Harnessing the Potential of Non-Apoptotic Cell Death Processes in the Treatment of Drug-Resistant Melanoma
Abstract
1. Introduction
2. Non-Apoptotic Mechanisms in Melanoma
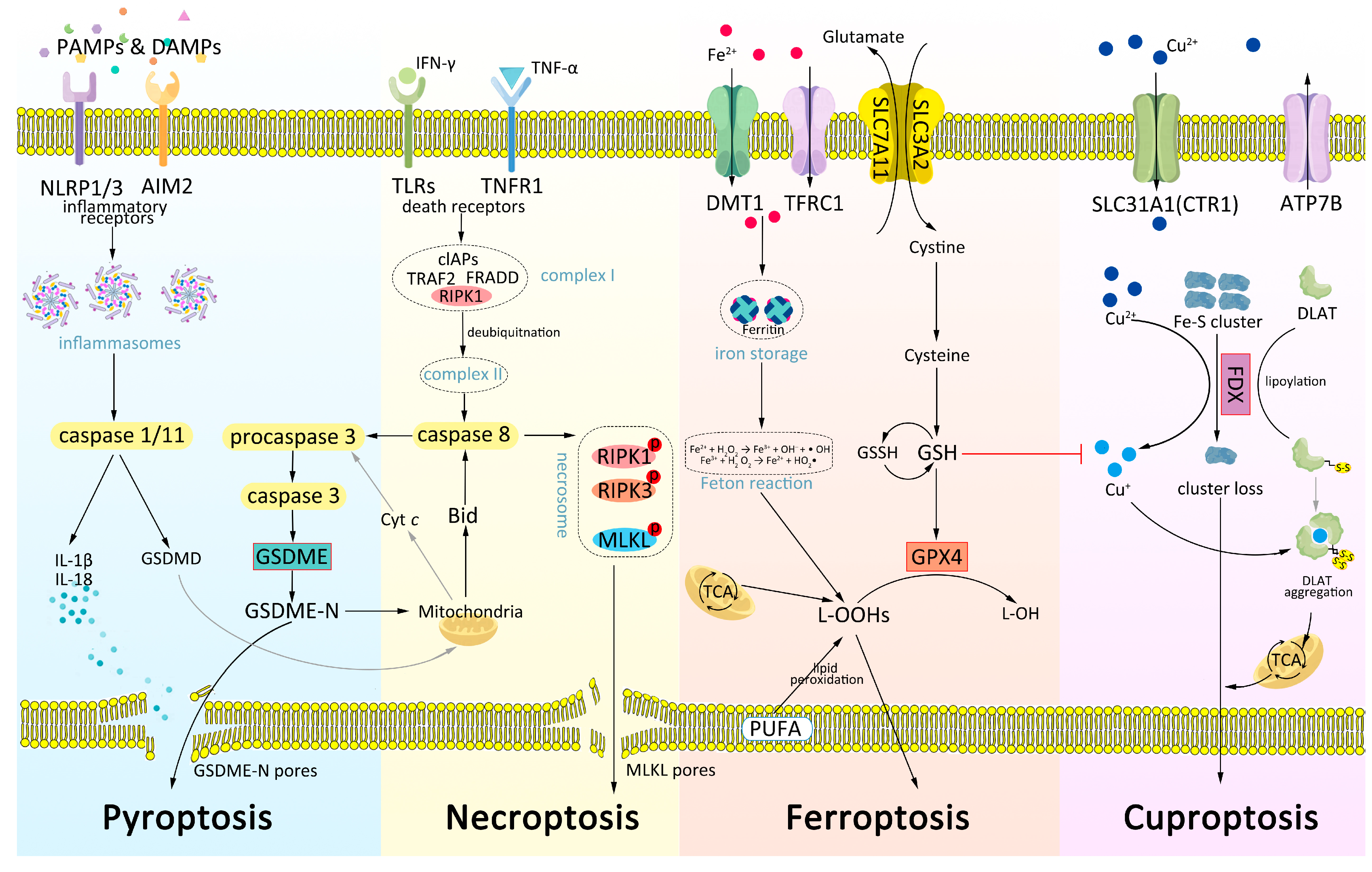
2.1. Pyroptosis
2.2. Necroptosis
2.3. Ferroptosis
2.4. Cuproptosis
2.5. Common Mechanisms among Pyroptosis, Ferroptosis, Necroptosis, and Cuproptosis
2.6. Crosstalks with Apoptosis and Autophagy
3. Interactions in the Tumor Microenvironment (TME)
3.1. Pyroptosis Strongly Promotes Maturation of DCs and Infiltration of T Cells
3.2. The Intricate Interplay between Ferroptosis and T Cells
3.3. Necroptosis Triggers Inflammatory Response
4. Addressing Drug-Resistant Melanoma by Targeting Non-Apoptotic Processes
4.1. Using Single Agents with High Anti-Tumor Capacity to Target Non-Apoptotic Processes in Cancer Cells
4.2. Activating Potent Response and Causing Immunogenic Cell Death
4.3. Synergizing with Other Therapies to Enhance Treatment Efficacy
4.4. Sensitizing Resistant Cells to Existing Therapy
4.5. Delaying the Development of Drug Resistance
4.6. Targeting Cancer Stem Cells
4.7. Building Prognosis Models to Predict Drug Efficiency and Clinical Outcomes
5. Future Perspectives
5.1. Considering Multiple Forms of Cell Death as a Whole
5.2. The Mechanisms of Non-Apoptotic Cell Death in Melanoma Need to Be Further Clarified
5.3. The Mechanisms of More Forms of Cell Death Are Waiting to Be Explored
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Wang, Y.; Wang, L.; Yin, P.; Lin, Y.; Zhou, M. Burden of melanoma in China, 1990–2017: Findings from the 2017 global burden of disease study. Int. J. Cancer 2020, 147, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Trager, M.H.; Queen, D.; Samie, F.H.; Carvajal, R.D.; Bickers, D.R.; Geskin, L.J. Advances in Prevention and Surveillance of Cutaneous Malignancies. Am. J. Med. 2020, 133, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prevention, Rates of New Melanomas–Deadly Skin Cancers–Have Doubled over Last Three Decades. Available online: cdc.gov/media/releases/2015 (accessed on 17 May 2023).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 17 May 2023).
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Yan, G.; Elbadawi, M.A.; Efferth, T. Multiple cell death modalities and their key features (Review). World Acad. Sci. J. 2020, 2, 39–48. [Google Scholar] [CrossRef]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Liao, Y.; Jia, X.; Ren, Y.; Deji, Z.; Gesang, Y.; Ning, N.; Feng, H.; Yu, H.; Wei, A. Suppressive role of microRNA-130b-3p in ferroptosis in melanoma cells correlates with DKK1 inhibition and Nrf2-HO-1 pathway activation. Hum. Cell 2021, 34, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, H.; Wu, H.; Liu, C.; Li, S.; Li, B.; Su, J.; Luo, S.; Li, Q. Gambogenic acid antagonizes the expression and effects of long non-coding RNA NEAT1 and triggers autophagy and ferroptosis in melanoma. Biomed. Pharmacother. 2022, 154, 113636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Zhao, R.; Li, G. Systematic analysis of the aberrances and functional implications of cuproptosis in cancer. iScience 2023, 26, 106319. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Huang, Y.; Yu, J.; Wang, T.; Li, C.; Yang, L.; Zhang, P.; Shi, L.; Yin, Y.; et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed. Pharmacother. 2023, 159, 114301. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Zheng, H.; Chen, X.; Liu, X.; Xie, Q.; Cai, X.; Zhang, Z.; Li, R. Nano-enabled photosynthesis in tumours to activate lipid peroxidation for overcoming cancer resistances. Biomaterials 2022, 285, 121561. [Google Scholar] [CrossRef]
