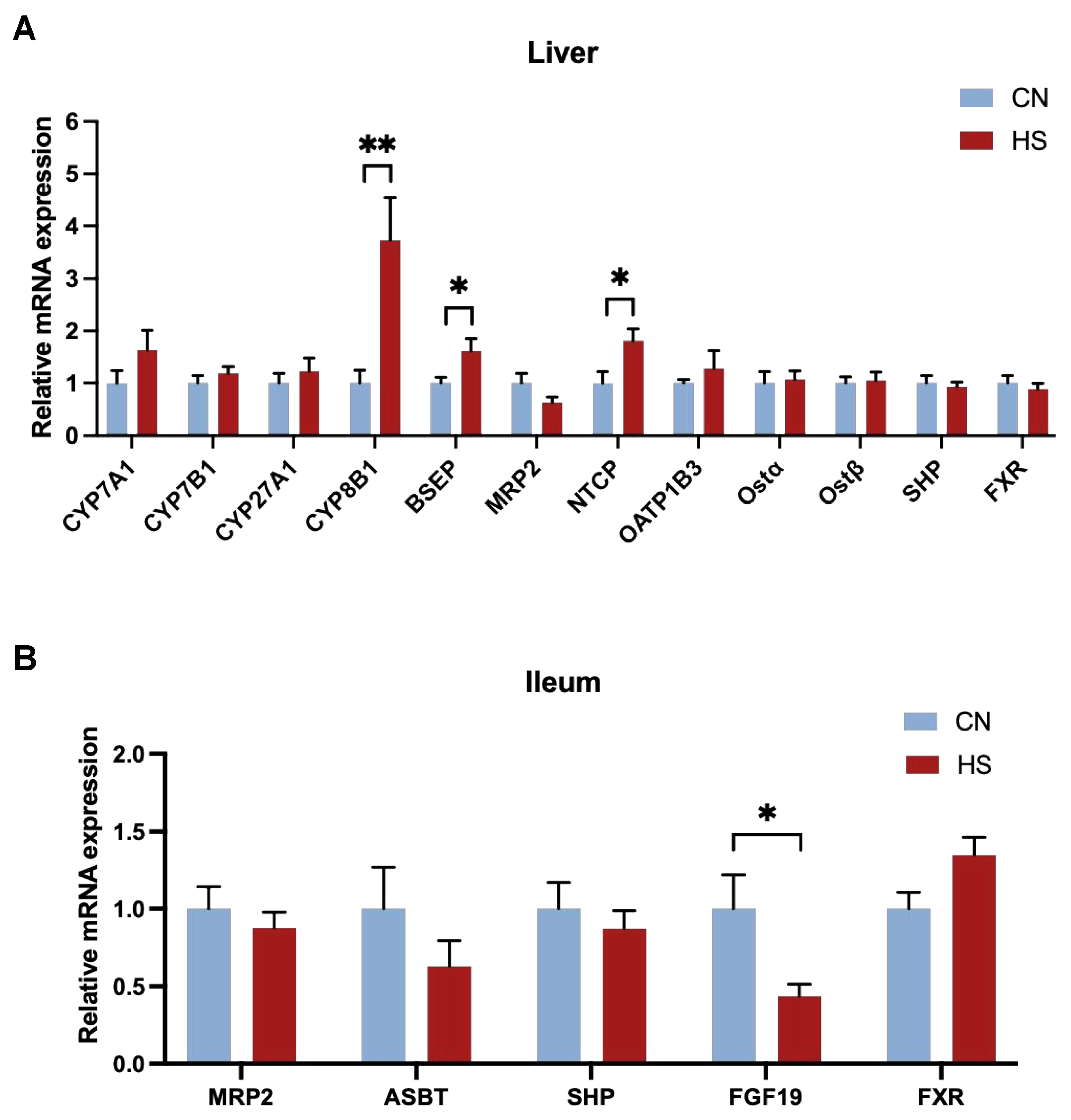
2.3. Chronic HS-Disrupted BAs Metabolism in the Liver and Serum
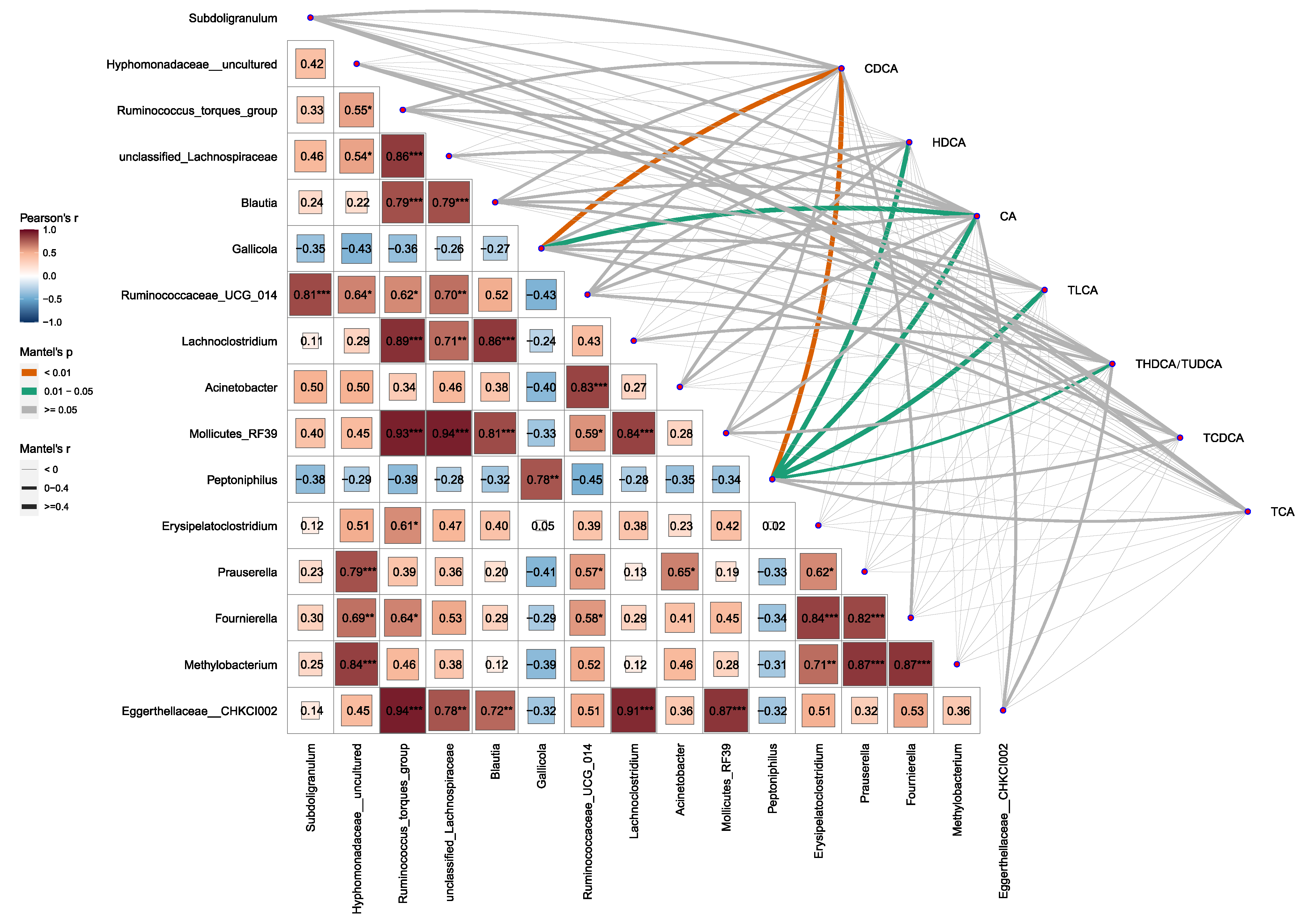
2.4. Alterations in Gut Microbiota Composition Are Associated with BA Metabolism
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Determination of Neutrophil to Lymphocyte (H/L) and Erythrocyte Hematocrit (HCT)
4.3. Measurement of Hepatic Cholesterol (CHOL), Triglyceride (TG), and Serum Total Bile Acids (TBA)
4.4. Determination of Plasma Biochemical Indicators Total Antioxidant Capacity (T-AOC)
4.5. Sections and Hematoxylin & Eosin (H&E) Staining of Liver and Duodenum
4.6. Serum Targeted Metabolomics of BA
4.7. Gut Microbiota Analysis
4.8. RNA Isolation and Real-Time PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Wang, Y.; Wei, L.; Wu, F.; Yin, F. Heat Stress Exposure Changed Liver Lipid Metabolism and Abdominal Fat Deposition in Broilers. Ital. J. Anim. Sci. 2022, 21, 1326–1333. [Google Scholar] [CrossRef]
- Nawaz, A.; Amoah, K.; Leng, Q.; Zheng, J.; Zhang, W.; Zhang, L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Yin, C.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens. Animals 2021, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wen, X.; Meng, Q.; Liu, L.; Xie, J.; Zhang, H.; Everaert, N. Heat Affects Cholesterol and Bile Acid Alterations in Cholesterol and Bile Acids Metabolism in Large White Pigs during Short-Term Heat Exposure. Animals 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Feng, J.; Zhang, M.; Li, X.; Ma, D.; Chang, S. Effects of High Ambient Temperature on the Community Structure and Composition of Ileal Microbiome of Broilers. Poult. Sci. 2018, 97, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of Gut Microbiota Structure in Heat-Stressed Broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Chiang, J.; Ferrell, J. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.; Patterson, A. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X.; Schoonjans, K. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 2021, 3, 411–425. [Google Scholar] [CrossRef]
