Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation
Abstract
1. Introduction
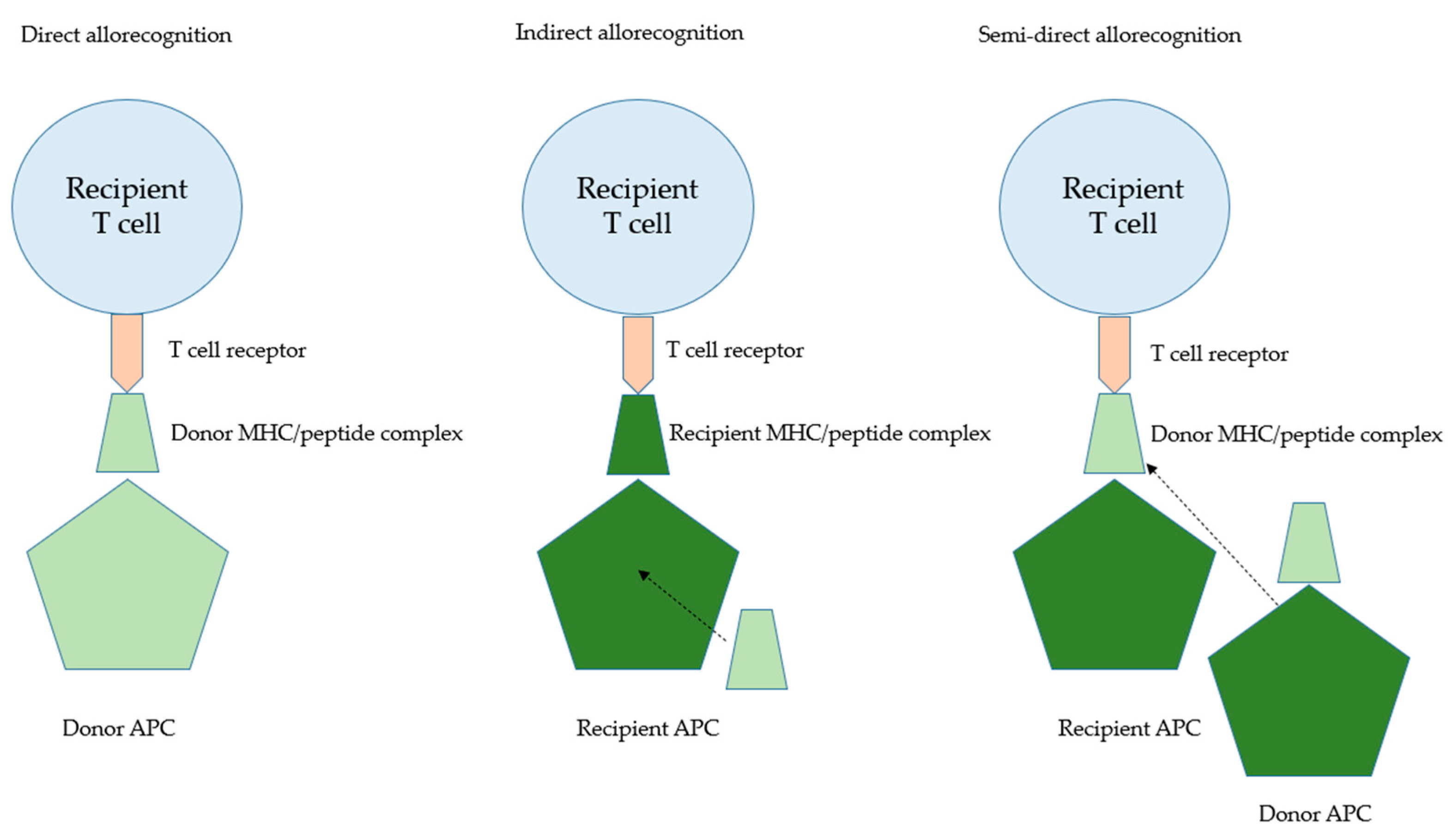
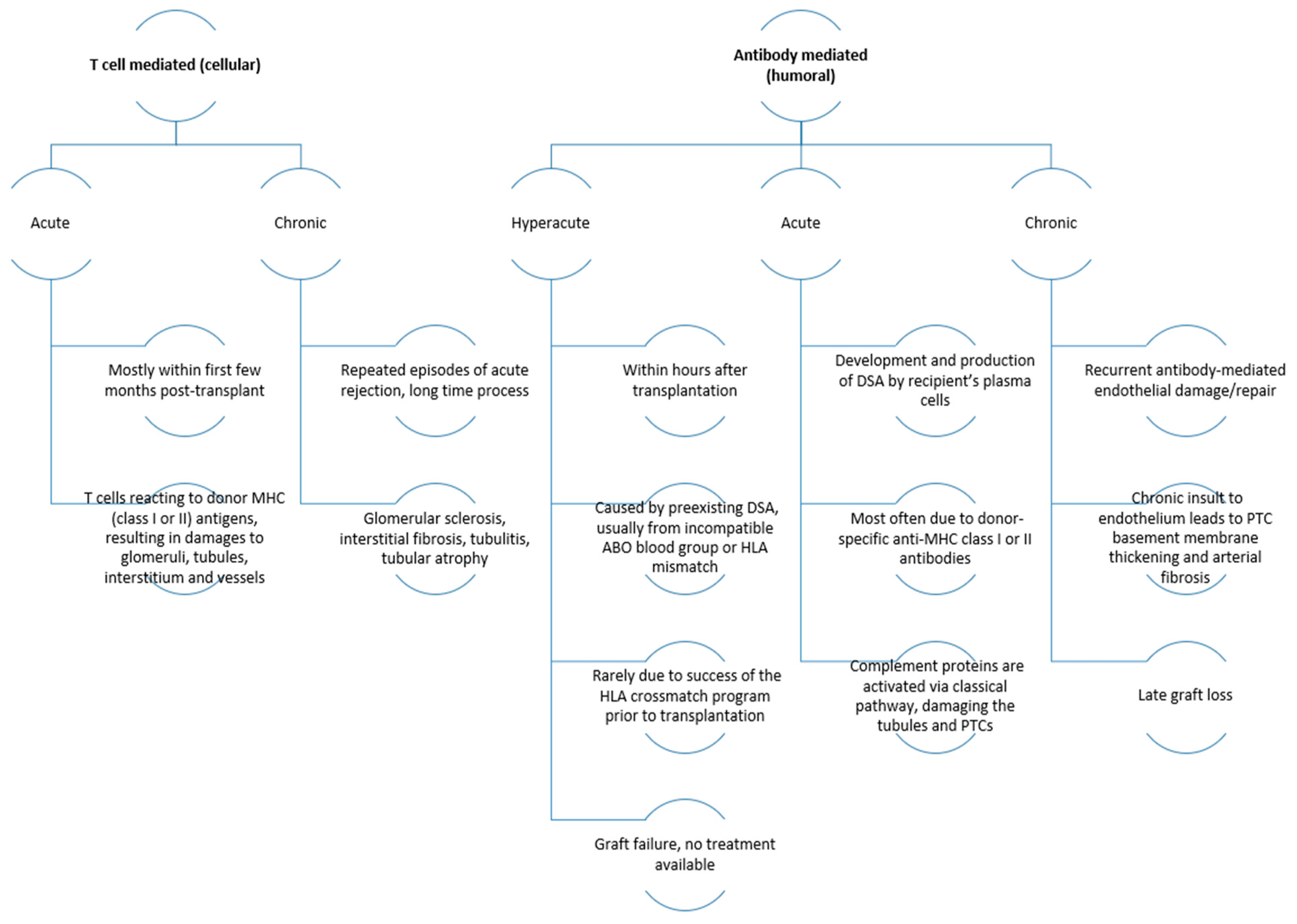
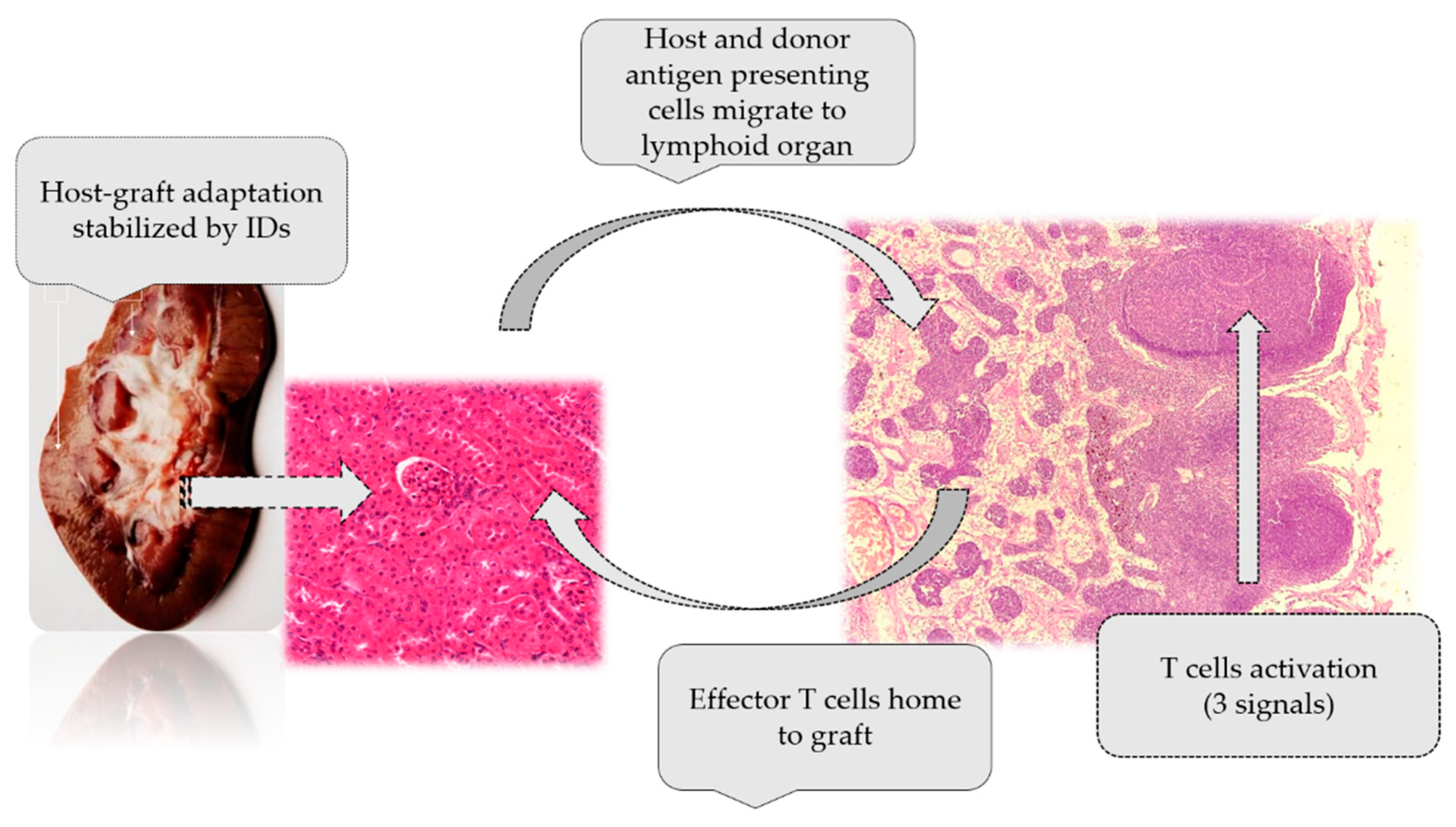
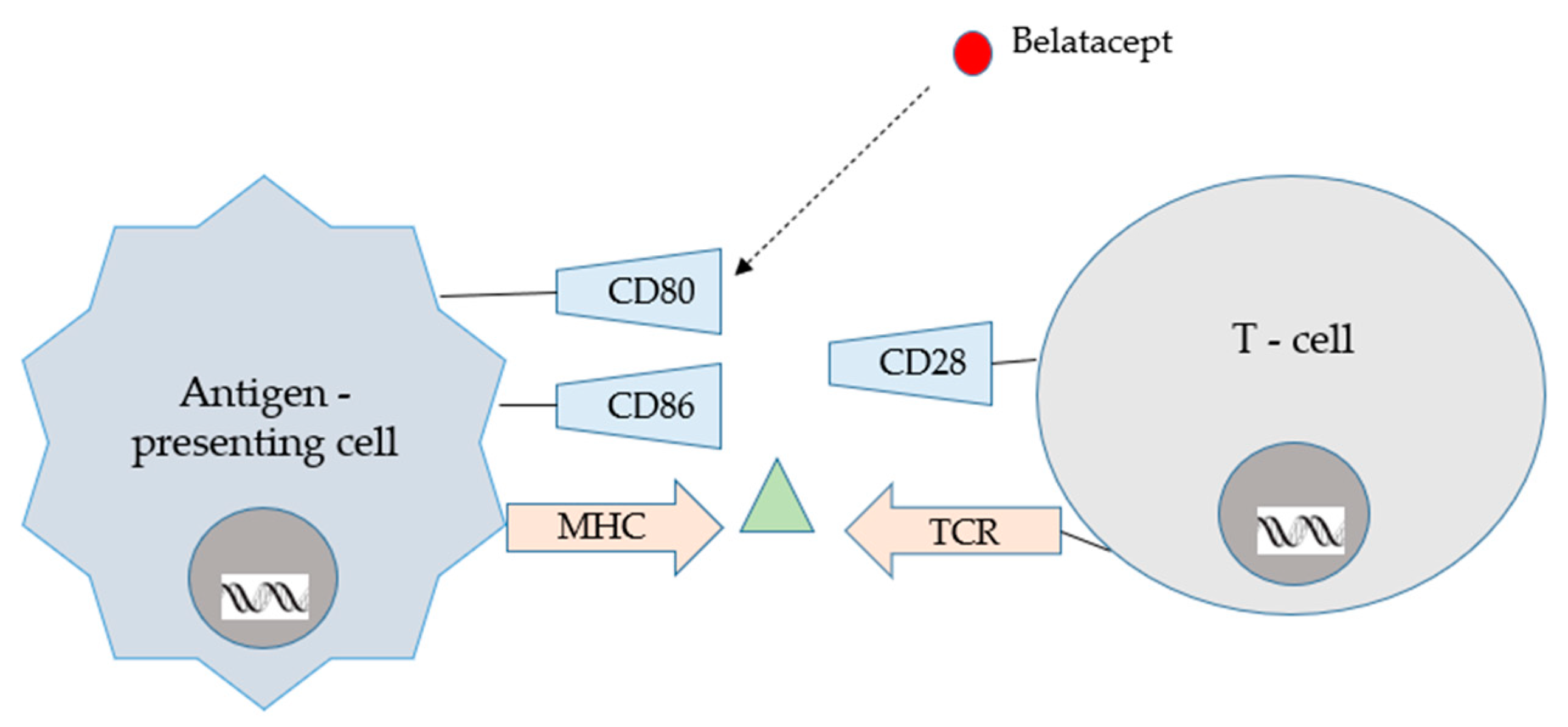
2. Immunological Aspects of Transplantation
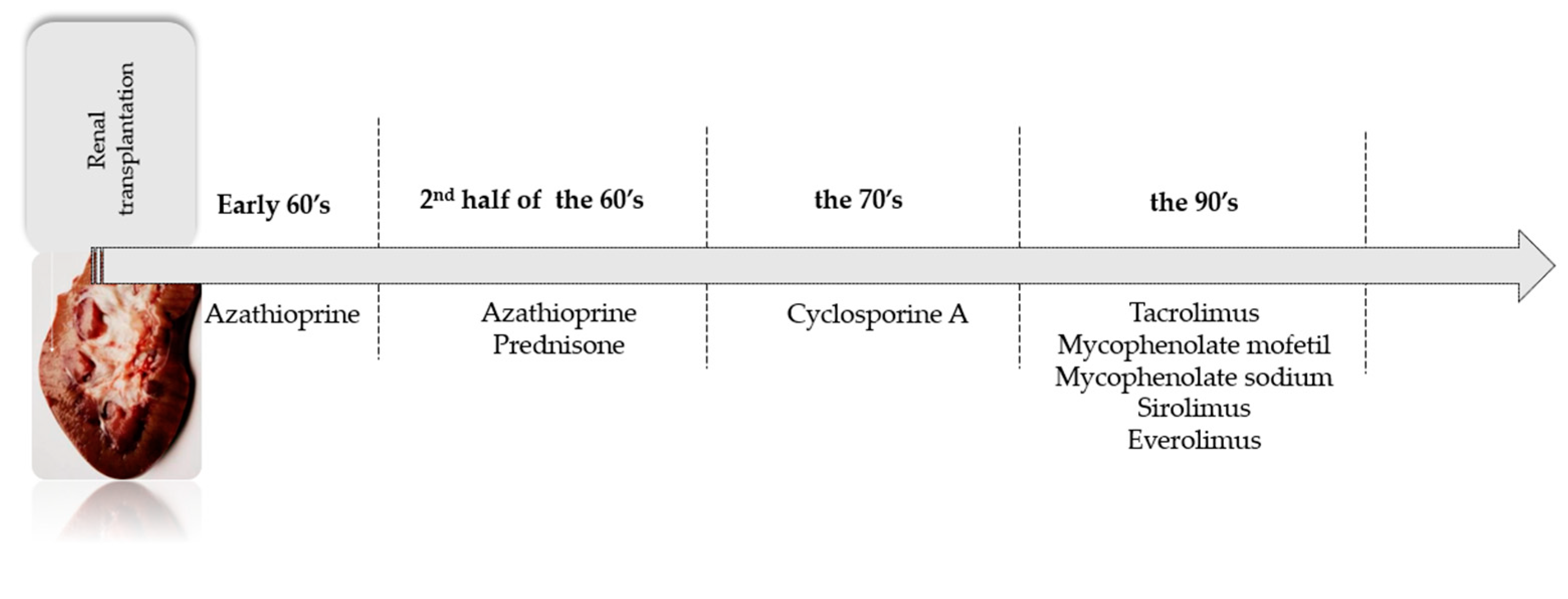
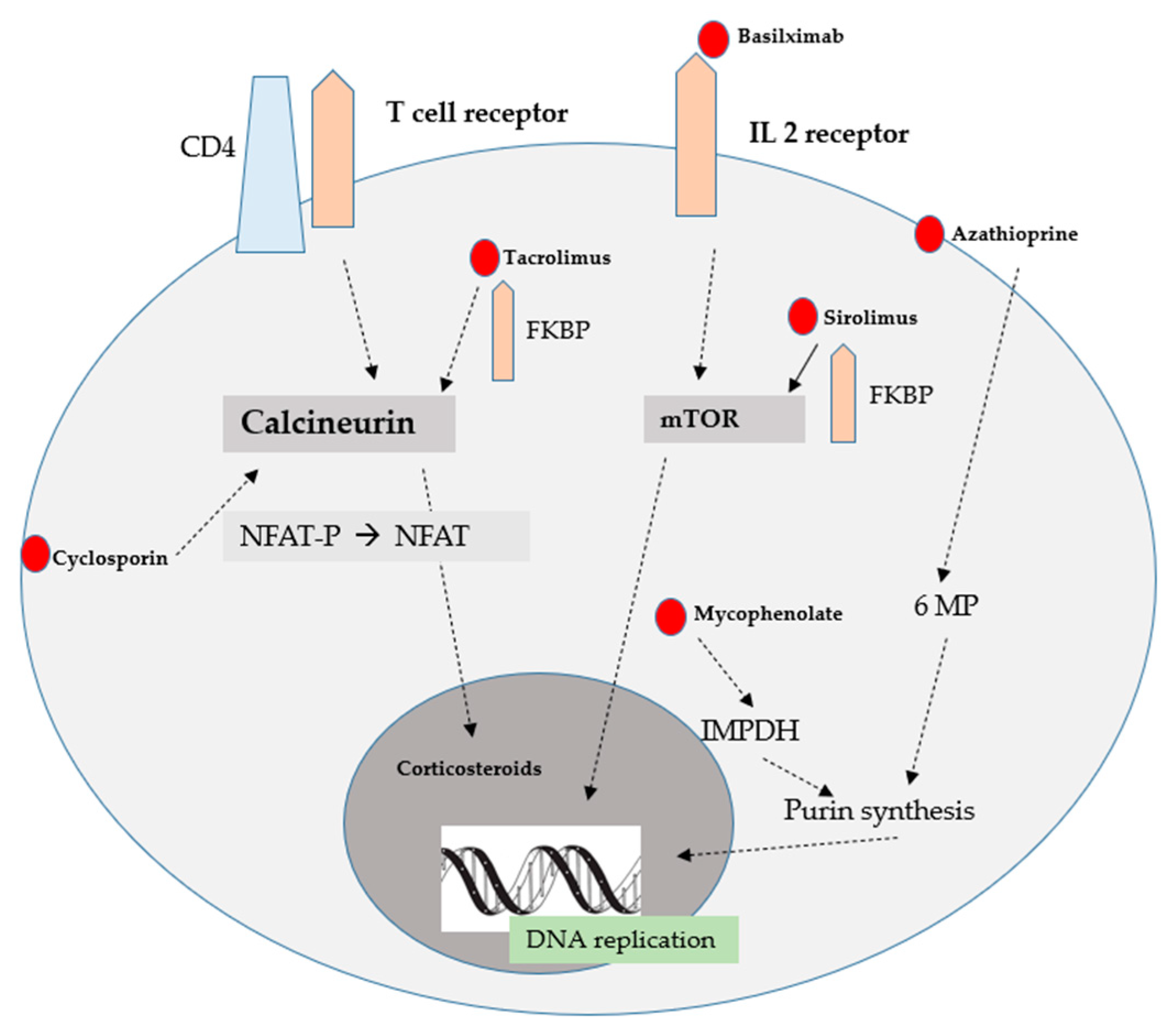
3. Classes of Immunosuppressive Drugs
3.1. Immunosuppressive Drugs—Induction Therapy
3.2. Immunosuppressive Drugs—Maintenance Therapy
CNIs
3.3. Mammalian Target of Rapamycin Inhibitors
3.4. Antiproliferatives
3.5. Glucocorticosteroids
4. Conversions
5. Complications Associated with Usage of Immunosuppressive Drugs
6. Other Options of Immunosuppressive Treatment Used by Renal Transplant Recipients
7. Future Possibilities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meneghini, M.; Bestard, O.; Grinyo, J.M. Immunosuppressive Drugs Modes of Action. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101757. [Google Scholar] [CrossRef]
- Lee, R.A.; Gabardi, S. Current Trends in Immunosuppressive Therapies for Renal Transplant Recipients. Am. J. Health Syst. Pharm. 2012, 69, 1961–1975. [Google Scholar] [CrossRef]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Viklicky, O.; Slatinska, J.; Novotny, M.; Hruba, P. Developments in Immunosuppression. Curr. Opin. Organ Transplant. 2021, 26, 91–96. [Google Scholar] [CrossRef]
- Banas, B.; Krämer, B.K.; Krüger, B.; Kamar, N.; Undre, N. Long-Term Kidney Transplant Outcomes: Role of Prolonged-Release Tacrolimus. Transplant. Proc. 2020, 52, 102–110. [Google Scholar] [CrossRef]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. CJASN 2018, 13, 182–192. [Google Scholar] [CrossRef]
- Dinavahi, R.; George, A.; Tretin, A.; Akalin, E.; Ames, S.; Bromberg, J.S.; Deboccardo, G.; Dipaola, N.; Lerner, S.M.; Mehrotra, A.; et al. Antibodies Reactive to Non-HLA Antigens in Transplant Glomerulopathy. J. Am. Soc. Nephrol. JASN 2011, 22, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, P.L.; Tripathi, S.; Chandraker, A. Regulatory T Cells and Kidney Transplantation. Clin. J. Am. Soc. Nephrol. CJASN 2018, 13, 1760–1764. [Google Scholar] [CrossRef]
- Callemeyn, J.; Lamarthée, B.; Koenig, A.; Koshy, P.; Thaunat, O.; Naesens, M. Allorecognition and the Spectrum of Kidney Transplant Rejection. Kidney Int. 2022, 101, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, J.D.; Chen, C.; Schwartz, R.H. High Frequency and Nonrandom Distribution of Alloreactivity in T Cell Clones Selected for Recognition of Foreign Antigen in Association with Self Class II Molecules. J. Immunol. 1986, 136, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kyo, M.; Hatori, M.; Takahara, S.; Kyakuno, M.; Nakamura, T.; Okada, M.; Kokado, Y.; Toki, K.; Ding, X.Q.; Miki, T.; et al. Morphological Findings in Non-Episode Biopsies of Kidney Transplant Allografts Treated with FK506 or Cyclosporine. Transpl. Int. 1998, 11 (Suppl. S1), S100–S103. [Google Scholar] [CrossRef]