- Celis, U.M.; García-Gasca, T.; Mejía, C. Apoptosis-Induced Compensatory Proliferation in Cancer; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Erkes, D.A.; Cai, W.; Sanchez, I.M.; Purwin, T.J.; Rogers, C.; Field, C.O.; Berger, A.C.; Hartsough, E.J.; Rodeck, U.; Alnemri, E.S.; et al. Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov. 2020, 10, 254–269. [Google Scholar] [CrossRef]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef]
- Vernon, M.; Wilski, N.A.; Kotas, D.; Cai, W.; Pomante, D.; Tiago, M.; Alnemri, E.S.; Aplin, A.E. Raptinal Induces Gasdermin E-Dependent Pyroptosis in Naïve and Therapy-Resistant Melanoma. Mol. Cancer Res. 2022, 20, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Nguyen, M.Q.; Wilski, N.A.; Purwin, T.J.; Vernon, M.; Tiago, M.; Aplin, A.E. A Genome-Wide Screen Identifies PDPK1 as a Target to Enhance the Efficacy of MEK1/2 Inhibitors in NRAS Mutant Melanoma. Cancer Res. 2022, 82, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Chun, N.; Ang, R.L.; Chan, M.; Fairchild, R.L.; Baldwin, W.M., 3rd; Horwitz, J.K.; Gelles, J.D.; Chipuk, J.E.; Kelliher, M.A.; Pavlov, V.I.; et al. T cell-derived tumor necrosis factor induces cytotoxicity by activating RIPK1-dependent target cell death. JCI Insight 2021, 6, e148643. [Google Scholar] [CrossRef]
- Hou, J.; Ju, J.; Zhang, Z.; Zhao, C.; Li, Z.; Zheng, J.; Sheng, T.; Zhang, H.; Hu, L.; Yu, X.; et al. Discovery of potent necroptosis inhibitors targeting RIPK1 kinase activity for the treatment of inflammatory disorder and cancer metastasis. Cell Death Dis. 2019, 10, 493. [Google Scholar] [CrossRef]
- Jin, L.; Liu, P.; Yin, M.; Zhang, M.; Kuang, Y.; Zhu, W. RIPK1: A rising star in inflammatory and neoplastic skin diseases. J. Dermatol. Sci. 2020, 99, 146–151. [Google Scholar] [CrossRef]
- Yang, L.; Joseph, S.; Sun, T.; Hoffmann, J.; Thevissen, S.; Offermanns, S.; Strilic, B. TAK1 regulates endothelial cell necroptosis and tumor metastasis. Cell Death Differ. 2019, 26, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Podder, B.; Guttà, C.; Rožanc, J.; Gerlach, E.; Feoktistova, M.; Panayotova-Dimitrova, D.; Alexopoulos, L.G.; Leverkus, M.; Rehm, M. TAK1 suppresses RIPK1-dependent cell death and is associated with disease progression in melanoma. Cell Death Differ. 2019, 26, 2520–2534. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Lv, J.; Yan, S.; Chang, K.J.; Wang, G. A Novel Naphthyridine Derivative, 3u, Induces Necroptosis at Low Concentrations and Apoptosis at High Concentrations in Human Melanoma A375 Cells. Int. J. Mol. Sci. 2018, 19, 2975. [Google Scholar] [CrossRef] [PubMed]
- Geserick, P.; Wang, J.; Schilling, R.; Horn, S.; Harris, P.A.; Bertin, J.; Gough, P.J.; Feoktistova, M.; Leverkus, M. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 2015, 6, e1884. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Raes, L.; Stremersch, S.; Brans, T.; Fraire, J.C.; Roelandt, R.; Declercq, W.; Vandenabeele, P.; Raemdonck, K.; Braeckmans, K.; et al. Delivery of Mixed-Lineage Kinase Domain-Like Protein by Vapor Nanobubble Photoporation Induces Necroptotic-Like Cell Death in Tumor Cells. Int. J. Mol. Sci. 2019, 20, 4254. [Google Scholar] [CrossRef] [PubMed]