- Joyce, S.; Gahan, C. Bile Acid Modifications at the Microbe-Host Interface: Potential for Nutraceutical and Pharmaceutical Interventions in Host Health. Annu. Rev. Food Sci. Technol. 2016, 7, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.; Karpen, S. Intestinal Transport and Metabolism of Bile Acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Xie, G.; Jia, W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhuri, S.; Klaassen, C. Molecular Regulation of Bile Acid Homeostasis. Drug Metab. Dispos. 2022, 50, 425–455. [Google Scholar] [CrossRef]
- Staley, C.; Weingarten, A.; Khoruts, A.; Sadowsky, M. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (FXR): Structures and Ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Fang, W.; Wen, X.; Meng, Q.; Wu, W.; Everaert, N.; Xie, J.; Zhang, H. Alteration in Bile Acids Profile in Large White Pigs during Chronic Heat Exposure. J. Therm. Biol. 2019, 84, 375–383. [Google Scholar] [CrossRef]
- Tang, S.; Xie, J.; Fang, W.; Wen, X.; Yin, C.; Meng, Q.; Zhong, R.; Chen, L.; Zhang, H. Chronic Heat Stress Induces the Disorder of Gut Transport and Immune Function Associated with Endoplasmic Reticulum Stress in Growing Pigs. Anim. Nutr. 2022, 11, 228–241. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, B.; He, H.; Wang, J.; Liu, X.; Guo, J.; Li, P.; Madigosky, S. The Effect of Bile Salt Diet Supplementation on Genes Related to Fat Metabolism in Yellow-Feathered Broilers. Vet. World 2022, 15, 911–918. [Google Scholar] [CrossRef]
- Yin, C.; Xia, B.; Tang, S.; Cao, A.; Liu, L.; Zhong, R.; Chen, L.; Zhang, H. The Effect of Exogenous Bile Acids on Antioxidant Status and Gut Microbiota in Heat-Stressed Broiler Chickens. Front. Nutr. 2021, 8, 747136. [Google Scholar] [CrossRef]
- Selvam, R.; Suresh, S.; Saravanakumar, M.; Chandrasekaran, C.V.; Prashanth, D. Alleviation of Heat Stress by a Polyherbal Formulation, Phytocee: Impact on Zootechnical Parameters, Cloacal Temperature, and Stress Markers. Pharmacogn. Res. 2018, 10, 1–8. [Google Scholar]
- Jumah, M.; Abd El-Hack, M.; Abdelnour, S.; Hendy, Y.; Ghanem, H.; Alsafy, S.; Khafaga, A.; Noreldin, A. Potential use of chromium to combat thermal stress in animals: A review. Sci. Total Environ. 2019, 707, 135996. [Google Scholar] [CrossRef]
