Innovative Strategy for Truly Reversible Capture of Polluting Gases—Application to Carbon Dioxide
Abstract
:1. Introduction—Air Pollutants and Environmental Impacts
2. Greenhouse Gases and Global Warming
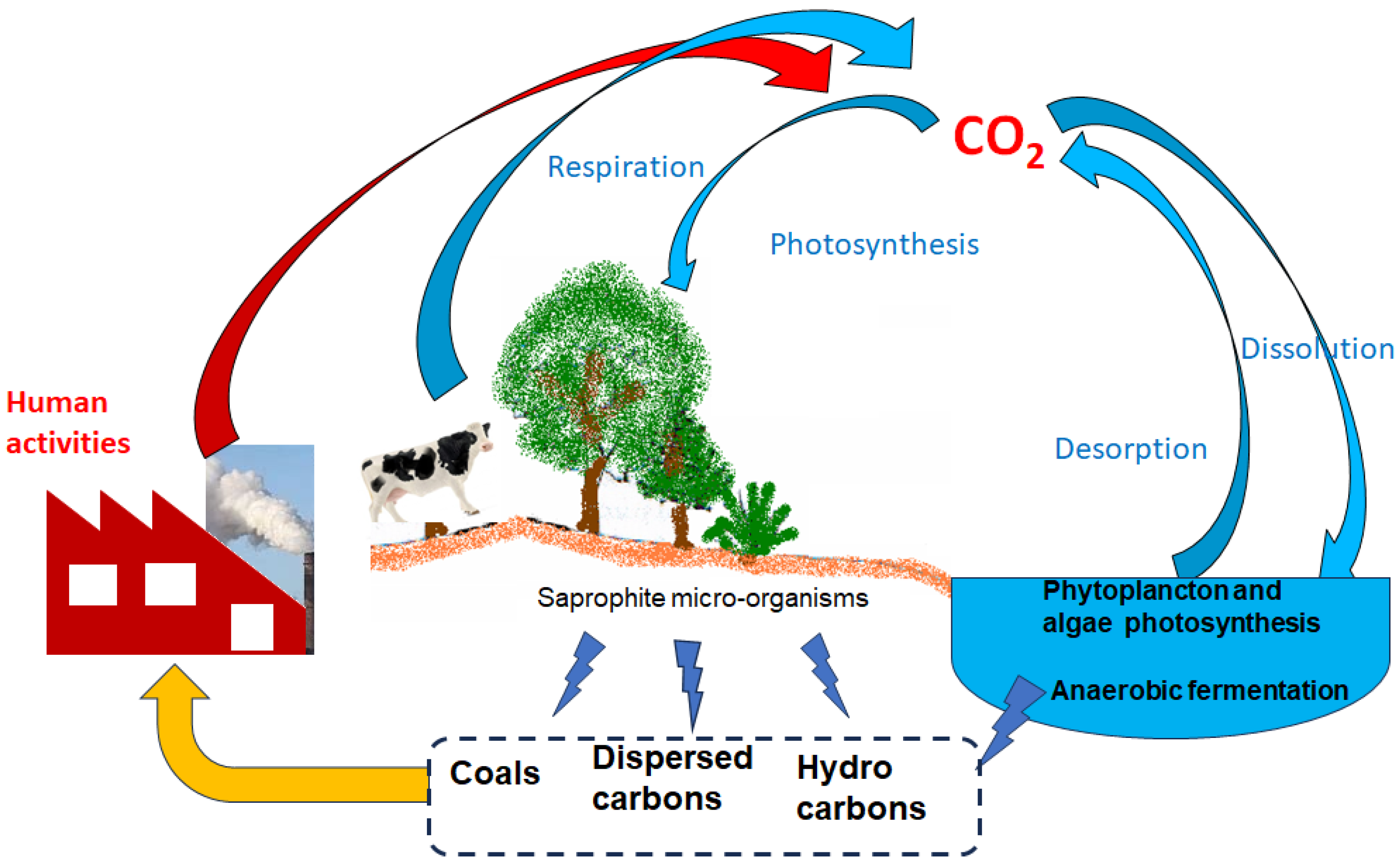
3. CO2 Natural Cycle and Major Emission Sources
4. Strategies for CO2 Capture and Potential Valorisation Routes
5. CO2 Absorption Methods
6. CO2 Adsorption on Solids
7. Design of Reversible CO2 Capture
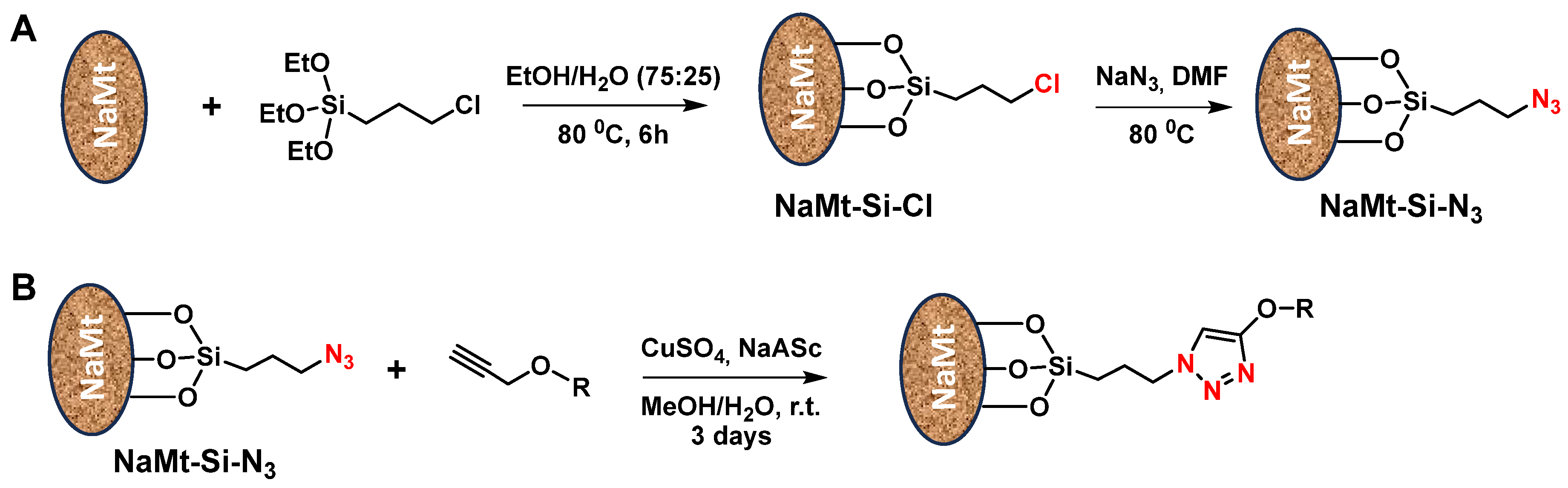
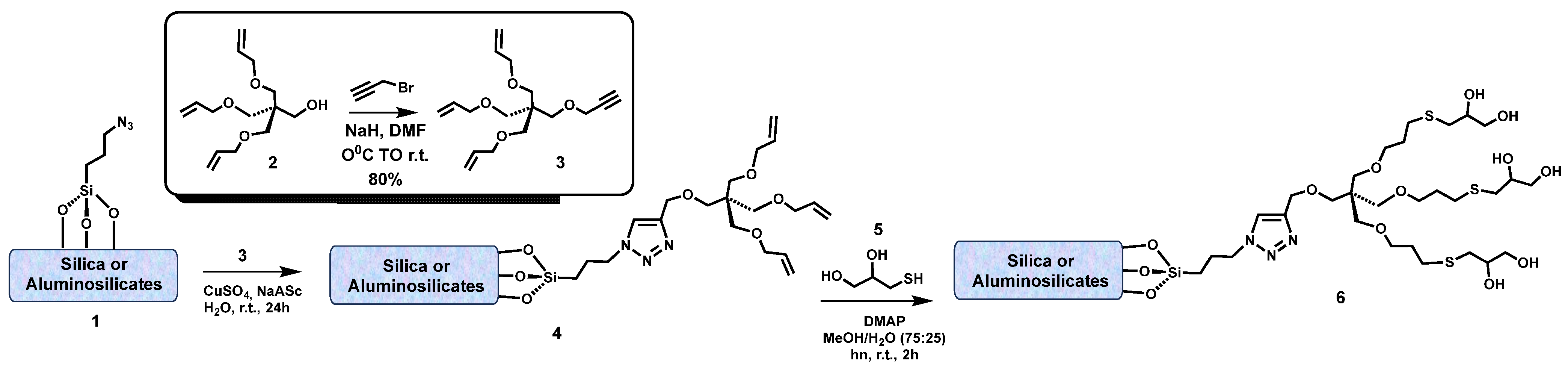
8. Interactions on Clay-Supported Polyalcohols
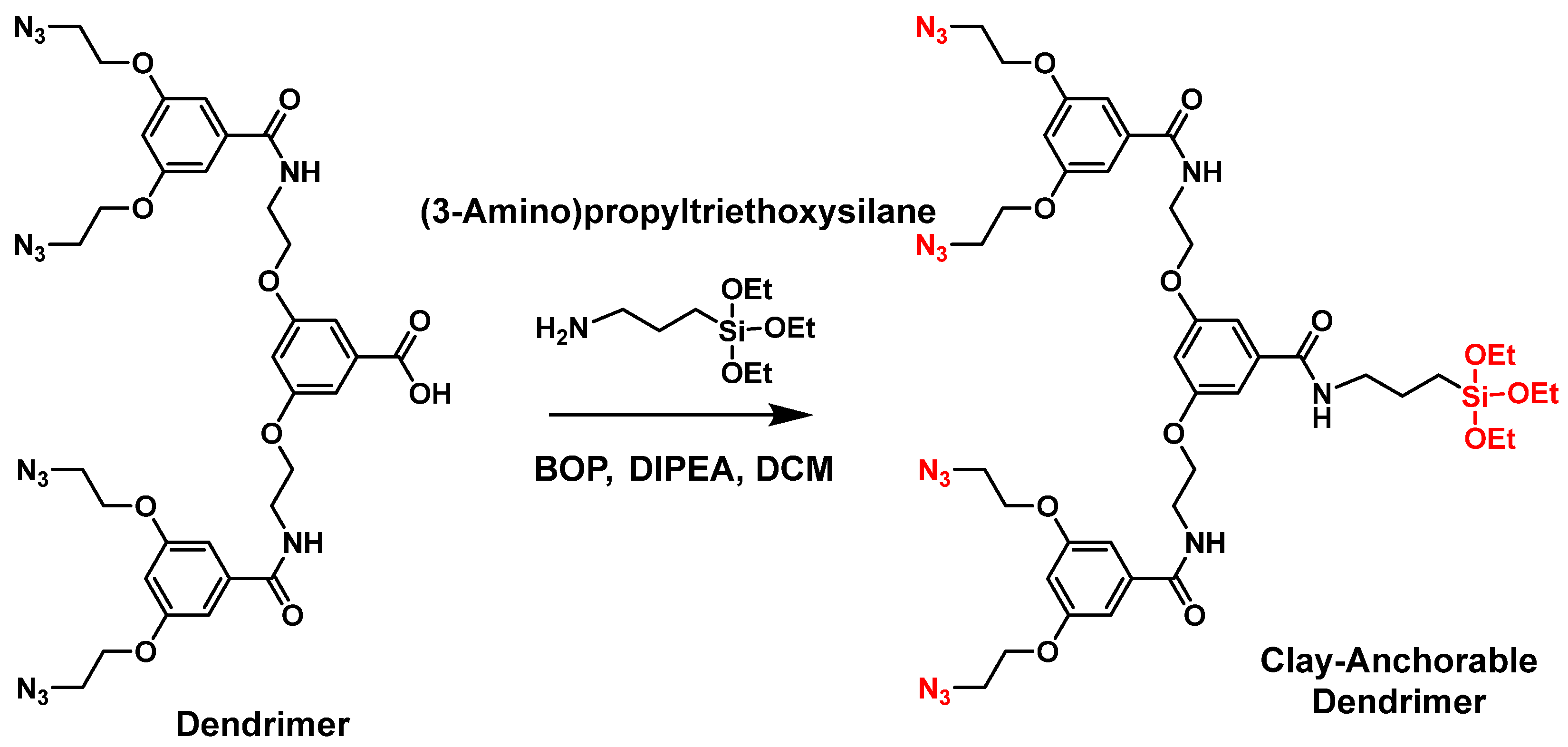
9. Chemical Grafting
10. CO2 Capture for Further Applications and Storage
11. Potential CO2 Adsorbents
12. CO2 Retention Capacity and Parameter Effects
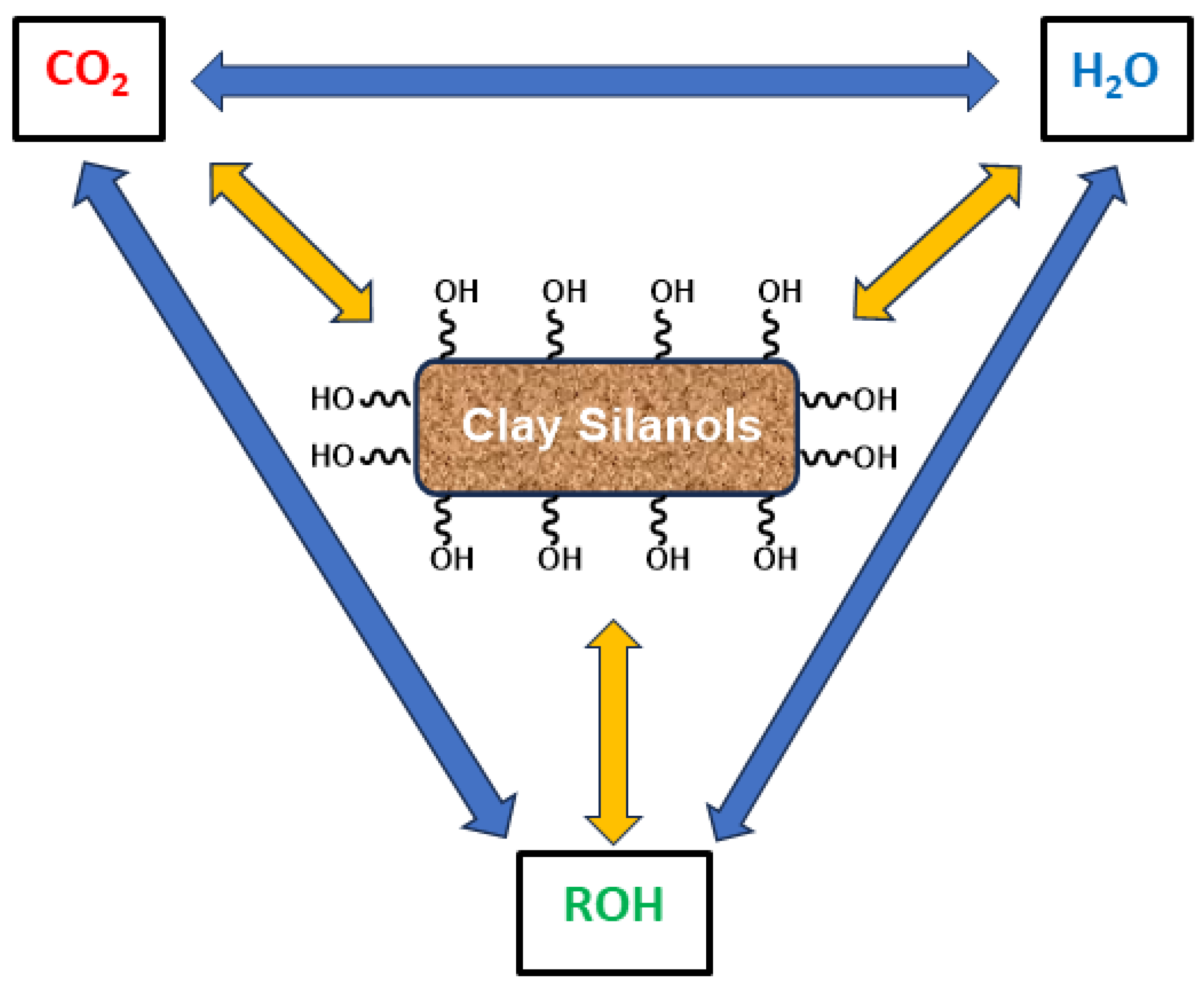
13. Hydroxyl Affinity towards CO2 and Water
14. Metal-Carbonate Association
15. Conclusions
Funding
Conflicts of Interest
References
- Felzer, B.S.; Cronin, T.; Reilly, J.M.; Melillo, J.M.; Wang, X. Impacts of ozone on trees and crops. Comptes Rendus Geosci. 2007, 339, 784–798. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Fujita, E.M. Review of volatile organic compound source apportionment by chemical mass balance. Atmos. Environ. 2001, 35, 1567–1584. [Google Scholar] [CrossRef]
- Shah, J.J.; Singh, H.B. Distribution of volatile organic chemicals in outdoor and indoor air: A national VOCs data base. Environ. Sci. Technol. 1988, 22, 1381–1388. [Google Scholar] [CrossRef]
- Mølhave, L.; Clausen, G.; Berglund, B.; De Ceaurriz, J.; Kettrup, A.; Lindvall, T.; Maroni, M.; Pickering, A.C.; Risse, U.; Rothweiler, H.; et al. Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations*. Indoor Air 1997, 7, 225–240. [Google Scholar] [CrossRef]
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef]
- Harmelen, T.v.; Horssen, A.v.; Jozwicka, M.; Pulles, T.; Odeh, N.; Adams, M. Air Pollution Impacts from Carbon Capture and Storage (CCS); Denmark. 2011. Available online: http://www.eea.europa.eu/publications/carbon-capture-and-storage (accessed on 15 November 2011).
- Herzog, H.J.; Adams, E.E.; Auerbach, D.; Caulfield, J. Environmental impacts of ocean disposal of CO2. Energy Convers. Manag. 1996, 37, 999–1005. [Google Scholar] [CrossRef]
- Kaithwas, A.; Prasad, M.; Kulshreshtha, A.; Verma, S. Industrial wastes derived solid adsorbents for CO2 capture: A mini review. Chem. Eng. Res. Des. 2012, 90, 1632–1641. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Sayari, A. Adsorption of CO2 from dry gases on MCM-41 silica at ambient temperature and high pressure. 2: Adsorption of CO2/N2, CO2/CH4 and CO2/H2 binary mixtures. Chem. Eng. Sci. 2009, 64, 3729–3735. [Google Scholar] [CrossRef]
- Cui, L.; Liu, C.; Yao, B.; Edwards, P.P.; Xiao, T.; Cao, F. A review of catalytic hydrogenation of carbon dioxide: From waste to hydrocarbons. Front. Chem. 2022, 10, 1037997. [Google Scholar] [CrossRef]
- Yu, X.; Catanescu, C.O.; Bird, R.E.; Satagopan, S.; Baum, Z.J.; Lotti Diaz, L.M.; Zhou, Q.A. Trends in Research and Development for CO2 Capture and Sequestration. ACS Omega 2023, 8, 11643–11664. [Google Scholar] [CrossRef]
- Florides, G.A.; Christodoulides, P. Global warming and carbon dioxide through sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, A.F. Needs, resources and climate change: Clean and efficient conversion technologies. Progress. Energy Combust. Sci. 2011, 37, 15–51. [Google Scholar] [CrossRef]
- Allen, M.R.; Frame, D.J.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Progress. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Farhat, S.C.; Silva, C.A.; Orione, M.A.; Campos, L.M.; Sallum, A.M.; Braga, A.L. Air pollution in autoimmune rheumatic diseases: A review. Autoimmun. Rev. 2011, 11, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mace, M.J.; Fyson, C.L.; Schaeffer, M.; Hare, W.L. Large-Scale Carbon Dioxide Removal to Meet the 1.5°C Limit: Key Governance Gaps, Challenges and Priority Responses. Glob. Policy 2021, 12, 67–81. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Khosroabadi, F.; Aslani, A.; Bekhrad, K.; Zolfaghari, Z. Analysis of Carbon Dioxide Capturing Technologies and their technology developments. Clean. Eng. Technol. 2021, 5, 100279. [Google Scholar] [CrossRef]
- Kikkawa, S.; Amamoto, K.; Fujiki, Y.; Hirayama, J.; Kato, G.; Miura, H.; Shishido, T.; Yamazoe, S. Direct Air Capture of CO2 Using a Liquid Amine–Solid Carbamic Acid Phase-Separation System Using Diamines Bearing an Aminocyclohexyl Group. ACS Environ. Au 2022, 2, 354–362. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Chen, G.; Wang, T.; Zhang, G.; Liu, G.; Jin, W. Membrane materials targeting carbon capture and utilization. Adv. Membr. 2022, 2, 100025. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Vega, A.; Ordóñez, S. A kinetic study of CO2 desorption from basic materials: Correlation with adsorption properties. Chem. Eng. J. 2011, 175, 341–348. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Azzouz, A.; Messad, D.; Nistor, D.; Catrinescu, C.; Zvolinschi, A.; Asaftei, S. Vapor phase aldol condensation over fully ion-exchanged montmorillonite-rich catalysts. Appl. Catal. A General. 2003, 241, 1–13. [Google Scholar] [CrossRef]