- Nanni, V.; Di Marco, G.; Sacchetti, G.; Canini, A.; Gismondi, A. Oregano Phytocomplex Induces Programmed Cell Death in Melanoma Lines via Mitochondria and DNA Damage. Foods 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Cotella, D.; Santoro, C.; Corà, D.; Barlev, N.A.; Piacentini, M.; Corazzari, M. Aldo-keto reductases protect metastatic melanoma from ER stress-independent ferroptosis. Cell Death Dis. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liang, X.; Yang, N.; Yang, J.; Zhang, J.; Pan, X.; Wei, Y.; Liu, Z.; Shen, Q. Boosting cancer immunotherapy by biomineralized nanovaccine with ferroptosis-inducing and photothermal properties. Biomater. Sci. 2023, 11, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, M.; Zhang, K.; Zhang, J.; Gao, T.; Connell, D.O.; Yao, F.; Mu, C.; Cai, B.; Shang, Y.; et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, H.; Zhu, Z.; Liu, Z.; Ma, C.; Lee, Y.J.; Bartlett, D.L.; Guo, Z.S. Ferroptosis Inducer Improves the Efficacy of Oncolytic Virus-Mediated Cancer Immunotherapy. Biomedicines 2022, 10, 1425. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, L.; Zhang, P.; Luo, M.; Du, J.; Gao, T.; O’Connell, D.; Wang, G.; Wang, H.; Yang, Y. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol. Carcinog. 2018, 57, 1566–1576. [Google Scholar] [CrossRef]
- Luo, M.; Wu, L.; Zhang, K.; Wang, H.; Zhang, T.; Gutierrez, L.; O’Connell, D.; Zhang, P.; Li, Y.; Gao, T.; et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018, 25, 1457–1472. [Google Scholar] [CrossRef]
- Sato, M.; Onuma, K.; Domon, M.; Hasegawa, S.; Suzuki, A.; Kusumi, R.; Hino, R.; Kakihara, N.; Kanda, Y.; Osaki, M.; et al. Loss of the cystine/glutamate antiporter in melanoma abrogates tumor metastasis and markedly increases survival rates of mice. Int. J. Cancer 2020, 147, 3224–3235. [Google Scholar] [CrossRef]
- Leu, J.I.; Murphy, M.E.; George, D.L. Targeting ErbB3 and Cellular NADPH/NADP(+) Abundance Sensitizes Cutaneous Melanomas to Ferroptosis Inducers. ACS Chem. Biol. 2022, 17, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.; Maric, D.; Zuhra, K.; Bogaerts, A.; Szabo, C.; Vanden Berghe, W.; Hoogewijs, D. Cytoglobin Silencing Promotes Melanoma Malignancy but Sensitizes for Ferroptosis and Pyroptosis Therapy Response. Antioxidants 2022, 11, 1548. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhou, Y.; Huang, H.; Lin, Y.; Zeng, Y.; Han, S.; Huang, K.; Liu, Q.; Zhu, W.; Yuan, Z.; et al. Nobiletin Induces Ferroptosis in Human Skin Melanoma Cells Through the GSK3β-Mediated Keap1/Nrf2/HO-1 Signalling Pathway. Front. Genet. 2022, 13, 865073. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Chen, Y.; Wang, H.; Tian, Y.; Yi, X.; Shi, Q.; Zhao, T.; Zhang, B.; Gao, T.; et al. Targeting Wnt/β-Catenin Signaling Exacerbates Ferroptosis and Increases the Efficacy of Melanoma Immunotherapy via the Regulation of MITF. Cells 2022, 11, 3580. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21–3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381. [Google Scholar] [CrossRef]
- Xiong, C.; Ling, H.; Hao, Q.; Zhou, X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023, 30, 876–884. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Zhou, B.; Cosco, D.; Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.Y.; Liu, X.S.; Chen, H.Z.; Ai, Y.L.; Cheng, K.; Sun, R.Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Yao, F.; Cui, X.; Zhang, Y.; Bei, Z.; Wang, H.; Zhao, D.; Wang, H.; Yang, Y. Iron regulatory protein 1 promotes ferroptosis by sustaining cellular iron homeostasis in melanoma. Oncol. Lett. 2021, 22, 657. [Google Scholar] [CrossRef]
