A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase
Abstract
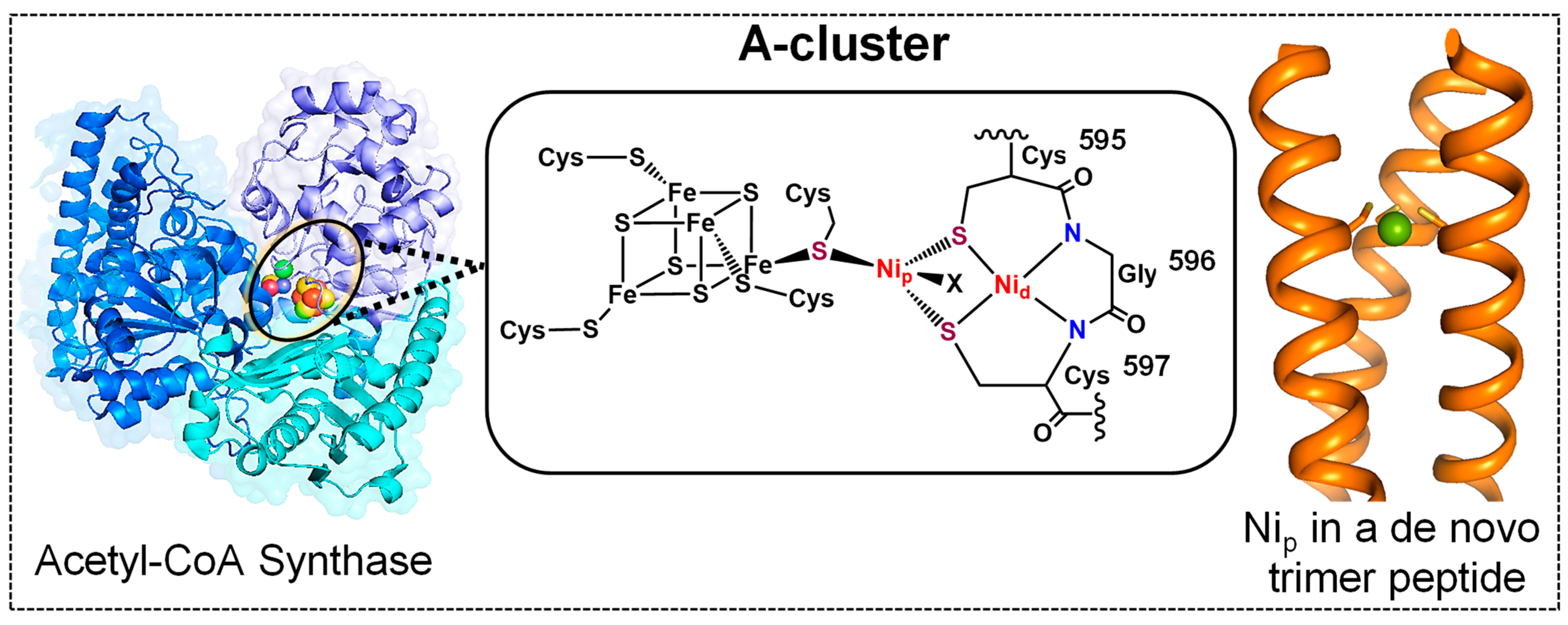
1. Introduction
2. Results and Discussion
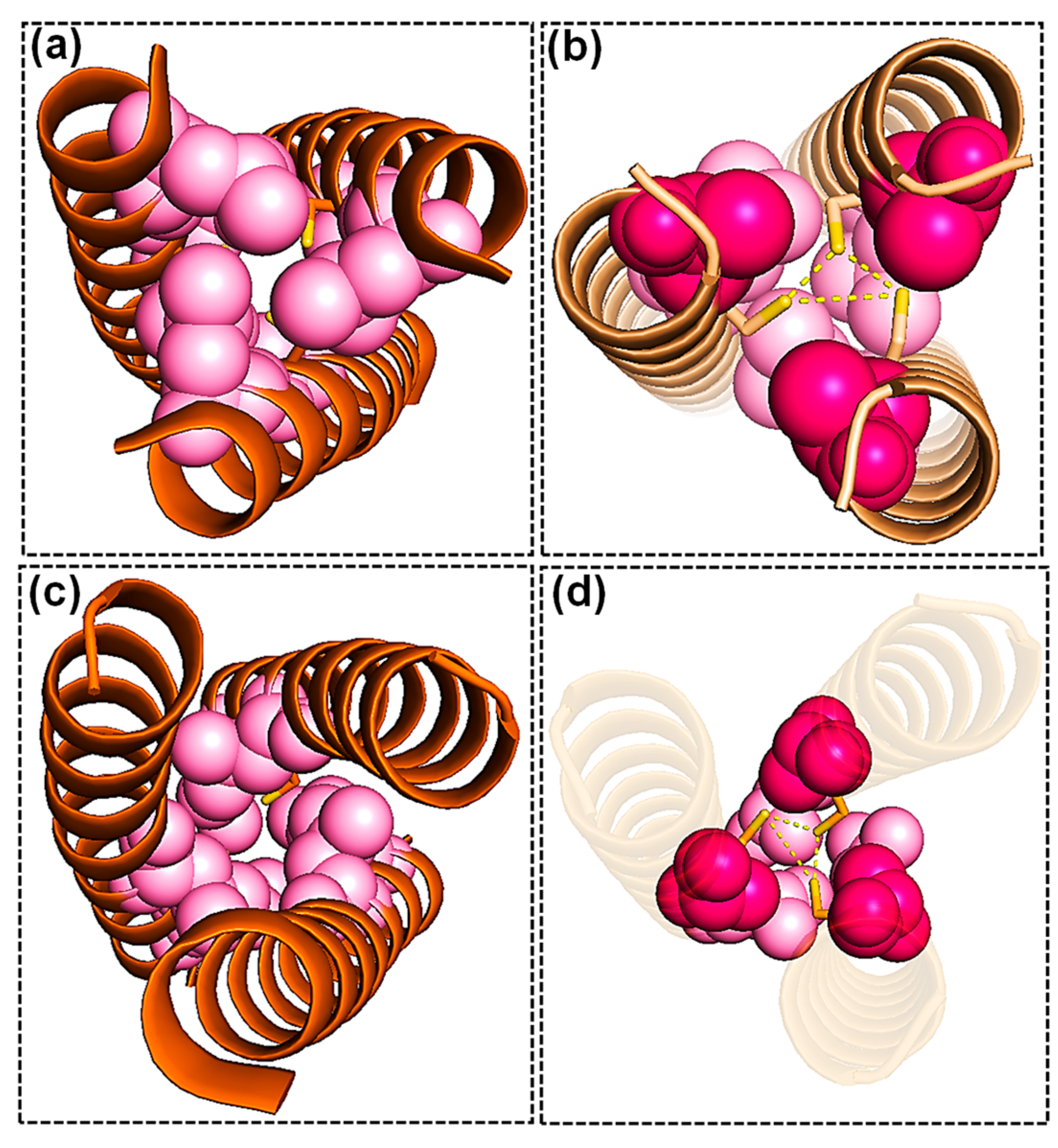
2.1. Scaffold Choice to Host the Ni(Cys)3 Site
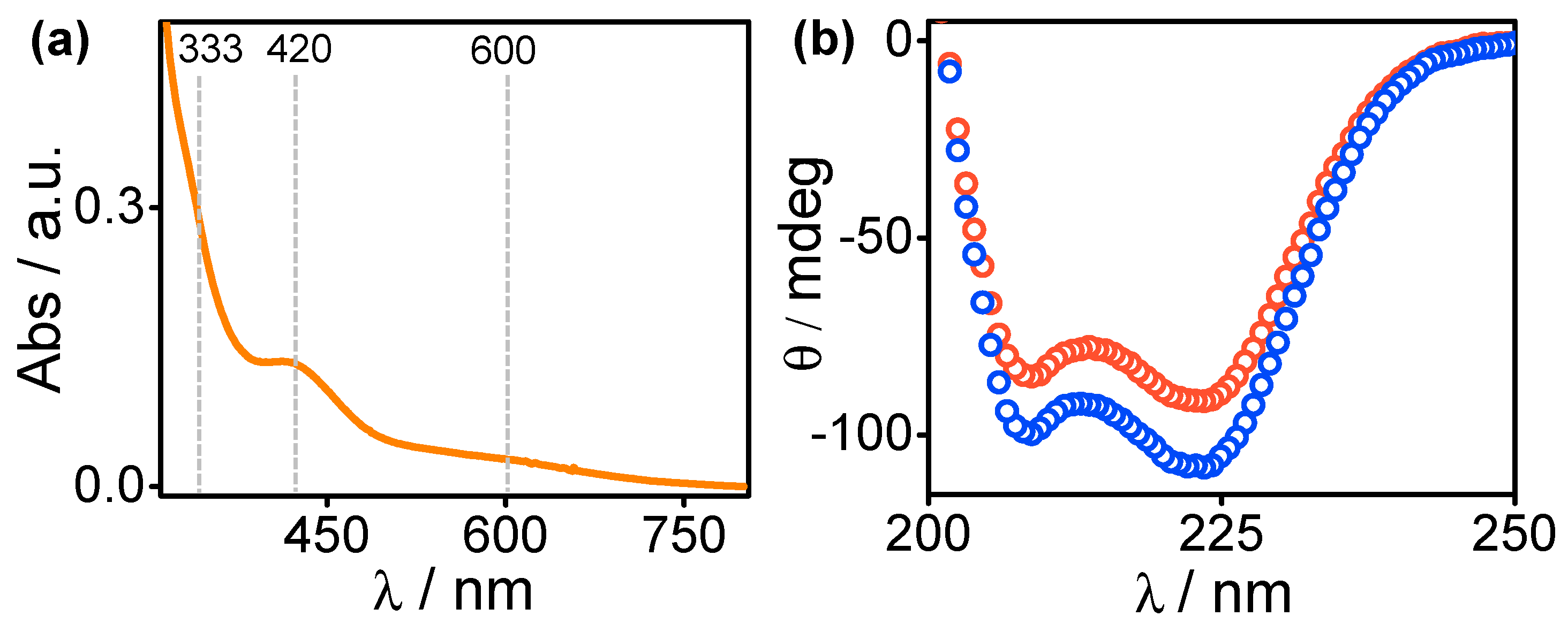
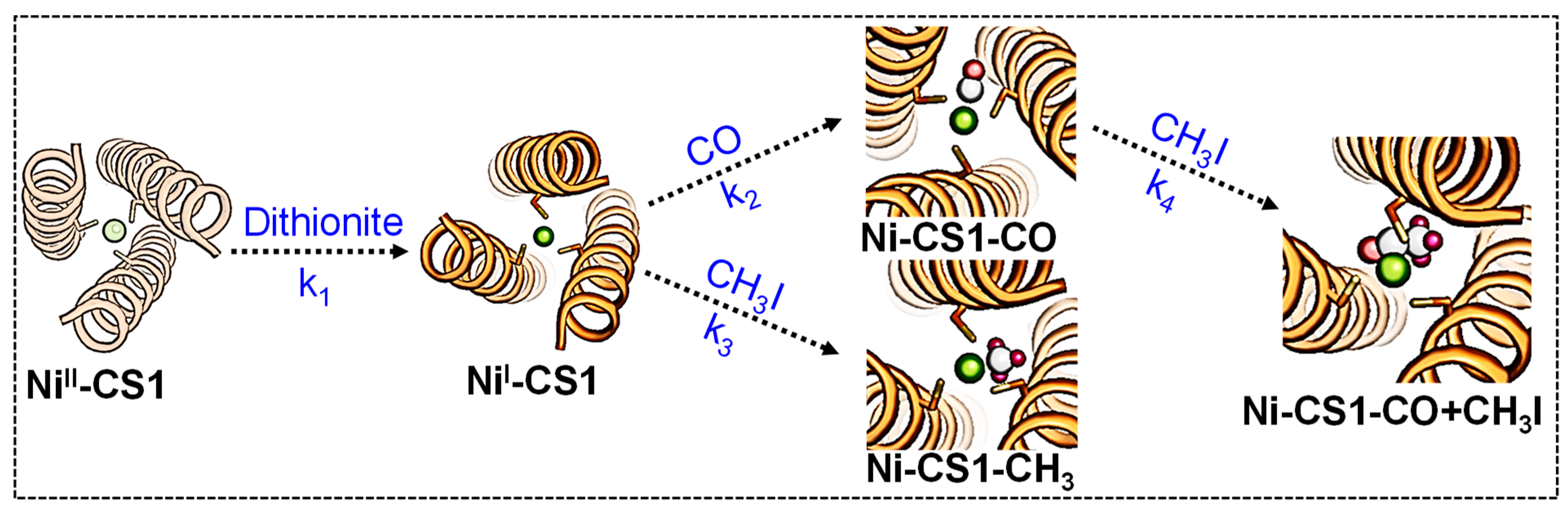
2.2. Ni Binding
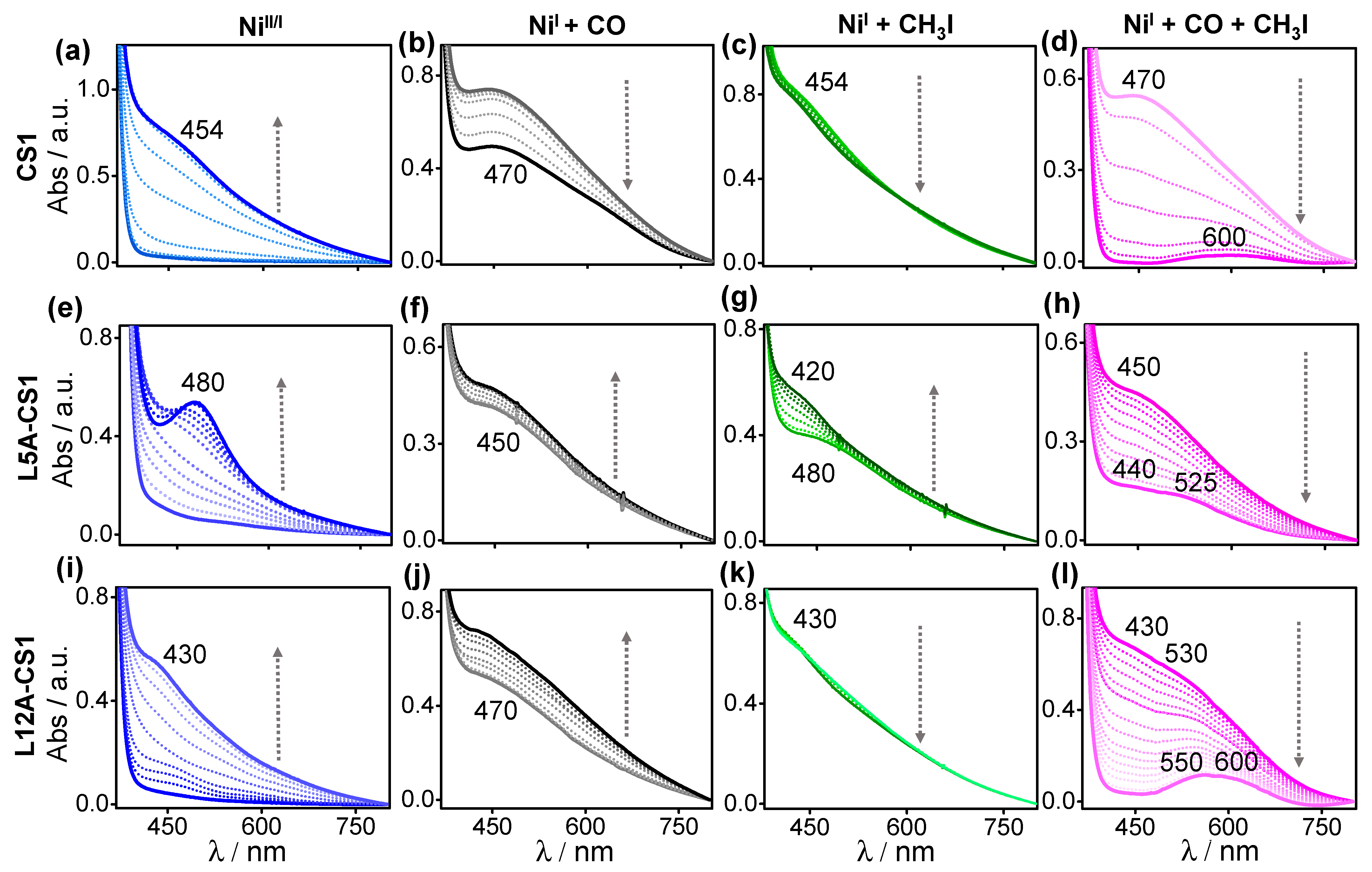
2.3. Characteristics of Ligand-Bound States
2.4. The Effect of Outer Sphere Steric on Ligand Binding
3. Materials and Methods
3.1. Experimental Procedures
3.2. General Procedures
3.3. Peptide Synthesis
3.4. Mass Spectrometry
3.5. DTNB Assay
3.6. Sample Preparations
3.7. UV-Vis Spectroscopy
3.8. CD Spectroscopy
3.9. FTIR Spectroscopy
3.10. EPR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Can, M.; Armstrong, F.A.; Ragsdale, S.W. Structure, Function, and Mechanism of the Nickel Metalloenzymes, CO Dehydrogenase, and Acetyl-CoA Synthase. Chem. Rev. 2014, 114, 4149–4174. [Google Scholar] [CrossRef]
- Svetlitchnyi, V.; Dobbek, H.; Meyer-Klaucke, W.; Meins, T.; Thiele, B.; Römer, P.; Huber, R.; Meyer, O. A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc. Natl. Acad. Sci. USA 2004, 101, 446–451. [Google Scholar] [CrossRef]
- Doukov, T.I.; Iverson, T.M.; Seravalli, J.; Ragsdale, S.W.; Drennan, C.L. A Ni-Fe-Cu Center in a Bifunctional Carbon Monoxide Dehydrogenase/ Acetyl-CoA Synthase. Science 2002, 298, 567–572. [Google Scholar] [CrossRef]