- Yamasaki, A. An Overview of CO2 Mitigation Options for Global Warming—Emphasizing CO2 Sequestration Options. J. Chem. Eng. Jpn. 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Gui, X.; Tang, Z.; Fei, W. Solubility of CO2 in Alcohols, Glycols, Ethers, and Ketones at High Pressures from (288.15 to 318.15) K. J. Chem. Eng. Data 2011, 56, 2420–2429. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef]
- Galán Sánchez, L.M.; Meindersma, G.W.; de Haan, A.B. Solvent Properties of Functionalized Ionic Liquids for CO2 Absorption. Chem. Eng. Res. Des. 2007, 85, 31–39. [Google Scholar] [CrossRef]
- Jassim, M.S.; Rochelle, G.; Eimer, D.; Ramshaw, C. Carbon Dioxide Absorption and Desorption in Aqueous Monoethanolamine Solutions in a Rotating Packed Bed. Ind. Eng. Chem. Res. 2007, 46, 2823–2833. [Google Scholar] [CrossRef]
- Lee, S.; Filburn, T.P.; Gray, M.; Park, J.-W.; Song, H.-J. Screening Test of Solid Amine Sorbents for CO2 Capture. Ind. Eng. Chem. Res. 2008, 47, 7419–7423. [Google Scholar] [CrossRef]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Thermal and Chemical Stability of Regenerable Solid Amine Sorbent for CO2 Capture. Energy Fuels 2006, 20, 1514–1520. [Google Scholar] [CrossRef]
- Monks, P.S.; Granier, C.; Fuzzi, S.; Stohl, A.; Williams, M.L.; Akimoto, H.; Amann, M.; Baklanov, A.; Baltensperger, U.; Bey, I.; et al. Atmospheric composition change—global and regional air quality. Atmos. Environ. 2009, 43, 5268–5350. [Google Scholar] [CrossRef]
- Ochoa-Fernández, E.; Rusten, H.K.; Jakobsen, H.A.; Rønning, M.; Holmen, A.; Chen, D. Sorption enhanced hydrogen production by steam methane reforming using Li2ZrO3 as sorbent: Sorption kinetics and reactor simulation. Catal. Today 2005, 106, 41–46. [Google Scholar] [CrossRef]
- Azzouz, A.; Aruş, V.-A.; Platon, N.; Ghomari, K.; Nistor, I.-D.; Shiao, T.C.; Roy, R. Polyol-modified layered double hydroxides with attenuated basicity for a truly reversible capture of CO2. Adsorption 2013, 19, 909–918. [Google Scholar] [CrossRef]
- Schlink, U.; Herbarth, O.; Richter, M.; Dorling, S.; Nunnari, G.; Cawley, G.; Pelikan, E. Statistical models to assess the health effects and to forecast ground-level ozone. Environ. Model. Softw. 2006, 21, 547–558. [Google Scholar] [CrossRef]
- Rosseinsky, M.J. Recent developments in metal–organic framework chemistry: Design, discovery, permanent porosity and flexibility. Microporous Mesoporous Mater. 2004, 73, 15–30. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Losch, J. Adsorption of CO2 on Zeolites at Moderate Temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Nousir, S.; Platon, N.; Ghomari, K.; Sergentu, A.-S.; Shiao, T.C.; Hersant, G.; Bergeron, J.-Y.; Roy, R.; Azzouz, A. Correlation between the hydrophilic character and affinity towards carbon dioxide of montmorillonite-supported polyalcohols. J. Colloid. Interface Sci. 2013, 402, 215–222. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Shiao, T.C.; Hersant, G.; Bergeron, J.-Y.; Roy, R. Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int. J. Green. Gas. Control 2013, 17, 140–147. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo-montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Hersant, G.; Bergeron, J.-Y.; Shiao, T.C.; Rej, R.; Roy, R. Preparation and characterization of hydrophilic organo-montmorillonites through incorporation of non-ionic polyglycerol dendrimers derived from soybean oil. Mater. Res. Bull. 2013, 48, 3466–3473. [Google Scholar] [CrossRef]
- Azzouz, A.; Assaad, E.; Ursu, A.-V.; Sajin, T.; Nistor, D.; Roy, R. Carbon dioxide retention over montmorillonite–dendrimer materials. Appl. Clay Sci. 2010, 48, 133–137. [Google Scholar] [CrossRef]
- Azzouz, A. Achievement in hydrogen storage on adsorbents with high surface-to-bulk ratio—Prospects for Si-containing matrices. Int. J. Hydrog. Energy 2012, 37, 5032–5049. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Bouazizi, N.; Roy, R. Metal-organoclays as potentials adsorbents for hydrogen storage. In Proceedings of the 2014 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech, Washington, DC, USA, 15–18 June 2014; Paper number 396. pp. 72–75. [Google Scholar]
- Makhoukhi, B.; Didi, M.A.; Moulessehoul, H.; Azzouz, A.; Villemin, D. Diphosphonium ion-exchanged montmorillonite for Telon dye removal from aqueous media. Appl. Clay Sci. 2010, 50, 354–361. [Google Scholar] [CrossRef]
- Azzouz, A.; Kotbi, A.; Niquette, P.; Sajin, T.; Ursu, V.; Rami, A.; Monette, F.; Hausler, R. Ozonation of oxalic acid catalyzed by ion-exchanged montmorillonites in moderately acidic media. Reac Kinet. Mech. Catal. 2010, 99, 289. [Google Scholar] [CrossRef]