- Hong, X.; Roh, W.; Sullivan, R.J.; Wong, K.H.K.; Wittner, B.S.; Guo, H.; Dubash, T.D.; Sade-Feldman, M.; Wesley, B.; Horwitz, E.; et al. The Lipogenic Regulator SREBP2 Induces Transferrin in Circulating Melanoma Cells and Suppresses Ferroptosis. Cancer Discov. 2021, 11, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Miller, G. Molecular Pathways: The Necrosome-A Target for Cancer Therapy. Clin. Cancer Res. 2017, 23, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Werthmöller, N.; Frey, B.; Wunderlich, R.; Fietkau, R.; Gaipl, U.S. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015, 6, e1761. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, Z.; Rahmati, M.; Khataee, A.; Moosavi, M.A. Differential effects of N-TiO(2) nanoparticle and its photo-activated form on autophagy and necroptosis in human melanoma A375 cells. J. Cell. Physiol. 2020, 235, 8246–8259. [Google Scholar] [CrossRef]
- Wang, L.; Karges, J.; Wei, F.; Xie, L.; Chen, Z.; Gasser, G.; Ji, L.; Chao, H. A mitochondria-localized iridium(iii) photosensitizer for two-photon photodynamic immunotherapy against melanoma. Chem. Sci. 2023, 14, 1461–1471. [Google Scholar] [CrossRef]
- Kang, R.; Zeng, L.; Zhu, S.; Xie, Y.; Liu, J.; Wen, Q.; Cao, L.; Xie, M.; Ran, Q.; Kroemer, G.; et al. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe 2018, 24, 97–108.e4. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Noulala, C.G.T.; Samba, A.R.M.; Tankeo, S.B.; Abdelfatah, S.; Fotso, G.W.; Happi, E.N.; Ngadjui, B.T.; Beng, V.P.; Kuete, V.; et al. The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem. Biol. Interact. 2021, 333, 109334. [Google Scholar] [CrossRef]
- Vergani, E.; Beretta, G.L.; Aloisi, M.; Costantino, M.; Corno, C.; Frigerio, S.; Tinelli, S.; Dugo, M.; Accattatis, F.M.; Granata, A.; et al. Targeting of the Lipid Metabolism Impairs Resistance to BRAF Kinase Inhibitor in Melanoma. Front. Cell Dev. Biol. 2022, 10, 927118. [Google Scholar] [CrossRef]
- Lin, A.; Sahun, M.; Biscop, E.; Verswyvel, H.; De Waele, J.; De Backer, J.; Theys, C.; Cuypers, B.; Laukens, K.; Berghe, W.V.; et al. Acquired non-thermal plasma resistance mediates a shift towards aerobic glycolysis and ferroptotic cell death in melanoma. Drug Resist. Updat. 2023, 67, 100914. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Liu, Y.; Tao, H.; Banerjee, S.; Srinivasan, S.; Nemeth, E.; Czaja, M.J.; He, P. Integrated regulation of stress responses, autophagy and survival by altered intracellular iron stores. Redox Biol. 2022, 55, 102407. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; van Oppen, L.M.; Schöckel, L.; Bossenbroek, H.M.; van Emst-de Vries, S.E.; Hermeling, J.C.; Grefte, S.; Kopitz, C.; Heroult, M.; Hgm Willems, P.; et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Wang, Y.; Cheng, H.; Su, J.; Li, Q. Gambogenic acid induces ferroptosis in melanoma cells undergoing epithelial-to-mesenchymal transition. Toxicol. Appl. Pharmacol. 2020, 401, 115110. [Google Scholar] [CrossRef] [PubMed]
- Hammerová, J.; Uldrijan, S.; Táborská, E.; Vaculová, A.H.; Slaninová, I. Necroptosis modulated by autophagy is a predominant form of melanoma cell death induced by sanguilutine. Biol. Chem. 2012, 393, 647–658. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Chi, G.F.; Nguenang, G.S.; Abdelfatah, S.; Tchangna Sop, R.V.; Ngadjui, B.T.; Kuete, V.; Efferth, T. Cytotoxicity of a naturally occuring spirostanol saponin, progenin III, towards a broad range of cancer cell lines by induction of apoptosis, autophagy and necroptosis. Chem. Biol. Interact. 2020, 326, 109141. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.Y.; Tian, M.; Li, A.X.; Chen, X.S.; Wang, X.L.; Zhang, Y.; Cheng, Y. Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacol. Sin. 2019, 40, 1237–1244. [Google Scholar] [CrossRef]