- Moustafa, E.; Alsanie, W.; Gaber, A.; Kamel, N.; Alaqil, A.; Abbas, A. Blue-Green Algae (Spirulina platensis) Alleviates the Negative Impact of Heat Stress on Broiler Production Performance and Redox Status. Animals 2021, 11, 1243. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Hu, Y.; Sun, L.; Wang, S.; Fu, W.; Ni, Y.; Zhao, R. Exogenous Administration of Chronic Corticosterone Affects Hepatic Cholesterol Metabolism in Broiler Chickens Showing Long or Short Tonic Immobility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Frey, M.; Kohli, R. The Role of FGF19 and MALRD1 in Enterohepatic Bile Acid Signaling. Front. Endocrinol. 2022, 12, 799648. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, K.; Chaudhari, A.; Schreier, L.; Miska, K.; Proszkowiec-Weglarz, M. The Supplementation of FloraMax-B11 Did Not Affect the Bile Acid Neosynthesis and the Enterohepatic Circulation in Broiler Chickens. Animals 2022, 12, 2901. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front. Immunol. 2023, 14, 1127743. [Google Scholar] [CrossRef]
- Liu, W.; Pan, Z.; Zhao, Y.; Guo, Y.; Qiu, S.; Balasubramanian, B.; Jha, R. Effects of Heat Stress on Production Performance, Redox Status, Intestinal Morphology and Barrier-Related Gene Expression, Cecal Microbiome, and Metabolome in Indigenous Broiler Chickens. Front. Physiol. 2022, 13, 890520. [Google Scholar] [CrossRef]
- Collins, S.; Stine, J.; Bisanz, J.; Okafor, C.; Patterson, A. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Jiang, W.; Hu, Y.; Jia, Y.; Zhao, R. GR-Mediated Transcriptional Regulation of M6A Metabolic Genes Contributes to Diet-Induced Fatty Liver in Hens. J. Anim. Sci. Biotechnol. 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; An, Z.; Shi, C.; Li, P.; Liu, L. A Sensitive and Efficient Method for Simultaneous Profiling of Bile Acids and Fatty Acids by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 178, 112815. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shu, T.; Liu, G.; Mei, H.; Zhu, X.; Huang, X.; Zhang, L.; Jiang, Z. Quantitative Profiling of 19 Bile Acids in Rat Plasma, Liver, Bile and Different Intestinal Section Contents to Investigate Bile Acid Homeostasis and the Application of Temporal Variation of Endogenous Bile Acids. J. Steroid Biochem. Mol. Biol. 2017, 172, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile Acid Metabolism Is Altered in Multiple Sclerosis and Supplementation Ameliorates Neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Control | HS | p-Value | |