- Dinca, M.; Dailly, A.; Liu, Y.; Brown, C.M.; Neumann, D.A.; Long, J.R. Hydrogen storage in a microporous metal-organic framework with exposed Mn2+ coordination sites. J. Am. Chem. Soc. 2006, 128, 16876–16883. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ursu, A.-V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49. [Google Scholar] [CrossRef]
- Bujdák, J.; Hackett, E.; Giannelis, E.P. Effect of Layer Charge on the Intercalation of Poly(ethylene oxide) in Layered Silicates: Implications on Nanocomposite Polymer Electrolytes. Chem. Mater. 2000, 12, 2168–2174. [Google Scholar] [CrossRef]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef]
- Innocenzi, P.; Martucci, A.; Guglielmi, M.; Bearzotti, A.; Traversa, E.; Pivin, J.C. Mesoporous silica thin films for alcohol sensors. J. Eur. Ceram. Soc. 2001, 21, 1985–1988. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Arroyo, L.J.; Li, H.; Teppen, B.J.; Boyd, S.A. A Simple Method for Partial Purification of Reference Clays. Clays Clay Miner. 2005, 53, 511–519. [Google Scholar] [CrossRef]
- Nistor, D.; Dron, P.I.; Surpăţeanu, G.G.; Siminiceanu, I.; Miron, N.D.; Azzouz, A. Optimized procedure for clay pillaring with aluminum species used in depollution. J. Therm. Anal. Calorim. 2006, 84, 527–530. [Google Scholar] [CrossRef]
- Moreno, M.; Benavente, E.; González, G.; Lavayen, V.; Torres, C.M.S. Functionalization of Bentonite by Intercalation of Surfactants. Mol. Cryst. Liq. Cryst. 2006, 448, 123/[725]–131/[733]. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, T.; Miyamoto, N.; Kuroda, K. Intercalation of a cationic azobenzene into montmorillonite. Appl. Clay Sci. 2003, 22, 179–185. [Google Scholar] [CrossRef]
- Aranda, P.; Ruiz-Hitzky, E. Poly(ethylene oxide)/NH4+-smectite nanocomposites. Appl. Clay Sci. 1999, 15, 119–135. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Pinnavaia, T.J. Clay Nanolayer Reinforcement of a Silicone Elastomer. Chem. Mater. 2001, 13, 3760–3765. [Google Scholar] [CrossRef]
- Ayyappan, S.; Subbanna, G.N.; Gopalan, R.S.; Rao, C.N.R. Nanoparticles of nickel and silver produced by the polyol reduction of the metal salts intercalated in montmorillonite. Solid. State Ion. 1996, 84, 271–281. [Google Scholar] [CrossRef]
- Sánchez, V.; Benavente, E.; Santa Ana, M.A.; González, G. High Electronic Conductivity Molybdenum Disulfide-Dialkylamine Nanocomposites. Chem. Mater. 1999, 11, 2296–2298. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, X.; Yang, S.; Blake, A.J.; Dailly, A.; Champness, N.R.; Hubberstey, P.; Schroder, M. Exceptionally high H2storage by a metal-organic polyhedral framework. Chem. Commun. 2009, 9, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 Saturation Uptake in Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; Fierro, C.M.; Nieuwenhuyzen, M.; James, S.L. A pillared-grid MOF with large pores based on the Cu2(O2CR)4 paddle-wheel. CrystEngComm 2007, 9, 449–451. [Google Scholar] [CrossRef]
- Liu, Y.; Kabbour, H.; Brown, C.M.; Neumann, D.A.; Ahn, C.C. Increasing the Density of Adsorbed Hydrogen with Coordinatively Unsaturated Metal Centers in Metal−Organic Frameworks. Langmuir 2008, 24, 4772–4777. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.K.; Liu, Y.; Brown, C.M.; Kepert, C.J. Neutron Powder Diffraction Study of D2 Sorption in Cu3(1,3,5-benzenetricarboxylate)2. J. Am. Chem. Soc. 2006, 128, 15578–15579. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Spencer, E.C.; Eckert, J.; Howard, J.A.K.; Yaghi, O.M. Gas Adsorption Sites in a Large-Pore Metal-Organic Framework. Science 2005, 309, 1350. [Google Scholar] [CrossRef]
- Forster, P.M.; Eckert, J.; Heiken, B.D.; Parise, J.B.; Yoon, J.W.; Jhung, S.H.; Chang, J.-S.; Cheetham, A.K. Adsorption of Molecular Hydrogen on Coordinatively Unsaturated Ni(II) Sites in a Nanoporous Hybrid Material. J. Am. Chem. Soc. 2006, 128, 16846–16850. [Google Scholar] [CrossRef]
- Shen, W.; He, H.; Zhu, J.; Yuan, P.; Frost, R.L. Grafting of montmorillonite with different functional silanes via two different reaction systems. J. Colloid. Interface Sci. 2007, 313, 268–273. [Google Scholar] [CrossRef]
- He, H.; Duchet, J.; Galy, J.; Gerard, J.-F. Grafting of swelling clay materials with 3-aminopropyltriethoxysilane. J. Colloid. Interface Sci. 2005, 288, 171–176. [Google Scholar] [CrossRef]
- Gray, M.L.; Yee, S.; Champagne, K.J. Amine Enriched Solid Sorbents for Carbon Dioxide Capture. U.S. Patent 6,547,854, 15 April 2003. [Google Scholar]