- Cardile, A.; Zanrè, V.; Campagnari, R.; Asson, F.; Addo, S.S.; Orlandi, E.; Menegazzi, M. Hyperforin Elicits Cytostatic/Cytotoxic Activity in Human Melanoma Cell Lines, Inhibiting Pro-Survival NF-κB, STAT3, AP1 Transcription Factors and the Expression of Functional Proteins Involved in Mitochondrial and Cytosolic Metabolism. Int. J. Mol. Sci. 2023, 24, 1263. [Google Scholar] [CrossRef]
- Ahmed, F.; Tseng, H.Y.; Ahn, A.; Gunatilake, D.; Alavi, S.; Eccles, M.; Rizos, H.; Gallagher, S.J.; Tiffen, J.C.; Hersey, P.; et al. Repurposing Melanoma Chemotherapy to Activate Inflammasomes in the Treatment of BRAF/MAPK Inhibitor Resistant Melanoma. J. Investig. Dermatol. 2022, 142, 1444–1455.e10. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C.; Li, Y.; Huang, D.; Wu, X.; Kou, X.; Wang, X.; Wu, Q.; Gong, C. A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat. Commun. 2023, 14, 779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Liang, M.Y.; Yang, S.C.; Ma, X.B.; Wan, S.C.; Yang, Q.C.; Wang, S.; Xu, Z.; Sun, Z.J. Bioengineering of BRAF and COX2 inhibitor nanogels to boost the immunotherapy of melanoma via pyroptosis. Chem. Commun. 2023, 59, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Li, K.; Qian, B.; Li, Y.; Zhang, D.; Cui, W. Pyroptosis correlates with tumor immunity and prognosis. Commun. Biol. 2022, 5, 917. [Google Scholar] [CrossRef]
- Wang, G.; Xie, L.; Li, B.; Sang, W.; Yan, J.; Li, J.; Tian, H.; Li, W.; Zhang, Z.; Tian, Y.; et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat. Commun. 2021, 12, 5733. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, T.; Ye, J.; Sun, F.; Hou, B.; Saeed, M.; Gao, J.; Wang, Y.; Zhu, Q.; Xu, Z.; et al. Acidity-Activatable Dynamic Nanoparticles Boosting Ferroptotic Cell Death for Immunotherapy of Cancer. Adv. Mater. 2021, 33, e2101155. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Xiao, L.; Ma, X.; Ye, L.; Su, P.; Xiong, W.; Bi, E.; Wang, Q.; Xian, M.; Yang, M.; Qian, J.; et al. IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J. Clin. Investig. 2022, 132, e153247. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S.; Johnson, T.; Qi, R.; Zhang, S.; Zhang, J.; Yang, K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021, 35, 109235. [Google Scholar] [CrossRef]
- Drijvers, J.M.; Gillis, J.E.; Muijlwijk, T.; Nguyen, T.H.; Gaudiano, E.F.; Harris, I.S.; LaFleur, M.W.; Ringel, A.E.; Yao, C.H.; Kurmi, K.; et al. Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8(+) T Cells. Cancer Immunol. Res. 2021, 9, 184–199. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Van Lint, S.; Roose, K.; Van Parys, A.; Vandenabeele, P.; Grooten, J.; Tavernier, J.; De Koker, S.; Saelens, X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018, 9, 3417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, C.; Fedorov, A.; Qiao, L.; Bao, H.; Beknazarov, N.; Wang, S.; Gautam, A.; Williams, R.M.; Crawford, J.C.; et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 2022, 606, 594–602. [Google Scholar] [CrossRef] [PubMed]