- Darnault, C.; Volbeda, A.; Kim, E.J.; Legrand, P.; Vernède, X.; Lindahl, P.A.; Fontecilla-Camps, J.C. Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open α subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase. Nat. Struct. Mol. Biol. 2003, 10, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Linck, R.C.; Spahn, C.W.; Rauchfuss, T.B.; Wilson, S.R. Structural Analogues of the Bimetallic Reaction Center in Acetyl CoA Synthase: A Ni−Ni Model with Bound CO. J. Am. Chem. Soc. 2003, 125, 8700–8701. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Bhaduri, S.; Jiang, J.; Holm, R.H. Sulfur bridging interactions of cis-planar NII-S2N2 coordination units with nickel(II), copper(I,II), zinc(II), and mercury(II): A library of bridging modes, including NII(m2-SR)2MI,II rhombs. Inorg. Chem. 2004, 43, 5833–5849. [Google Scholar] [CrossRef] [PubMed]
- Harrop, T.C.; Olmstead, M.M.; Mascharak, P.K. Structural Models of the Bimetallic Subunit at the A-Cluster of Acetyl Coenzyme A Synthase/CO Dehydrogenase: Binuclear Sulfur-Bridged Ni−Cu and Ni−Ni Complexes and Their Reactions with CO. J. Am. Chem. Soc. 2004, 126, 14714–14715. [Google Scholar] [CrossRef]
- Harrop, T.C.; Olmstead, M.M.; Mascharak, P.K. Binding of CO to structural models of the bimetallic subunit at the A-cluster of acetyl coenzyme A synthase/CO dehydrogenase. Chem. Commun. 2004, 10, 1744–1745. [Google Scholar] [CrossRef]