- Khatri, R.A.; Chuang, S.S.C.; Soong, Y.; Gray, M. Carbon Dioxide Capture by Diamine-Grafted SBA-15: A Combined Fourier Transform Infrared and Mass Spectrometry Study. Ind. Eng. Chem. Res. 2005, 44, 3702–3708. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Odian, G.; Tomalia, D.A. Thermal polymerization of a 2-(carboxyalkyl)-2-oxazoline. Macromolecules 1988, 21, 1556–1562. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications. and the Future; Cambridge University Press: New York, NY, USA, 2012; p. 420. [Google Scholar]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective*. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; VÖGtle, F. "Cascade"- and "Nonskid-Chain-like" Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanoparticle Res. 2009, 11, 1251–1310. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendrons/dendrimers: Quantized, nano-element like building blocks for soft-soft and soft-hard nano-compound synthesis. Soft Matter 2010, 6, 456–474. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendritic effects: Dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J. Chem. 2012, 36, 264–281. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. In Quest of a Systematic Framework For Unifying And Defining Nanoscience. Mod. Phys. Lett. B 2014, 28, 1430002. [Google Scholar] [CrossRef]
- Husin, N.M.; Hasni, R.; Arif, E.N.; Imran, M. On Topological Indices of Certain Families of Nanostar Dendrimers. Molecules 2016, 21, 821. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A. Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s. Molecules 2016, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef]
- Ramírez-Crescencio, F.; Enciso, E.A.; Hasan, M.; da Costa, C.V.; Annunziata, O.; Redón, R.; Coffer, L.J.; Simanek, E.E. Thermoregulated Coacervation, Metal-Encapsulation and Nanoparticle Synthesis in Novel Triazine Dendrimers. Molecules 2016, 21, 599. [Google Scholar] [CrossRef]
- Kaga, S.; Arslan, M.; Sanyal, R.; Sanyal, A. Dendrimers and Dendrons as Versatile Building Blocks for the Fabrication of Functional Hydrogels. Molecules 2016, 21, 497. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Bifunctional Phosphorus Dendrimers and Their Properties. Molecules 2016, 21, 538. [Google Scholar] [CrossRef]
- Stenström, P.; Andrén, C.O.; Malkoch, M. Fluoride-Promoted Esterification (FPE) Chemistry: A Robust Route to Bis-MPA Dendrons and Their Postfunctionalization. Molecules 2016, 21, 366. [Google Scholar] [CrossRef]
- Lee, C.; Ji, K.; Simanek, E.E. Functionalization of a Triazine Dendrimer Presenting Four Maleimides on the Periphery and a DOTA Group at the Core. Molecules 2016, 21, 335. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Reyna, L.A.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35, 61. [Google Scholar] [CrossRef]
- Da Silva Santos, S.; Igne Ferreira, E.; Giarolla, J. Dendrimer Prodrugs. Molecules 2016, 21, 686. [Google Scholar] [CrossRef]
- Arus, A.V.; Tahir, M.N.; Sennour, R.; Shiao, T.C.; Sallam, L.M.; Nistor, I.D.; Roy, R.; Azzouz, A. Cu0and Pd0loaded Organo-Bentonites as Sponge-like Matrices for Hydrogen Reversible Capture at Ambient Conditions. ChemistrySelect 2016, 1, 1452–1461. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Bouazizi, N.; Roy, R. Metal-inorganic-organic matrices as efficient sorbents for hydrogen storage. ChemSusChem 2015, 8, 800–803. [Google Scholar] [CrossRef]
- Tahir, M.N.; Sennour, R.; Arus, V.A.; Sallam, L.M.; Roy, R.; Azzouz, A. Metal organoclays with compacted structure for truly physical capture of hydrogen. Appl. Surf. Sci. 2017, 398, 116–124. [Google Scholar] [CrossRef]
- Shiao, C.T.; Rej, R.; Rose, M.; Pavan, M.G.; Roy, R. Synthesis of Dense and Chiral Dendritic Polyols Using Glyconanosynthon Scaffolds. Molecules 2016, 21, 448. [Google Scholar] [CrossRef]
- Roy, R.; Shiao, T.C. Glyconanosynthons as powerful scaffolds and building blocks for the rapid construction of multifaceted, dense and chiral dendrimers. Chem. Soc. Rev. 2015, 44, 3924–3941. [Google Scholar] [CrossRef]
- Mousavifar, L.; Roy, R. Design, Synthetic Strategies, and Therapeutic Applications of Heterofunctional Glycodendrimers. Molecules 2021, 26, 2428. [Google Scholar] [CrossRef]
- Sharma, R.; Naresh, K.; Chabre, Y.M.; Rej, R.; Saadeh, N.K.; Roy, R. “Onion peel” dendrimers: A straightforward synthetic approach towards highly diversified architectures. Polym. Chem. 2014, 5, 4321–4331. [Google Scholar] [CrossRef]
- Liang, Z.; Fadhel, B.; Schneider, C.J.; Chaffee, A.L. Adsorption of CO2 on mesocellular siliceous foam iteratively functionalized with dendrimers. Adsorption 2009, 15, 429–437. [Google Scholar] [CrossRef]
- Delaney, S.; Knowles, G.; Chaffee, A. Hybrid Mesoporous Materials for Carbon Dioxide Separation. ACS Div. Fuel Chem. Prepr. 2002, 47, 65–66. [Google Scholar]
- Reynhardt, J.P.K.; Yang, Y.; Sayari, A.; Alper, H. Periodic Mesoporous Silica-Supported Recyclable Rhodium-Complexed Dendrimer Catalysts. Chem. Mater. 2004, 16, 4095–4102. [Google Scholar] [CrossRef]
- Reynhardt, J.P.K.; Yang, Y.; Sayari, A.; Alper, H. Polyamidoamine Dendrimers Prepared Inside the Channels of Pore-Expanded Periodic Mesoporous Silica. Adv. Funct. Mater. 2005, 15, 1641–1646. [Google Scholar] [CrossRef]