- Bamoulid, J.; Staeck, O.; Halleck, F.; Khadzhynov, D.; Brakemeier, S.; Dürr, M.; Budde, K. The Need for Minimization Strategies: Current Problems of Immunosuppression. Transpl. Int. 2015, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.J.; Joh, J.-W.; Kwon, C.H.D.; Jang, K.-T.; An, J.; Ki, C.-S.; Kang, E.-S.; Shin, M.; Kim, B.N.; et al. Graft-versus-Host Disease after Kidney Transplantation. J. Korean Surg. Soc. 2011, 80 (Suppl. S1), S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ding, S.; Guo, H.; Li, S.; Lu, X.; Chen, Z.; Chen, Z.K.; Ming, C.; Gong, N. Graft-versus-Host-Disease after Kidney Transplantation: A Case Report and Literature Review. Medicine 2017, 96, e7333. [Google Scholar] [CrossRef] [PubMed]
- Lechler, R.I.; Batchelor, J.R. Restoration of Immunogenicity to Passenger Cell-Depleted Kidney Allografts by the Addition of Donor Strain Dendritic Cells. J. Exp. Med. 1982, 155, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Morris, P.J.; Austyn, J.M. Migration of Dendritic Leukocytes from Cardiac Allografts into Host Spleens. A Novel Pathway for Initiation of Rejection. J. Exp. Med. 1990, 171, 307–314. [Google Scholar] [CrossRef]
- Duneton, C.; Winterberg, P.D.; Ford, M.L. Activation and Regulation of Alloreactive T Cell Immunity in Solid Organ Transplantation. Nat. Rev. Nephrol. 2022, 18, 663–676. [Google Scholar] [CrossRef]
- Oluwole, S.; Hardy, M.A.; Wang, T.; Satake, K.; Todd, G.; Reemtsma, K.; Nowygrod, R. Donor Pretreatment: Rat Heart Allograft Survival and Measurement of Passenger Leukocyte Depletion with Indium-111. Transplantation 1980, 30, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.-C.; Zhu, X.; Lin, Y.J.; Hara, H.; Ezzelarab, M.; Epperley, M.; Quader, M.A.; Cooper, D.K.C. Attempted Depletion of Passenger Leukocytes by Irradiation in Pigs. J. Transplant. 2011, 2011, 928759. [Google Scholar] [CrossRef]
- Baker, R.J.; Hernandez-Fuentes, M.P.; Brookes, P.A.; Chaudhry, A.N.; Cook, H.T.; Lechler, R.I. Loss of Direct and Maintenance of Indirect Alloresponses in Renal Allograft Recipients: Implications for the Pathogenesis of Chronic Allograft Nephropathy. J. Immunol. 2001, 167, 7199–7206. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Fan, Z.; Koulmanda, M.; Thronley, T.; Strom, T.B. Innate Immunity for Better or Worse Govern the Allograft Response. Curr. Opin. Organ Transplant. 2015, 20, 8–12. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Alexander, S.I. Rejection of the Kidney Allograft. N. Engl. J. Med. 2010, 363, 1451–1462. [Google Scholar] [CrossRef]
- Sandal, S.; Bae, S.; McAdams-DeMarco, M.; Massie, A.B.; Lentine, K.L.; Cantarovich, M.; Segev, D.L. Induction Immunosuppression Agents as Risk Factors for Incident Cardiovascular Events and Mortality after Kidney Transplantation. Am. J. Transplant. 2019, 19, 1150–1159. [Google Scholar] [CrossRef]
- Wagner, S.J.; Brennan, D.C. Induction Therapy in Renal Transplant Recipients: How Convincing Is the Current Evidence? Drugs 2012, 72, 671–683. [Google Scholar] [CrossRef]
- Bauer, A.C.; Franco, R.F.; Manfro, R.C. Immunosuppression in Kidney Transplantation: State of the Art and Current Protocols. Curr. Pharm. Des. 2020, 26, 3440–3450. [Google Scholar] [CrossRef]