- O’Day, S.; Gonzalez, R.; Lawson, D.; Weber, R.; Hutchins, L.; Anderson, C.; Haddad, J.; Kong, S.; Williams, A.; Jacobson, E. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J. Clin. Oncol. 2009, 27, 5452–5458. [Google Scholar] [CrossRef]
- Chang, M.T.; Tsai, L.C.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Phyto-sesquiterpene lactones DET and DETD-35 induce ferroptosis in vemurafenib sensitive and resistant melanoma via GPX4 inhibition and metabolic reprogramming. Pharmacol. Res. 2022, 178, 106148. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Zhang, L.; Ma, K.; Riegman, M.; Chen, F.; Ingold, I.; Conrad, M.; Turker, M.Z.; Gao, M.; Jiang, X.; et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 2016, 11, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Huang, Y.; Wang, J.; Lin, H.; Cao, F.; Li, S.; Li, Y.; Li, Z.; Liu, X. A self-assembling CXCR4-targeted pyroptosis nanotoxin for melanoma therapy. Biomater. Sci. 2023, 11, 2200–2210. [Google Scholar] [CrossRef]
- Sun, L.; Moore, E.; Berman, R.; Clavijo, P.E.; Saleh, A.; Chen, Z.; Van Waes, C.; Davies, J.; Friedman, J.; Allen, C.T. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology 2018, 7, e1488359. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, H.P.; Xie, X.; Mao, Q.; Liu, Y.Q.; He, X.H.; Peng, C.; Jiang, Q.L.; Huang, W. Novel HSP90-PI3K Dual Inhibitor Suppresses Melanoma Cell Proliferation by Interfering with HSP90-EGFR Interaction and Downstream Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 1845. [Google Scholar]
- Nyakas, M.; Fleten, K.G.; Haugen, M.H.; Engedal, N.; Sveen, C.; Farstad, I.N.; Flørenes, V.A.; Prasmickaite, L.; Mælandsmo, G.M.; Seip, K. AXL inhibition improves BRAF-targeted treatment in melanoma. Sci. Rep. 2022, 12, 5076. [Google Scholar]
- Vadarevu, H.; Juneja, R.; Lyles, Z.; Vivero-Escoto, J.L. Light-Activated Protoporphyrin IX-Based Polysilsesquioxane Nanoparticles Induce Ferroptosis in Melanoma Cells. Nanomaterials 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ye, L.; Zhou, Q.; He, Y.; Zhang, Y.; Deng, G.; Chen, X.; Liu, H. Inhibiting SCD expression by IGF1R during lorlatinib therapy sensitizes melanoma to ferroptosis. Redox Biol. 2023, 61, 102653. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ichikawa, J.; Ochiai, T.; Yoshida, Y.; Haro, H.; Suzuki-Karasaki, Y. Combined Anticancer Effect of Plasma-Activated Infusion and Salinomycin by Targeting Autophagy and Mitochondrial Morphology. Front. Oncol. 2021, 11, 593127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, W.; Wei, M.; Sun, Y.; Wang, N.; Ma, L.; Yu, X.; Gao, R.; Wang, R.; Zhang, Y.; et al. A Novel Anticancer Stem Cell Compound Derived from Pleuromutilin Induced Necroptosis of Melanoma Cells. J. Med. Chem. 2021, 64, 15825–15845. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, S.; Chen, C.; Zhu, L.; Li, S.; Song, Z.; Liang, J.; Tang, C.; Xu, N.; Liu, T.; et al. DDTC-Cu(I) based metal-organic framework (MOF) for targeted melanoma therapy by inducing SLC7A11/GPX4-mediated ferroptosis. Colloids Surf. B Biointerfaces 2023, 225, 113253. [Google Scholar] [CrossRef]
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581. [Google Scholar] [CrossRef]
- O’Day, S.J.; Eggermont, A.M.; Chiarion-Sileni, V.; Kefford, R.; Grob, J.J.; Mortier, L.; Robert, C.; Schachter, J.; Testori, A.; Mackiewicz, J.; et al. Final results of phase III SYMMETRY study: Randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol. 2013, 31, 1211–1218. [Google Scholar] [CrossRef]