- Acosta, E.J.; Carr, C.S.; Simanek, E.E.; Shantz, D.F. Engineering Nanospaces: Iterative Synthesis of Melamine-Based Dendrimers on Amine-Functionalized SBA-15 Leading to Complex Hybrids with Controllable Chemistry and Porosity. Adv. Mater. 2004, 16, 985–989. [Google Scholar] [CrossRef]
- Bussetti, S.; Ferreiro, E. Adsorption of Poly(Vinyl Alcohol) on Montmorillonite. Clays Clay Miner. 2004, 52, 334–340. [Google Scholar] [CrossRef]
- Zhao, X.; Urano, K.; Ogasawara, S. Adsorption of polyethylene glycol from aqueous solution on montmorillonite clays. Colloid. Polym. Sci. 1989, 267, 899–906. [Google Scholar] [CrossRef]
- Lupu, D.; Radu Biriş, A.; Mişan, I.; Jianu, A.; Holzhüter, G.; Burkel, E. Hydrogen uptake by carbon nanofibers catalyzed by palladium. Int. J. Hydrog. Energy 2004, 29, 97–102. [Google Scholar] [CrossRef]
- Yamanaka, S.; Fujikane, M.; Uno, M.; Murakami, H.; Miura, O. Hydrogen content and desorption of carbon nano-structures. J. Alloys Compd. 2004, 366, 264–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Lusk, M.T.; Dillon, A.C.; Heben, M.J.; Zhang, S.B. Boron-Based Organometallic Nanostructures: Hydrogen Storage Properties and Structure Stability. Nano Lett. 2008, 8, 157–161. [Google Scholar] [CrossRef]
- Zhao, Y.; Heben, M.J.; Dillon, A.C.; Simpson, L.J.; Blackburn, J.L.; Dorn, H.C.; Zhang, S.B. Nontrivial Tuning of the Hydrogen-Binding Energy to Fullerenes with Endohedral Metal Dopants. J. Phys. Chem. C 2007, 111, 13275–13279. [Google Scholar] [CrossRef]
- Dillon, A.C.; Heben, M.J. Hydrogen storage using carbon adsorbents: Past, present and future. Appl. Phys. A 2001, 72, 133–142. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Zhou, Y. Storage of Hydrogen on Carbon Materials: Experiments and Analyses. Chem. Eng. Commun. 2006, 193, 564–579. [Google Scholar] [CrossRef]
- Touaibia, M.; Shiao, T.C.; Papadopoulos, A.; Vaucher, J.; Wang, Q.; Benhamioud, K.; Roy, R. Tri- and hexavalent mannoside clusters as potential inhibitors of type 1 fimbriated bacteria using pentaerythritol and triazole linkages. Chem. Commun. 2007, 4, 380–382. [Google Scholar] [CrossRef]
- Touaibia, M.; Wellens, A.; Shiao, T.C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. Mannosylated G(0) Dendrimers with Nanomolar Affinities to Escherichia coli FimH. ChemMedChem 2007, 2, 1190–1201. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Song, C. CO2 Conversion and Utilization: An Overview. In CO2 Conversion and Utilization; American Chemical Society: Washington, DC, USA, 2002; Volume 809, pp. 2–30. [Google Scholar]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Lee, J.P.; Lee, J.S.; Park, S.C. Two-phase methanization of food wastes in pilot scale. Appl. Biochem. Biotechnol. 1999, 77–79, 585–593. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Dorner, R.; Hardy, D.; Williams, F.; Willauer, H. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 2010, 3, 1514. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel Heterogeneous Catalysts for CO2 Hydrogenation to Liquid Fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Minyukova, T.P.; Dokuchits, E.V. Hydrogen for CO2 processing in heterogeneous catalytic reactions. Int. J. Hydrog. Energy 2023, 48, 22462–22483. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; Petrus van Bavel, A.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct conversion of CO2 to a jet fuel over CoFe alloy catalysts. Innov. 2021, 2, 100170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Duan, Y.-H.; Zhang, J.-F.; Tan, Y.-S. Catalytic conversion of CO2 into high value-added hydrocarbons over tandem catalyst. J. Fuel Chem. Technol. 2022, 50, 538–563. [Google Scholar] [CrossRef]
- Sharma, P.; Sebastian, J.; Ghosh, S.; Creaser, D.; Olsson, L. Recent advances in hydrogenation of CO2 into hydrocarbons via methanol intermediate over heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1665–1697. [Google Scholar] [CrossRef]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Yusuf, N.; Almomani, F.; Qiblawey, H. Catalytic CO2 conversion to C1 value-added products: Review on latest catalytic and process developments. Fuel 2023, 345, 128178. [Google Scholar] [CrossRef]
- Shi, Y.; Ni, R.; Zhao, Y. Review on Multidimensional Adsorbents for CO2 Capture from Ambient Air: Recent Advances and Future Perspectives. Energy Fuels 2023, 37, 6365–6381. [Google Scholar] [CrossRef]
- de Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Guégan, R. Intercalation of a Nonionic Surfactant (C10E3) Bilayer into a Na-Montmorillonite Clay. Langmuir 2010, 26, 19175–19180. [Google Scholar] [CrossRef]
- Jankovič, L.; Dimos, K.; Bujdák, J.; Koutselas, I.; Madejová, J.; Gournis, D.; Karakassides, M.A.; Komadel, P. Synthesis and characterization of low dimensional ZnS- and PbS-semiconductor particles on a montmorillonite template. Phys. Chem. Chem. Phys. PCCP 2010, 12, 14236–14244. [Google Scholar] [CrossRef] [PubMed]