- Hafeez, M.S.; Haq, M.U.; Bakhthiyar, S.S.; Azhar, K.; Awan, A.A.Y.; Ramana Murthy, B.V.; Abbas, R. Outcomes after Anti-Thymocyte Globulin vs. Basiliximab Induction before Deceased Donor Kidney Transplants. Transpl. Immunol. 2022, 75, 101733. [Google Scholar] [CrossRef]
- Muromonab-CD3. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Favi, E.; Molinari, P.; Alfieri, C.; Castellano, G.; Ferraresso, M.; Cresseri, D. Case Report: Eculizumab plus Obinutuzumab Induction in a Deceased Donor Kidney Transplant Recipient with DEAP-HUS. Front. Immunol. 2022, 13, 1073808. [Google Scholar] [CrossRef] [PubMed]
- Jarmi, T.; Abdelmoneim, Y.; Li, Z.; Jebrini, A.; Elrefaei, M. Basiliximab Is Associated with a Lower Incidence of De Novo Donor-Specific HLA Antibodies in Kidney Transplant Recipients: A Single-Center Experience. Transpl. Immunol. 2023, 77, 101778. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Hoorn, E.J.; Le, T.H.; Blijdorp, C.J.; Burger, D. Extracellular Vesicles in Kidney Diseases: Moving Forward. Kidney360 2023, 4, 245–257. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and Immunosuppression: A Review of Novel Transplant-Related Immunosuppressant Drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Wijnsma, K.L.; Duineveld, C.; Wetzels, J.F.M.; van de Kar, N.C.A.J. Eculizumab in Atypical Hemolytic Uremic Syndrome: Strategies toward Restrictive Use. Pediatr. Nephrol. 2019, 34, 2261–2277. [Google Scholar] [CrossRef]
- Siedlecki, A.M.; Isbel, N.; Vande Walle, J.; James Eggleston, J.; Cohen, D.J. Global aHUS Registry Eculizumab Use for Kidney Transplantation in Patients With a Diagnosis of Atypical Hemolytic Uremic Syndrome. Kidney Int. Rep. 2019, 4, 434–446. [Google Scholar] [CrossRef]
- Lombardi, Y.; François, H. Belatacept in Kidney Transplantation: What Are the True Benefits? A Systematic Review. Front. Med. Lausanne 2022, 9, 942665. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Tedesco-Silva, H.; Riella, L.V. Belatacept in Kidney Transplantation—Past and Future Perspectives. J. Bras. Nefrol. 2017, 39, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Ishikura, K.; Sako, M.; Ito, S.; Nozu, K.; Iijima, K. Rituximab Therapy for Refractory Steroid-Resistant Nephrotic Syndrome in Children. Pediatr. Nephrol. 2020, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Mehta, A.A. Rituximab in Kidney Disease and Transplant. Anim. Models Exp. Med. 2019, 2, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Agrawal, N.; Xue, Y.; Chanchlani, R.; Pradhan, S.; Raina, R.; Marks, S.D. Use of Rituximab in Paediatric Nephrology. Arch. Dis. Child. 2021, 106, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Boonpheng, B.; Hansrivijit, P.; Thongprayoon, C.; Mao, S.A.; Vaitla, P.K.; Bathini, T.; Choudhury, A.; Kaewput, W.; Mao, M.A.; Cheungpasitporn, W. Rituximab or Plasmapheresis for Prevention of Recurrent Focal Segmental Glomerulosclerosis after Kidney Transplantation: A Systematic Review and Meta-Analysis. World J. Transplant. 2021, 11, 303–319. [Google Scholar] [CrossRef]