- Ito, M.; Kotera, M.; Matsumoto, T.; Tatsumi, K. Dinuclear nickel complexes modeling the structure and function of the acetyl CoA synthase active site. Proc. Natl. Acad. Sci. USA 2009, 106, 11862–11866. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ito, M.; Kotera, M.; Tatsumi, K. A dinuclear nickel complex modeling of the Nid(II)-Nip(I) state of the active site of acetyl CoA synthase. Dalton Trans. 2010, 39, 2995–2997. [Google Scholar] [CrossRef]
- Dougherty, W.G.; Rangan, K.; O’Hagan, M.J.; Yap, G.P.A.; Riordan, C.G. Binuclear Complexes Containing a Methylnickel Moiety: Relevance to Organonickel Intermediates in Acetyl Coenzyme A Synthase Catalysis. J. Am. Chem. Soc. 2008, 130, 13510–13511. [Google Scholar] [CrossRef]
- Krishnan, R.; Riordan, C.G. Cys-Gly-Cys Tripeptide Complexes of Nickel: Binuclear Analogues for the Catalytic Site in Acetyl Coenzyme A Synthase. J. Am. Chem. Soc. 2004, 126, 4484–4485. [Google Scholar] [CrossRef]
- Laplaza, C.E.; Holm, R.H. Helix−Loop−Helix Peptides as Scaffolds for the Construction of Bridged Metal Assemblies in Proteins: The Spectroscopic A-Cluster Structure in Carbon Monoxide Dehydrogenase. J. Am. Chem. Soc. 2001, 123, 10255–10264. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, K.B.; Laplaza, C.E.; Holm, R.H.; Hedman, B.; Hodgson, K.O. Structural Characterization of Metallopeptides Designed as Scaffolds for the Stabilization of Nickel(II)-Fe4S4 Bridged Assemblies by