- Beall, G.W. The use of organo-clays in water treatment. Appl. Clay Sci. 2003, 24, 11–20. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Jankovič, Ľ.; Madejová, J.; Komadel, P.; Jochec-Mošková, D.; Chodák, I. Characterization of systematically selected organo-montmorillonites for polymer nanocomposites. Appl. Clay Sci. 2011, 51, 438–444. [Google Scholar] [CrossRef]
- Gelfer, M.; Burger, C.; Fadeev, A.; Sics, I.; Chu, B.; Hsiao, B.S.; Heintz, A.; Kojo, K.; Hsu, S.L.; Si, M.; et al. Thermally Induced Phase Transitions and Morphological Changes in Organoclays. Langmuir 2004, 20, 3746–3758. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Pham, T.N.; Gérard, J.-F. Synthesis and physical properties of new surfactants based on ionic liquids: Improvement of thermal stability and mechanical behaviour of high density polyethylene nanocomposites. J. Colloid. Interface Sci. 2011, 354, 555–562. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Jiang, X.; Lu, F.; Zhou, Y.; Yin, W.; Ji, X. Modification of montmorillonite surfaces using a novel class of cationic gemini surfactants. J. Colloid. Interface Sci. 2009, 332, 16–21. [Google Scholar] [CrossRef]
- Zhou, Q.; Pramoda, K.P.; Lee, J.-M.; Wang, K.; Loo, L.S. Role of interface in dispersion and surface energetics of polymer nanocomposites containing hydrophilic POSS and layered silicates. J. Colloid. Interface Sci. 2011, 355, 222–230. [Google Scholar] [CrossRef]
- Costa, A.S.; Imae, T.; Takagi, K.; Kikuta, K. Intercalation of dendrimers in the interlayer of hydrotalcite clay sheets. In Proceedings of Surface and Colloid Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 113–119. [Google Scholar]
- Rodlert, M.; Plummer, C.J.G.; Garamszegi, L.; Leterrier, Y.; Grünbauer, H.J.M.; Månson, J.-A.E. Hyperbranched polymer/montmorillonite clay nanocomposites. Polymer 2004, 45, 949–960. [Google Scholar] [CrossRef]
- Rodlert, M.; Plummer, C.J.G.; Grünbauer, H.J.M.; Månson, J.A.E. Hyperbranched Polymer/Clay Nanocomposites. Adv. Eng. Mater. 2004, 6, 715–719. [Google Scholar] [CrossRef]
- Lingaiah, S.; Shivakumar, K.N.; Sadler, R.; Sharpe, M. A method of visualization of dispersion of nanoplatelets in nanocomposites. Compos. Sci. Technol. 2005, 65, 2276–2280. [Google Scholar] [CrossRef]
- Liu, P. Hyperbranched aliphatic polyester grafted attapulgite via a melt polycondensation process. Appl. Clay Sci. 2007, 35, 11–16. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers. Manag. 2008, 49, 346–356. [Google Scholar] [CrossRef]
- Grande, C.A.; Rodrigues, A.E. Electric Swing Adsorption for CO2 removal from flue gases. Int. J. Greenh. Gas. Control 2008, 2, 194–202. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Zou, Y.; Rodrigues, A.E. Adsorbent Materials for Carbon Dioxide. Adsorpt. Sci. Technol. 2001, 19, 255–266. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Indira, V.; Abhitha, K. A review on polymer based adsorbents for CO2 capture. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1114, 012081. [Google Scholar] [CrossRef]
- Pham Ngoc, K.; Nguyen Tien, T. Understanding Interaction Capacity of CO2 with Organic Compounds at Molecular Level: A Theoretical Approach. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Iyad, K., Janah, S., Hassan, S., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous materials as adsorbents for CO2 capture: Synthesis and modification. Carbon. Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef] [PubMed]
- Hospital-Benito, D.; Moya, C.; Gazzani, M.; Palomar, J. Direct air capture based on ionic liquids: From molecular design to process assessment. Chem. Eng. J. 2023, 468, 143630. [Google Scholar] [CrossRef]
- Varghese, A.M.; Karanikolos, G.N. CO2 capture adsorbents functionalized by amine—bearing polymers: A review. Int. J. Greenh. Gas. Control 2020, 96, 103005. [Google Scholar] [CrossRef]
- Lyu, H.; Li, H.; Hanikel, N.; Wang, K.; Yaghi, O.M. Covalent Organic Frameworks for Carbon Dioxide Capture from Air. J. Am. Chem. Soc. 2022, 144, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Fracaroli, A.M.; Furukawa, H.; Suzuki, M.; Dodd, M.; Okajima, S.; Gándara, F.; Reimer, J.A.; Yaghi, O.M. Metal-organic frameworks with precisely designed interior for carbon dioxide capture in the presence of water. J. Am. Chem. Soc. 2014, 136, 8863–8866. [Google Scholar] [CrossRef]
- Jiang, W.; Takeda, K. Crystal-size effect on the kinetics of CO2 adsorption in metal organic frameworks studied by NMR. Phys. Chem. Chem. Phys. PCCP 2022, 24, 21210–21215. [Google Scholar] [CrossRef]
- Gassensmith, J.J.; Furukawa, H.; Smaldone, R.A.; Forgan, R.S.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Strong and Reversible Binding of Carbon Dioxide in a Green Metal–Organic Framework. J. Am. Chem. Soc. 2011, 133, 15312–15315. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Sneddon, G.; Ganin, A.Y.; Yiu, H.H.P. Sustainable CO2 Adsorbents Prepared by Coating Chitosan onto Mesoporous Silicas for Large-Scale Carbon Capture Technology. Energy Technol. 2015, 3, 249–258. [Google Scholar] [CrossRef]