- Arslansoyu Camlar, S.; Soylu, A.; Kavukçu, S. Cyclosporine in Pediatric Nephrology. Iran J. Kidney Dis. 2018, 12, 319–330. [Google Scholar]
- Farouk, S.S.; Rein, J.L. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv. Chronic Kidney Dis. 2020, 27, 56–66. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Kuca, K. Mechanism of Cyclosporine A Nephrotoxicity: Oxidative Stress, Autophagy, and Signalings. Food Chem. Toxicol. 2018, 118, 889–907. [Google Scholar] [CrossRef]
- Bentata, Y. Tacrolimus: 20 Years of Use in Adult Kidney Transplantation. What We Should Know about Its Nephrotoxicity. Artif. Organs 2020, 44, 140–152. [Google Scholar] [CrossRef]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and Toxicity—A Review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Wilk, A.; Szypulska-Koziarska, D.; Kędzierska-Kapuza, K.; Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Ciechanowski, K.; Wiszniewska, B. Effect of Long-Term Immunosuppressive Therapy on Native Rat Liver Morphology and Hepatocyte- Apoptosis. Transpl. Immunol. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Kabat-Koperska, J.; Kolasa-Wołosiuk, A.; Wojciuk, B.; Wojciechowska-Koszko, I.; Roszkowska, P.; Krasnodębska-Szponder, B.; Paczkowska, E.; Safranow, K.; Gołembiewska, E.; Machaliński, B.; et al. The Influence of Intrauterine Exposure to Immunosuppressive Treatment on Changes in the Immune System in Juvenile Wistar Rats. Drug Des. Devel. Ther. 2016, 10, 2279–2288. [Google Scholar] [CrossRef]
- Wilk, A.; Szypulska-Koziarska, D.; Kędzierska-Kapuza, K.; Sieńko, J.; Kolasa-Wołosiuk, A.; Ciechanowski, K.; Wiszniewska, B. The Comparison of Parameters of Oxidative Stress in Native Rat Livers Between Different Immunosuppressive Regimens. Med. Sci. Monit. 2019, 25, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, K.; Sindrewicz, K.; Sporniak-Tutak, K.; Bober, J.; Stańczyk-Dunaj, M.; Dołęgowska, B.; Kaliszczak, R.; Sieńko, J.; Kabat-Koperska, J.; Gołembiewska, E.; et al. Effect of Immunosuppressive Therapy on Proteinogram in Rats. Med. Sci. Monit. 2016, 22, 1987–1998. [Google Scholar] [CrossRef]
- Ha, E.; Mun, K.C. Effects of Cyclosporine on Metalloproteinase in Endothelial Cells. Transplant. Proc. 2012, 44, 991–992. [Google Scholar] [CrossRef]
- Waller, J.R.; Brook, N.R.; Bicknell, G.R.; Nicholson, M.L. Differential Effects of Modern Immunosuppressive Agents on the Development of Intimal Hyperplasia. Transpl. Int. 2004, 17, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Surówka, A.; Szumilas, K.; Wilk, A.; Misiakiewicz-Has, K.; Ciechanowski, K.; Kędzierska-Kapuza, K. The Effect of Chronic Immunosuppressive Regimens Treatment on Aortal Media Morphology and the Balance between Matrix Metalloproteinases (Mmp-2 and Mmp-9) and Their Inhibitors in the Abdominal Aorta of Rats. Int. J. Environ. Res. Public. Health 2022, 19, 6399. [Google Scholar] [CrossRef]
- Kajiwara, M.; Masuda, S. Role of MTOR Inhibitors in Kidney Disease. Int. J. Mol. Sci. 2016, 17, 975. [Google Scholar] [CrossRef]