|---|---|---|---|
| ALT (U/L) | 8.10 ± 1.37 | 9.20 ± 1.93 | 0.159 |
| AST (U/L) | 209.20 ± 23.63 | 245.38 ± 44.95 * | 0.043 |
| AST/ALT | 26.57 ± 5.50 | 23.79 ± 10.10 | 0.459 |
| ALP (U/L) | 936.90 ± 442.13 | 581.67 ± 206.72 * | 0.042 |
| GLU (mmol/L) | 11.44 ± 0.88 | 10.36 ± 0.89 * | 0.020 |
| T-CHO (mmol/L) | 3.30 ± 0.82 | 3.55 ± 0.68 | 0.526 |
| TG (mmol/L) | 0.66 ± 0.13 | 0.60 ± 0.04 | 0.170 |
| LDH (U/L) | 391.25 ± 42.09 | 507.11 ± 112.97 * | 0.016 |
| Taxa Name | Group with Higher Abundance | LDA_Score | p-Value |
|---|---|---|---|
| Phylum | |||
| Firmicutes | HS | 4.707 | 0.018 |
| Class | |||
| Alphaproteobacteria | CN | 4.178 | 0.018 |
| Negativicutes | HS | 3.768 | 0.0484 |
| Order | |||
| Micrococcales | CN | 4.365 | 0.048 |
| Rhizobiales | CN | 3.772 | 0.035 |
| Family | |||
| Ruminococcaceae | CN | 4.429 | 0.002 |
| Lachnospiraceae | CN | 4.209 | 0.0023 |
| Microbacteriaceae | CN | 3.896 | 0.004 |
| Clostridiaceae_1 | HS | 4.549 | 0.035 |
| Veillonellaceae | HS | 3.769 | 0.025 |
| Genus | |||
| Subdoligranulum | CN | 4.314 | 0.002 |
| Lactococcus | CN | 4.107 | 0.001 |
| Ruminococcus__torques_group | CN | 3.913 | 0.002 |
| Leucobacter | CN | 3.854 | 0.004 |
| Proteiniclasticum | HS | 4.263 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, H.; Cong, W.; Zhang, K.; Jia, Y.; Wu, L. Chronic Heat Stress Affects Bile Acid Profile and Gut Microbiota in Broilers. Int. J. Mol. Sci. 2023, 24, 10238. https://doi.org/10.3390/ijms241210238
Zhang Y, Chen H, Cong W, Zhang K, Jia Y, Wu L. Chronic Heat Stress Affects Bile Acid Profile and Gut Microbiota in Broilers. International Journal of Molecular Sciences. 2023; 24(12):10238. https://doi.org/10.3390/ijms241210238
Chicago/Turabian StyleZhang, Yuting, Huimin Chen, Wei Cong, Ke Zhang, Yimin Jia, and Lei Wu. 2023. "Chronic Heat Stress Affects Bile Acid Profile and Gut Microbiota in Broilers" International Journal of Molecular Sciences 24, no. 12: 10238. https://doi.org/10.3390/ijms241210238