X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2002, 124, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Manesis, A.C.; Shafaat, H.S. Electrochemical, Spectroscopic, and Density Functional Theory Characterization of Redox Activity in Nickel-Substituted Azurin: A Model for Acetyl-CoA Synthase. Inorg. Chem. 2015, 54, 7959–7967. [Google Scholar] [CrossRef] [PubMed]
- Manesis, A.C.; O’Connor, M.J.; Schneider, C.R.; Shafaat, H.S. Multielectron Chemistry within a Model Nickel Metalloprotein: Mechanistic Implications for Acetyl-CoA Synthase. J. Am. Chem. Soc. 2017, 139, 10328–10338. [Google Scholar] [CrossRef]
- Manesis, A.C.; Musselman, B.W.; Keegan, B.C.; Shearer, J.; Lehnert, N.; Shafaat, H.S. A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase. Inorg. Chem. 2019, 58, 8969–8982. [Google Scholar] [CrossRef] [PubMed]
- Koebke, K.J.; Pinter, T.B.; Pitts, W.C.; Pecoraro, V.L. Catalysis and Electron Transfer in De Novo Designed Metalloproteins. Chem. Rev. 2022, 122, 12046–12109. [Google Scholar] [CrossRef]
- Chakraborty, S.; Touw, D.S.; Peacock, A.F.A.; Stuckey, J.; Pecoraro, V.L. Structural Comparisons of Apo- and Metalated Three-Stranded Coiled Coils Clarify Metal Binding Determinants in Thiolate Containing Designed Peptides. J. Am. Chem. Soc. 2010, 132, 13240–13250. [Google Scholar] [CrossRef]