- Saran, U.; Foti, M.; Dufour, J.F. Cellular and Molecular Effects of the MTOR Inhibitor Everolimus. Clin. Sci. 2015, 129, 895–914. [Google Scholar] [CrossRef]
- Flechner, S.M. MTOR Inhibition and Clinical Transplantation: Kidney. Transplantation 2018, 102, S17–S18. [Google Scholar] [CrossRef] [PubMed]
- Tedesco-Silva, H.; Del Carmen Rial, M.; Cruz Santiago, J.; Mazzali, M.; Pacheco-Silva, A.; Torres, R. Optimizing the Clinical Utility of Sirolimus-Based Immunosuppression for Kidney Transplantation. Clin. Transplant. 2019, 33, e13464. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.S.; Joo, Y.H. Rapamycin Increases the Incidence of Neuropsychiatric Illness in Kidney Transplant Patients through the Suppression of Neural Stem Cells. Transl. Psychiatry 2020, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Kerns, K.A.; Ouellette, A.; Robinson, L.; Morris, H.D.; Kaczorowski, C.; Park, S.-I.; Mekvanich, T.; Kang, A.; McLean, J.S.; et al. Rapamycin Rejuvenates Oral Health in Aging Mice. eLife 2020, 9, e54318. [Google Scholar] [CrossRef]
- Mohammadi, O.; Kassim, T.A. Azathioprine; StatPearls: Treasure Land, FL, USA, 2022. [Google Scholar]
- Chandra, A.; Midtvedt, K.; Åsberg, A.; Eide, I.A. Immunosuppression and Reproductive Health After Kidney Transplantation. Transplantation 2019, 103, e325–e333. [Google Scholar] [CrossRef]
- Yung, S.; Zhang, Q.; Chau, M.K.M.; Chan, T.M. Distinct Effects of Mycophenolate Mofetil and Cyclophosphamide on Renal Fibrosis in NZBWF1/J Mice. Autoimmunity 2015, 48, 471–487. [Google Scholar] [CrossRef]
- Schwarze, M.L.; Houser, S.L.; Muniappan, A.; Allan, J.S.; Menard, M.T.; McMorrow, I.; Maloney, M.E.; Madsen, J.C. Effects of Mycophenolate Mofetil on Cardiac Allograft Survival and Cardiac Allograft Vasculopathy in Miniature Swine. Ann. Thorac. Surg. 2005, 80, 1787–1793. [Google Scholar] [CrossRef]
- Rathinam, S.R.; Gonzales, J.A.; Thundikandy, R.; Kanakath, A.; Murugan, S.B.; Vedhanayaki, R.; Lim, L.L.; Suhler, E.B.; Al-Dhibi, H.A.; Doan, T.; et al. Effect of Corticosteroid-Sparing Treatment With Mycophenolate Mofetil vs Methotrexate on Inflammation in Patients With Uveitis: A Randomized Clinical Trial. JAMA 2019, 322, 936–945. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, Complications, and Practice Delivery Issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- Friedman, E.A. “Early” Withdrawal of Glucocorticosteroids Is Well Tolerated by Kidney Transplant Recipients Without Increasing Allograft Rejection While Preserving Bone Integrity. Transplantation 2014, 98, 1255–1256. [Google Scholar] [CrossRef]
- Wiseman, A.C. Immunosuppressive Medications. Clin. J. Am. Soc. Nephrol. 2016, 11, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Huang, J. Effects of glycyrrihizic acid and prednisone on pathological and ultrastructural changes of kidney in rats with chronic aristolochic acid nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007, 27, 45–49. [Google Scholar] [PubMed]