- Wang, D.; Fu, Z.; Gao, L.; Zeng, J.; Xiang, Y.; Zhou, L.; Tong, X.; Wang, X.Q.; Lu, J. Increased IRF9-STAT2 Signaling Leads to Adaptive Resistance toward Targeted Therapy in Melanoma by Restraining GSDME-Dependent Pyroptosis. J. Investig. Dermatol. 2022, 142, 2476–2487.e9. [Google Scholar] [CrossRef]
- Ju, A.; Tang, J.; Chen, S.; Fu, Y.; Luo, Y. Pyroptosis-Related Gene Signatures Can Robustly Diagnose Skin Cutaneous Melanoma and Predict the Prognosis. Front. Oncol. 2021, 11, 709077. [Google Scholar] [CrossRef]
- Meng, J.; Huang, X.; Qiu, Y.; Zheng, X.; Huang, J.; Wen, Z.; Yao, J. Pyroptosis-related gene mediated modification patterns and immune cell infiltration landscapes in cutaneous melanoma to aid immunotherapy. Aging 2021, 13, 24379–24401. [Google Scholar] [CrossRef]
- Gullett, J.M.; Tweedell, R.E.; Kanneganti, T.D. It’s All in the PAN: Crosstalk, Plasticity, Redundancies, Switches, and Interconnectedness Encompassed by PANoptosis Underlying the Totality of Cell Death-Associated Biological Effects. Cells 2022, 11, 1495. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sundaram, B.; Sharma, B.R.; Lee, S.; Malireddi, R.K.S.; Nguyen, L.N.; Christgen, S.; Zheng, M.; Wang, Y.; Samir, P.; et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021, 37, 109858. [Google Scholar] [CrossRef] [PubMed]
- Machesky, L.M. Deadly actin collapse by disulfidptosis. Nat. Cell Biol. 2023, 25, 375–376. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Drug Type | Cell Death Forms | Cell Lines | In Vivo | Reference |
|---|---|---|---|---|---|
| Clinical Trials | |||||
| Elesclomol-Cucl2 | Cuproptosis inducer | Cuproptosis | M619 | No | [86] |
| Preclinical studies | |||||
| ML162 | GPX4 inhibition | Ferroptosis | A375, A2058, B16F10 | Yes | [47,87] |
| ML210 | GPX4 inhibition | Ferroptosis | A375, A2058, B16 | Yes | [40,83] |
| IFN-γ | Cytokine | Ferroptosis | A375, A2058, WM793B | Yes | [48] |
| Gambogenic acid | Natural compound | Ferroptosis + autophagy | A375, A2058, B16, B16F10 | No | [14,68] |
| Nobiletin | Natural compound | Ferroptosis | SK-MEL-28 | No | [46] |
| Gallic acid | Natural compound | Ferroptosis + apoptosis | A375 | No | [72,88] |
| Hyerforin | Natural compound | Ferroptosis + autophagy + apoptosis | A375, FO1, SK-MEL-28, M3Wo | No | [72] |
| α-MSH-PEG-C’ | Silica nanoparticles | Ferroptosis | M21 | Yes | [89] |
| 3u | Naphthyridine derivative | Necroptosis (in low concentration), apoptosis (in high concentration) | A375 | No | [31] |
| Oregano | Origanum vulgare L. hydroalcoholic extract | Necroptosis + apoptosis | A375, B16F10 | No | [34] |
| T22-PE24 | CXCR4 antagonist | Pyroptosis | A375, A2058, HMCB, ME4405 | Yes | [90] |
| CDNPs | Nanogels loaded with dabrafenib (BRAFi) and celecoxib (COX2i) | Pyroptosis | B16F10 | Yes | [75] |
| Nano-CD | Nano-CRISPR scaffold | Pyroptosis | A375, B16F10 | Yes | [74] |
| Erastin | System Xc inhibition | Ferroptosis | A375, G-361 | Yes | [13,41,42] |
| A375, A2058, WM793B | Yes | [48] | |||
| Erastin | System Xc inhibition | Ferroptosis | B16 | No | [38] |
| oncolytic vaccinia virus | Immunotherapy | ||||
| MiR-21-3p-AuNp | Nanoparticles | Ferroptosis | A375, A2058, WM793B | Yes | [48] |
| ICG001 | Wnt inhibitor | Ferroptosis | A375, A2058, B16F10 | Yes | [47] |
| RSL-3 | GPX4 inhibition | Ferroptosis | A375, G-361 | Yes | [13,41,42] |
| A375, A2058, WM793B | Yes | [48] | |||
| B16F10 | Yes | [78] | |||
| MeWo, A2058, SK-MEL-5, SKMEL-24, C8161, CHL-1, A375 | No | [35] | |||