- Du, P.; Yuan, P.; Liu, J.; Ye, B. Clay minerals on Mars: An up-to-date review with future perspectives. Earth-Sci. Rev. 2023, 243, 104491. [Google Scholar] [CrossRef]
- Tomkinson, T.; Lee, M.R.; Mark, D.F.; Smith, C.L. Sequestration of Martian CO2 by mineral carbonation. Nat. Commun. 2013, 4, 2662. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Rodrigues, A.r.E. Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 2002, 43, 1865–1876. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Effect of interlayer anion on the CO2 capture capacity of hydrotalcite-based sorbents. Sep. Purif. Technol. 2019, 219, 290–302. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Doping of hydrotalcite-based sorbents with different interlayer anions for CO2 capture. Sep. Purif. Technol. 2020, 235, 116140. [Google Scholar] [CrossRef]
- Lund, A.; Manohara, G.V.; Song, A.-Y.; Jablonka, K.M.; Ireland, C.P.; Cheah, L.A.; Smit, B.; Garcia, S.; Reimer, J.A. Characterization of Chemisorbed Species and Active Adsorption Sites in Mg–Al Mixed Metal Oxides for High-Temperature CO2 Capture. Chem. Mater. 2022, 34, 3893–3901. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, J.; Mittal, A.; Kumari, K.; Maken, S.; Kumar, N. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 2021, 19, 875–910. [Google Scholar] [CrossRef]
- Goeppert, A.; Zhang, H.; Czaun, M.; May, R.B.; Prakash, G.K.; Olah, G.A.; Narayanan, S.R. Easily regenerable solid adsorbents based on polyamines for carbon dioxide capture from the air. ChemSusChem 2014, 7, 1386–1397. [Google Scholar] [CrossRef]
- Inthawong, S.; Wongkoblap, A.; Intomya, W.; Tangsathitkulchai, C. The Enhancement of CO(2) and CH(4) Capture on Activated Carbon with Different Degrees of Burn-Off and Surface Chemistry. Molecules 2023, 28, 5433. [Google Scholar] [CrossRef]
- Danish, M.; Parthasarthy, V.; Al Mesfer, M.K. Comparative Study of CO(2) Capture by Adsorption in Sustainable Date Pits-Derived Porous Activated Carbon and Molecular Sieve. Int. J. Environ. Res. Public Health 2021, 18, 8497. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Casal, M.D.; Martín, C.F.; Fermoso, J.; Rubiera, F.; Pis, J.J. Different Approaches for the Development of Low-Cost CO2 Adsorbents. J. Environ. Eng. 2009, 135, 426–432. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh. Gases Sci. Technol. 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Díaz, E.; Muñoz, E.; Vega, A.; Ordóñez, S. Enhancement of the CO2 retention capacity of X zeolites by Na- and Cs-treatments. Chemosphere 2008, 70, 1375–1382. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.-H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 2011, 4, 444–452. [Google Scholar] [CrossRef]
- Qi, G.; Fu, L.; Choi, B.H.; Giannelis, E.P. Efficient CO2 sorbents based on silica foam with ultra-large mesopores. Energy Environ. Sci. 2012, 5, 7368–7375. [Google Scholar] [CrossRef]
- Ouargli-Saker, R.; Bouazizi, N.; Boukoussa, B.; Barrimo, D.; Paola-Nunes-Beltrao, A.; Azzouz, A. Metal-loaded SBA-16-like silica—Correlation between basicity and affinity towards hydrogen. Appl. Surf. Sci. 2017, 411, 476–486. [Google Scholar] [CrossRef]
- Terrab, I.; Ouargli-Saker, R.; Boukoussa, B.; Ghomari, K.; Hamacha, R.; Roy, R.; Azzouz, A.; Bengueddach, A. Assessment of the intrinsic interactions of mesoporous silica with carbon dioxide. Res. Chem. Intermed. 2017, 43, 3755–3786. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirila, D.; Arus, V.A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation—Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Bouazizi, N.; Ouargli, R.; Nousir, S.; Slama, R.B.; Azzouz, A. Properties of SBA-15 modified by iron nanoparticles as potential hydrogen adsorbents and sensors. J. Phys. Chem. Solids 2015, 77, 172–177. [Google Scholar] [CrossRef]
- Wang, J.; Stevens, L.A.; Drage, T.C.; Wood, J. Preparation and CO2 adsorption of amine modified Mg–Al LDH via exfoliation route. Chem. Eng. Sci. 2012, 68, 424–431. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
- Gupta, S.K.; Lesslie, R.D.; King, A.D., Jr. Solubility of alcohols in compressed gases. Comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases. I. Ethanol in compressed helium, hydrogen, argon, methane, ethylene, ethane, carbon dioxide, and nitrous oxide. J. Phys. Chem. 1973, 77, 2011–2015. [Google Scholar] [CrossRef]
- Massoudi, R.; King, A.D., Jr. Solubility of alcohols in compressed gases. Comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases. II. 1-Butanol, diethyl ether, and n-pentane in compressed nitrogen, argon, methane, ethane, and carbon dioxide at 25.deg. J. Phys. Chem. 1973, 77, 2016–2018. [Google Scholar] [CrossRef]