- Touw, D.S.; Nordman, C.E.; Stuckey, J.A.; Pecoraro, V.L. Identifying important structural characteristics of arsenic resistance proteins by using designed three-stranded coiled coils. Proc. Natl. Acad. Sci. USA 2007, 104, 11969–11974. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, D.N. The Design of Coiled-Coil Structures and Assemblies. In Advances in Protein Chemistry; Elsevier Academic Press: Cambridge, MA, USA, 2005; Volume 70, pp. 79–112. [Google Scholar]
- Lu, Y.; Chakraborty, S.; Miner, K.D.; Wilson, T.D.; Mukherjee, A.; Yu, Y.; Liu, J.; Marshall, N.M. 3.19—Metalloprotein Design. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 565–593. [Google Scholar]
- Malayam Parambath, S.; Williams, A.E.; Hunt, L.A.; Selvan, D.; Hammer, N.I.; Chakraborty, S. A De Novo-Designed Artificial Metallopeptide Hydrogenase: Insights into Photochemical Processes and the Role of Protonated Cys. ChemSusChem 2021, 14, 2237–2246. [Google Scholar] [CrossRef]
- Selvan, D.; Prasad, P.; Farquhar, E.R.; Shi, Y.; Crane, S.; Zhang, Y.; Chakraborty, S. Redesign of a Copper Storage Protein into an Artificial Hydrogenase. ACS Catal. 2019, 9, 5847–5859. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Seravalli, J.; Gutzman, H.; Swartz, D.J.; Ragsdale, S.W.; Bagley, K.A. Infrared studies of carbon monoxide binding to carbon monoxide dehydrogenase/acetyl-CoA synthase from Moorella thermoacetica. Biochemistry 2003, 42, 14822–14830. [Google Scholar] [CrossRef]