| HGF | Hydrophilic nanoparticle with polyphenol-iron and GW4869 inhibitor loaded | Ferroptosis | B16F10 | Yes | [77] |
| Fe@OVA-IR820 | Nanovaccines | Ferroptosis | B16-OVA | Yes | [36] |
| Curaxin CBL0137 | Molecular compound | Necroptosis | B16F10, B16OVA, YUMMER1.7 | Yes | [85] |
| MLKL-mRNA | Nanoparticles | Necroptosis | B16 | Yes | [84] |
| AZD1775 | WEE1 kinase inhibitor | Necroptosis | B16 | Yes | [91] |
| GSK2334470 | PDPK1i | Pyroptosis | WM1361A, WM1366 | Yes | [25] |
| trametinib | MEKi | ||||
| Raptinal | Caspase-3 activator | Pyroptosis | D4M3.A, YUMM1.7, A375, WM35, WM793, and 1205Lu | Yes | [24] |
| AUY-922 PI-103 DHP1808 | Hsp90i pan-PI3Ki | Pyroptosis | A375 | No | [92] |
| BGB324 | ALK inhibitor | Ferroptosis + autophagy + apoptosis | A375, WM1366, Melmet 1 | Yes | [93] |
| Iridium(III) complex Ir-pbt-Bpa | PDT | Ferroptosis | A375, B16F10 | Yes | [59] |
| PpIX-PSilQ NPs | Protoporphyrin IX-based PSilQ PLATFORM | Ferroptosis + apoptosis | A375 | Yes | [94] |
| Etoposide | Chemotherapy drug | Pyroptosis | A375, D4M3.A, YUMM1.7 | Yes | [22,53] |
| Doxorubicin | Chemotherapy drug | Pyroptosis | A375, D4M3.A, YUMM1.7 | Yes | [22,53] |
| PLX4720 | BRAFi | Pyroptosis | D4M3.A, YUMM1.7 | Yes | [22] |
| PD0325901 | MEKi | ||||
| RSL3 Lorlatinib | GPX4 inhibition ALK inhibitor | Ferroptosis | A375, A2058 | Yes | [95] |
| Salinomycin | Plasma-activated infusion | Necroptosis | A2058 | Yes | [96] |
| Compound 38 | Pleuromutilin derivative | Necroptosis | A375, B16F10 | Yes | [97] |
| Actinomycin-D | Chemotherapy drug | Pyroptosis | A375 | No | [53] |
| NIR-PMCs | Nanoparticles | Ferroptosis | A375, B16, B16F10 | Yes | [18] |
| Cu-BTC@DDTC | Nanoparticles | Ferroptosis | B16F10 | Yes | [98] |
| zVAD-fmk | Caspase inhibitor | Necroptosis | B16F10 | No | [57] |
| N-TiO2NPs | Nanoparticles | Necroptosis + autophagy | A375 | No | [58] |
| Soyauxinium chloride | Natural compound | Apoptosis + ferroptosis + necroptosis | A2058, SK-MEL-505, SK-MEL-28, MEL-2A, B16F1, B16F10 | No | [61] |
| Progenin III | Natural compound | Autophagy + ferroptosis + necroptosis | A2058, SK-MEL-505, SK-MEL-28, MEL-2A, B16F1, B16F10 | No | [70] |
| Sanguilutine | Natural compound | Autophagy + necroptosis | A375 | No | [69] |
| Salinomycin | Plasma-activated infusion | Necroptosis | A2058 | Yes | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Vargas, C.P.D.; Tian, X.; Chu, X.; Yin, C.; Wong, A.; Yang, Y. Harnessing the Potential of Non-Apoptotic Cell Death Processes in the Treatment of Drug-Resistant Melanoma. Int. J. Mol. Sci. 2023, 24, 10376. https://doi.org/10.3390/ijms241210376
Dong L, Vargas CPD, Tian X, Chu X, Yin C, Wong A, Yang Y. Harnessing the Potential of Non-Apoptotic Cell Death Processes in the Treatment of Drug-Resistant Melanoma. International Journal of Molecular Sciences. 2023; 24(12):10376. https://doi.org/10.3390/ijms241210376
Chicago/Turabian StyleDong, Linyinxue, Ceeane Paul Dagoc Vargas, Xuechen Tian, Xiayu Chu, Chenqi Yin, Aloysius Wong, and Yixin Yang. 2023. "Harnessing the Potential of Non-Apoptotic Cell Death Processes in the Treatment of Drug-Resistant Melanoma" International Journal of Molecular Sciences 24, no. 12: 10376. https://doi.org/10.3390/ijms241210376
APA StyleDong, L., Vargas, C. P. D., Tian, X., Chu, X., Yin, C., Wong, A., & Yang, Y. (2023). Harnessing the Potential of Non-Apoptotic Cell Death Processes in the Treatment of Drug-Resistant Melanoma. International Journal of Molecular Sciences, 24(12), 10376. https://doi.org/10.3390/ijms241210376