- Saharay, M.; Balasubramanian, S. Electron donor-acceptor interactions in ethanol-CO2 mixtures: An ab initio molecular dynamics study of supercritical carbon dioxide. J. Phys. Chem. B 2006, 110, 3782–3790. [Google Scholar] [CrossRef]
- Aylmore, L.A.G. Gas Sorption in Clay Mineral Systems. Clays Clay Miner. 1974, 22, 175–183. [Google Scholar] [CrossRef]
- Bouazizi, N.; Barrimo, D.; Nousir, S.; Ben Slama, R.; Roy, R.; Azzouz, A. Montmorillonite-supported Pd0, Fe0, Cu0 and Ag0 nanoparticles: Properties and affinity towards CO2. Appl. Surf. Sci. 2017, 402, 314–322. [Google Scholar] [CrossRef]
- Weitkamp, J.; Hunger, M. Chapter 22—Acid and Base Catalysis on Zeolites. In Studies in Surface Science and Catalysis; Čejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 787–835. [Google Scholar]
- Deng, W.; Zhang, L.; Li, L.; Chen, S.; Hu, C.; Zhao, Z.-J.; Wang, T.; Gong, J. Crucial Role of Surface Hydroxyls on the Activity and Stability in Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 2911–2915. [Google Scholar] [CrossRef]
- Lee, A.S.; Kitchin, J.R. Chemical and Molecular Descriptors for the Reactivity of Amines with CO2. Ind. Eng. Chem. Res. 2012, 51, 13609–13618. [Google Scholar] [CrossRef]



| Capture Methods * | Intial CO2 Content | Removal Process | Capture Yield (%) | Advantages-Drawbacks |
|---|---|---|---|---|
| Direct atmospheric capture (DAC) | 400 ppm | Chemosorption in liquid amines | Proportional to amine basicity |
|
| IPDA: 99% | ||||
| Chemical/physical adsorption | Water and/or alcohols | |||
| Chemorption on supported amines | Proportional to basicity * | |||
| Physisorption on supported polyol | Proportional to hydroxyl content | |||
| Pre-combustion ab/adsorption | 5–15% | Before complete fossil fuel combustion | Specific to each sorbent * IPDA: 99% | |
| Post-combustion ab/adsorption | 15–50% | Ab/absorption after fossil fuel combustion in air | Specific to each sorbent * IPDA: 99% | |
| Oxy-fuel combustion ab/adsorption | Variable | After recycled flue gas combustion in nearly pure oxygen | Almost 100% of N-free CO2 |
| Materials | CRC (mmol/g) a | Conditions | Full Regeneration | Ref. |
|---|---|---|---|---|
| OH-free activated carbon from longan seeds | 6.4 | 273 K/5 bars | [175] | |
| OH-rich counterpart | 8.0 | |||
| Activated carbon prepared by activation at 700 °C for 5 h | 4.54 | 25 °C/1 atm | [176] | |
| N-functionalized Carbon materials | 111 | 100 °C | [177] | |
| Metal-organic frameworks (IRMOF-74-III-CH2NH2) | 3.2 | 65% relative humidity 800 Torr | [162] | |
| Tetraethylenepentamine-MOF-177 | 4.60 Up to 48.71 | Post-combustion condition Pre-combustion at 1 bar | [178] | |
| Hyper-cross-linked polymers, covalent organic frameworks, conjugated microporous polymers and covalent triazine-based frameworks | 3–6 | 273 K/1 bar Polyethyleneimine insertion improved CO2 capture at higher temperature | [155] | |
| NaX@NaA zeolite core-shell microspheres | 5.60 | Direct Air Capture (DAC) | [178] | |
| Cesium-modified X zeolite | 0.227 | [179] | ||
| Tetraethylenepentamine supported by hollow mesoporous silica capsules | 6.7 | 1 atm dry CO2 Under simulated flue gas conditions (pre-humidified 10% CO2) | Reversibility and stability up to 50 adsorption–regeneration cycles | [180] |
| Amines supported by silica foam-based adsorbents | 5.8 | 1 atm of dry CO2 | [181] | |
| SBA-15 loaded with Fe°, Pd° or Cu°SBA-16 loaded with Fe° or Cu° | 0.002–0.004 * | T = 22–23 °C | [182,183] | |
| Chitosan/SBA or MCM-like silica | 0.98 | P = 1 atm/T = 25 °C | 75 °C with a >85 % CRC after 4 cycles | [166] |
| LDH-polyol composites | 1.5–2.5 | T ≤ 80 °C | [35] | |
| Bentonite NaMt HMt-1 | 2.39 3.82 1.28 | HMt-1: bentonite after 1 h acid activation | [184] | |
| Polyol dendrimer intercalated montmorillonite | 0.0117–0.164 ** | 15 mL/min dry carrier gas stream | 35–40 °C, or 20 °C upon forced convection or with KOH pills | [40,42,184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzouz, A.; Roy, R. Innovative Strategy for Truly Reversible Capture of Polluting Gases—Application to Carbon Dioxide. Int. J. Mol. Sci. 2023, 24, 16463. https://doi.org/10.3390/ijms242216463
Azzouz A, Roy R. Innovative Strategy for Truly Reversible Capture of Polluting Gases—Application to Carbon Dioxide. International Journal of Molecular Sciences. 2023; 24(22):16463. https://doi.org/10.3390/ijms242216463
Chicago/Turabian StyleAzzouz, Abdelkrim, and René Roy. 2023. "Innovative Strategy for Truly Reversible Capture of Polluting Gases—Application to Carbon Dioxide" International Journal of Molecular Sciences 24, no. 22: 16463. https://doi.org/10.3390/ijms242216463
APA StyleAzzouz, A., & Roy, R. (2023). Innovative Strategy for Truly Reversible Capture of Polluting Gases—Application to Carbon Dioxide. International Journal of Molecular Sciences, 24(22), 16463. https://doi.org/10.3390/ijms242216463