- Hirsch, J.; DeBeer, G.S.; Solomon, E.I.; Hedman, B.; Hodgson, K.O.; Burstyn, J.N. Raman and extended x-ray absorption fine structure characterization of a sulfur-ligated Cu (I) ethylene complex: Modeling the proposed ethylene binding site of Arabidopsis thaliana ETR1. Inorg. Chem. 2001, 40, 2439–2441. [Google Scholar] [CrossRef] [PubMed]
- Schebler, P.J.; Mandimutsira, B.S.; Riordan, C.G.; Liable-Sands, L.M.; Incarvito, C.D.; Rheingold, A.L. Organometallic Cobalt (II) and Nickel (II) complexes supported by thioether ligation: Unexpected nickel alkylation by the borato ligand phenyltris ((tert-butylthio) methyl) borate. J. Am. Chem. Soc. 2001, 123, 331–332. [Google Scholar] [CrossRef]
- Craft, J.L.; Mandimutsira, B.S.; Fujita, K.; Riordan, C.G.; Brunold, T.C. Spectroscopic and Computational Studies of a Ni+−CO Model Complex: Implications for the Acetyl-CoA Synthase Catalytic Mechanism. Inorg. Chem. 2003, 42, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Bender, G.; Stich, T.A.; Yan, L.; Britt, R.D.; Cramer, S.P.; Ragsdale, S.W. Infrared and EPR Spectroscopic Characterization of a Ni(I) Species Formed by Photolysis of a Catalytically Competent Ni(I)-CO Intermediate in the Acetyl-CoA Synthase Reaction. Biochemistry 2010, 49, 7516–7523. [Google Scholar] [CrossRef]