- Guglieri, M.; Bushby, K.; McDermott, M.P.; Hart, K.A.; Tawil, R.; Martens, W.B.; Herr, B.E.; McColl, E.; Speed, C.; Wilkinson, J.; et al. Effect of Different Corticosteroid Dosing Regimens on Clinical Outcomes in Boys With Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA 2022, 327, 1456. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.; Kamar, N.; Agarwal, A.; de Fijter, J.; Rostaing, L.; Berger, S.; Djamali, A.; et al. Conversion from Calcineurin Inhibitor to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021, 32, 3252–3264. [Google Scholar] [CrossRef]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A Phase III Study of Belatacept-Based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients (BENEFIT Study). Am. J. Transpl. 2010, 10, 535–546. [Google Scholar] [CrossRef]
- Durrbach, A.; Pestana, J.M.; Pearson, T.; Vincenti, F.; Garcia, V.D.; Campistol, J.; Rial Mdel, C.; Florman, S.; Block, A.; Di Russo, G.; et al. A Phase III Study of Belatacept versus Cyclosporine in Kidney Transplants from Extended Criteria Donors (BENEFIT-EXT Study). Am. J. Transpl. 2010, 10, 547–557. [Google Scholar] [CrossRef]
- Bertrand, D.; Chavarot, N.; Gatault, P.; Garrouste, C.; Bouvier, N.; Grall-Jezequel, A.; Jaureguy, M.; Caillard, S.; Lemoine, M.; Colosio, C.; et al. Opportunistic Infections after Conversion to Belatacept in Kidney Transplantation. Nephrol. Dial. Transpl. 2020, 35, 336–345. [Google Scholar] [CrossRef]
- Yazdi, M.; Kahwaji, J.M.; Meguerditchian, S.; Lee, R. Belatacept Conversion Protocols and Outcomes in Kidney Transplant Recipients. Transpl. Proc. 2021, 53, 976–983. [Google Scholar] [CrossRef]
- Vincenti, F.; Larsen, C.P.; Alberu, J.; Bresnahan, B.; Garcia, V.D.; Kothari, J.; Lang, P.; Urrea, E.M.; Massari, P.; Mondragon-Ramirez, G.; et al. Three-Year Outcomes from BENEFIT, a Randomized, Active-Controlled, Parallel-Group Study in Adult Kidney Transplant Recipients. Am. J. Transplant. Off. 2012, 12, 210–217. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.-C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343. [Google Scholar] [CrossRef]
- Kim, I.; Wu, G.; Chai, N.N.; Klein, A.S.; Jordan, S.C. Immunological Characterization of de Novo and Recall Alloantibody Suppression by CTLA4Ig in a Mouse Model of Allosensitization. Transpl. Immunol. 2016, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kumbala, D.; Zhang, R. Essential Concept of Transplant Immunology for Clinical Practice. World J. Transpl. 2013, 3, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Becker, T.; Arns, W.; Sommerer, C.; Reinke, P.; Eisenberger, U.; Kramer, S.; Fischer, W.; Gschaidmeier, H.; Pietruck, F. Everolimus-Based, Calcineurin-Inhibitor-Free Regimen in Recipients of de-Novo Kidney Transplants: An Open-Label, Randomised, Controlled Trial. Lancet 2011, 377, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Claes, K.; Meier-Kriesche, H.U.; Schold, J.D.; Vanrenterghem, Y.; Halloran, P.F.; Ekberg, H. Effect of Different Immunosuppressive Regimens on the Evolution of Distinct Metabolic Parameters: Evidence from the Symphony Study. Nephrol. Dial. Transpl. 2012, 27, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Holdaas, H.; Rostaing, L.; Serón, D.; Cole, E.; Chapman, J.; Fellstrøm, B.; Strom, E.H.; Jardine, A.; Midtvedt, K.; Machein, U.; et al. Conversion of Long-Term Kidney Transplant Recipients from Calcineurin Inhibitor Therapy to Everolimus: A Randomized, Multicenter, 24-Month Study. Transplantation 2011, 92, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhong, Q.; Feng, X.; Li, L.; Feng, S.; Fan, Y.; Song, T.; Huang, Z.; Wang, X.; Lin, T. Conversion From Calcineurin Inhibitors to Mammalian Target of Rapamycin Inhibitors in Kidney Transplant Recipients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Immunol. 2021, 12, 663602. [Google Scholar] [CrossRef]
- Reggiani, F.; Moroni, G.; Ponticelli, C. Cardiovascular Risk after Kidney Transplantation: Causes and Current Approaches to a Relevant Burden. J. Pers. Med. 2022, 12, 1200. [Google Scholar] [CrossRef]
- Al-Adra, D.; Al-Qaoud, T.; Fowler, K.; Wong, G. De Novo Malignancies after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. CJASN 2022, 17, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ison, M.G.; Danziger-Isakov, L. Long-Term Infectious Complications of Kidney Transplantation. Clin. J. Am. Soc. Nephrol. CJASN 2022, 17, 286–295. [Google Scholar] [CrossRef]
- Gupta, K.L.; Bagai, S.; Ramachandran, R.; Kumar, V.; Rathi, M.; Kohli, H.S.; Sharma, A.; Chakrabarti, A. Fungal Infection in Post-Renal Transplant Patient: Single-Center Experience. Indian J. Pathol. Microbiol. 2020, 63, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Loupy, A.; Segev, D.L. Antibody-Mediated Rejection: New Approaches in Prevention and Management. Am. J. Transpl. 2018, 18 (Suppl. S3), 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Tatapudi, V.S.; Weldon, E.P.; Min, E.S.; Ali, N.M.; Deterville, C.L.; Gelb, B.E.; Benstein, J.A.; Dagher, N.N.; Wu, M.; et al. IdeS (Imlifidase): A Novel Agent That Cleaves Human IgG and Permits Successful Kidney Transplantation Across High-Strength Donor-Specific Antibody. Ann. Surg. 2018, 268, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Tedesco Silva, H.; Busque, S.; O’Connell, P.; Friedewald, J.; Cibrik, D.; Budde, K.; Yoshida, A.; Cohney, S.; Weimar, W.; et al. Randomized Phase 2b Trial of Tofacitinib (CP-690,550) in de Novo Kidney Transplant Patients: Efficacy, Renal Function and Safety at 1 Year. Am. J. Transpl. 2012, 12, 2446–2456. [Google Scholar] [CrossRef]
- Shah, N.; Meouchy, J.; Qazi, Y. Bortezomib in Kidney Transplantation. Curr. Opin. Organ Transpl. 2015, 20, 652–656. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Castelli, R.; Cannavò, A.; Conforti, F.; Grava, G.; Cortelezzi, A. Immunomodulatory Drugs in Multiple Myeloma: From Molecular Mechanisms of Action to Clinical Practice. Immunopharmacol. Immunotoxicol. 2012, 34, 740–753. [Google Scholar] [CrossRef]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Taddeo, A.; Prim, D.; Bojescu, E.-D.; Segura, J.-M.; Pfeifer, M.E. Point-of-Care Therapeutic Drug Monitoring for Precision Dosing of Immunosuppressive Drugs. J. Appl. Lab. Med. 2020, 5, 738–761. [Google Scholar] [CrossRef]
- Johnston, A.; Holt, D.W. Therapeutic Drug Monitoring of Immunosuppressant Drugs. Br. J. Clin. Pharmacol. 1999, 47, 339–350. [Google Scholar] [CrossRef]