- Wood, C.W.; Woolfson, D.N. CC Builder 2.0: Powerful and accessible coiled-coil modeling. Protein Sci. 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Prasad, P.; Hunt, L.A.; Pall, A.E.; Ranasinghe, M.; Williams, A.E.; Stemmler, T.L.; Demeler, B.; Hammer, N.I.; Chakraborty, S. Photocatalytic Hydrogen Evolution by a De Novo Designed Metalloprotein that Undergoes Ni-Mediated Oligomerization Shift. Chem. Eur. J. 2023, 29, e202202902. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |



| k1 (s−1) | k2 (s−1) | k3 (s−1) | k4 (s−1) | |
|---|---|---|---|---|
| CS1 | 3.65 × 10−4 | 2.74 × 10−4 | 6.81 × 10−5 | 1.10 × 10−3 |
| L5A-CS1 | 1.28 × 10−3 | 5.10 × 10−4 | 9.86 × 10−5 | 5.22 × 10−4 |
| L12A-CS1 | 1.22 × 10−3 | 3.55 × 10−4 | 9.16 × 10−5 | 4.69 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvan, D.; Chakraborty, S. A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase. Int. J. Mol. Sci. 2023, 24, 10317. https://doi.org/10.3390/ijms241210317
Selvan D, Chakraborty S. A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase. International Journal of Molecular Sciences. 2023; 24(12):10317. https://doi.org/10.3390/ijms241210317
Chicago/Turabian StyleSelvan, Dhanashree, and Saumen Chakraborty. 2023. "A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase" International Journal of Molecular Sciences 24, no. 12: 10317. https://doi.org/10.3390/ijms241210317
APA StyleSelvan, D., & Chakraborty, S. (2023). A De Novo Designed Trimeric Metalloprotein as a Nip Model of the Acetyl-CoA Synthase. International Journal of Molecular Sciences, 24(12), 10317. https://doi.org/10.3390/ijms241210317