- Hougardy, J.-M.; Maufort, L.; Cotton, F.; Coussement, J.; Mikhalski, D.; Wissing, K.M.; Le Moine, A.; Broeders, N.; Abramowicz, D. Therapeutic Drug Monitoring of Enteric-Coated Mycophenolate Sodium by Limited Sampling Strategies Is Associated with a High Rate of Failure. Clin. Kidney J. 2016, 9, 319–323. [Google Scholar] [CrossRef]
- Mahalati, K.; Kahan, B.D. Clinical Pharmacokinetics of Sirolimus. Clin. Pharmacokinet. 2001, 40, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Zwart, T.C.; Gokoel, S.R.M.; van der Boog, P.J.M.; de Fijter, J.W.; Kweekel, D.M.; Swen, J.J.; Guchelaar, H.-J.; Moes, D.J.A.R. Therapeutic Drug Monitoring of Tacrolimus and Mycophenolic Acid in Outpatient Renal Transplant Recipients Using a Volumetric Dried Blood Spot Sampling Device. Br. J. Clin. Pharmacol. 2018, 84, 2889–2902. [Google Scholar] [CrossRef]
- Uhl, P.; Heilos, A.; Bond, G.; Meyer, E.; Böhm, M.; Puchhammer-Stöckl, E.; Arbeiter, K.; Müller-Sacherer, T.; Csaicsich, D.; Aufricht, C.; et al. Torque Teno Viral Load Reflects Immunosuppression in Paediatric Kidney-Transplanted Patients-a Pilot Study. Pediatr. Nephrol. 2021, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M. Torque Teno Virus Load as a Surrogate Marker for the Net State of Immunosuppression: The Beneficial Side of the Virome. Am. J. Transplant. 2020, 20, 1963–1964. [Google Scholar] [CrossRef]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Gröne, H.-J.; Friede, T.; et al. Absolute Quantification of Donor-Derived Cell-Free DNA as a Marker of Rejection and Graft Injury in Kidney Transplantation: Results from a Prospective Observational Study. Am. J. Transplant. 2019, 19, 3087–3099. [Google Scholar] [CrossRef]
- Argani, H. New Markers for Transplant Rejection. Exp. Clin. Transplant. 2020, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.; Sachs, D.H. Progress in Xenotransplantation: Overcoming Immune Barriers. Nat. Rev. Nephrol. 2022, 18, 745–761. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumilas, K.; Wilk, A.; Wiśniewski, P.; Gimpel, A.; Dziedziejko, V.; Kipp, M.; Pawlik, A. Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. Int. J. Mol. Sci. 2023, 24, 10301. https://doi.org/10.3390/ijms241210301
Szumilas K, Wilk A, Wiśniewski P, Gimpel A, Dziedziejko V, Kipp M, Pawlik A. Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. International Journal of Molecular Sciences. 2023; 24(12):10301. https://doi.org/10.3390/ijms241210301
Chicago/Turabian StyleSzumilas, Kamila, Aleksandra Wilk, Piotr Wiśniewski, Anna Gimpel, Violetta Dziedziejko, Markus Kipp, and Andrzej Pawlik. 2023. "Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation" International Journal of Molecular Sciences 24, no. 12: 10301. https://doi.org/10.3390/ijms241210301
APA StyleSzumilas, K., Wilk, A., Wiśniewski, P., Gimpel, A., Dziedziejko, V., Kipp, M., & Pawlik, A. (2023). Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. International Journal of Molecular Sciences, 24(12), 10301. https://doi.org/10.3390/ijms241210301






