The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant
Abstract
1. Introduction
2. Involvement of the SP/NK-1R System in Cancer
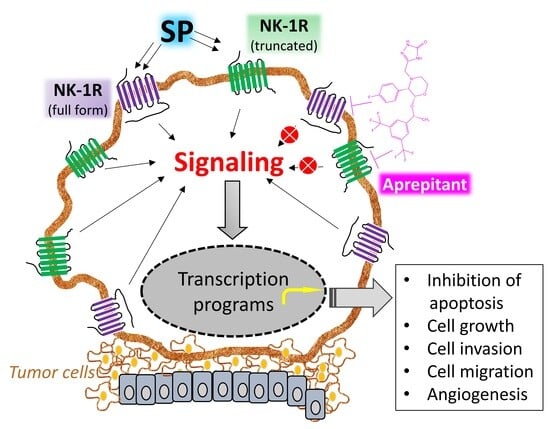
- NK-1R belongs to the rhodopsin-like G protein-coupled receptors family and shows a preferential affinity for SP [37]. This peptide is the natural ligand for NK-1R, so NK-1R is also named the SP receptor [38,39]. The SP high-affinity receptor NK-1R is widely distributed throughout the body and can bind to hemokinin-1, endokinins, and neurokinins. For a recent review focused on the structural dynamics and signaling of NK-1R, see [23].
- The 5’ flanking region of the tachykinin receptor 1 gene contains conserved gene promoter regulatory elements such as the octamer binding protein 2, nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), activating protein-1, -2, and -4, and cAMP responsive element binding sites. NF-κB favors cell survival, DNA transcription, and cancer progression, blocks apoptosis, and promotes cancer resistance and the synthesis of tumor-associated cytokines (interferon γ, macrophage inflammatory protein-1β, tumor necrosis factor α, interleukins 1β and 6) in tumor cells [42].
- NK-1R is coupled to Gαs, Gαq, Gαo, Gαi, and Gα12/13 proteins; activation of a specific G protein, which differs in the signaling/effector pathways they activate, is controlled by the conformations of NK-1R (each conformation shows a distinct affinity for antagonists and agonists) and type of ligands [43,44,45,46,47,48,49]. Aprepitant promotes conformational changes that interfere with the binding of SP, leading to a long-lasting inhibition of NK-1R [22,50,51,52]. The interaction between SP central and N-terminal regions with the NK-1R extracellular domain is crucial for signaling through Gs. NK-1R antagonists block access to the receptor binding site and hinder G-protein activation [53].
- SP binds to the extracellular loops of NK-1R [54] and non-peptide NK-1R antagonists between the receptor’s III and VI transmembrane segments; specific amino acid residues (His197, Gln165) control the binding of these antagonists as reported in recent detailed structural studies [48,52,53,55]. The amidated C-terminal of SP is involved in peptide activity since its deamidation suppressed the activity of SP. The C-terminal sequence contains a hydrophobic amino acid residue needed to activate NK-1R [53].
- Cancer cells express/overexpress NK-1R [11,56,57,58,59,60,61,62,63,64], which is involved in the viability of these cells. It has been suggested that the higher the number of NK-1R, the higher the tumor malignancy, and NK-1R mRNA expression is lower in benign compared to malignant tissues. NK-1R is not involved in the viability of normal cells. Tumor cells express and release SP, but SP is not involved in the viability of cancer cells [65].
- SP favors the proliferation of tumor cells [66,67,68,69,70,71,72]. The peptide, through NK-1R, promotes the proliferation via mitogen-activated protein kinases (MAPK) signaling and migration of both solid and non-solid cancer cells via the expression of matrix metalloproteinase 9 and exerts an antiapoptotic action by activating protein kinase B (this has been associated with a poor prognosis). SP favors the Warburg effect (glycolytic rate increase), which cancer cells use to maintain their high metabolism rate. SP promotes angiogenesis, leading to neovascularization [73]. Consequently, rapidly multiplying cancer cells use the nutrients and oxygen provided due to SP-induced angiogenesis. SP also increases the expression of NK-1R but not that of the other two tachykinin receptors (NK-2R and NK-3R).
- NK-1R shows two isoforms: full-length (407 amino acids) and truncated (311 amino acids; C-terminus 96 residues are lost) [74,75]. Isoforms can trigger different intracellular signaling pathways and play different roles in physiological and pathophysiological mechanisms [49]. The full-length form shows a ten-fold higher binding affinity for SP than the truncated form. Cells expressing the full-length form respond to nanomolar concentrations of SP, whereas those cells expressing the truncated isoform need micromolar concentrations of the peptide to elicit a signaling response. The full-length isoform is involved in NK-1R desensitization and internalization, whereas the truncated form partially disrupts signaling pathways but does not affect the SP binding domain. Truncation of the receptor leads to impairment in the receptor being internalized, thus imparting the receptor with (a) resistance to desensitization, (b) weaker interaction with G proteins and protein kinase K and other phosphorylation processes, and (c) delay in Ca++ release ultimately resulting in an inhibited response to SP.
- Tumor cells show a higher level of truncated and a lower level of full-length NK-1R than normal cells. MicroRNA-206 overexpression favors cancer cell proliferation, invasion, and migration via targeting the full-length form, whereas microRNA-22 blocks all these processes by targeting the truncated NK-1R form [67]. Furthermore, NK-1R is the predicted target of the miR-34 family, and the overexpression of microRNA-34b/c-5p has been shown to suppress cancer cell proliferation and promote apoptosis via NK-1R suppression.
- The truncated NK-1R isoform is involved in malignancy, tumor cell growth, metastasis, and apoptosis blockade [76]. In contrast, the full-length expression, inversely associated with invasion and metastasis, decreases cancer cell proliferation and attenuates apoptotic signals. Truncated NK-1 expression is positively regulated via Smad4 by tumor growth factor β and blocked with NK-1R antagonists (Aprepitant). SP promotes the activation of NF-κB, which upregulates the truncated form, induces a slight increase in the full-length isoform, and favors resistance to some chemotherapeutic agents and Aprepitant. This observation is vital since tumors showing overexpression of the NF-κB pathway may need to be treated with a higher dose of the NK-1R antagonist for mediating anticancer effects.
- SP is synthesized and released by cancer and immune cells, and it is released from nerve terminals and circulates in the bloodstream [62,77,78,79]. SP-immunoreactive fibers have been associated with tumor differentiation status. SP acts through autocrine, paracrine, neuroendocrine, and endocrine (from the tumor mass) mechanisms. SP and NK-1R have also been observed in the nuclei of cancer cells; their physiological significance is currently unknown.
- Following the interaction of SP with NK-1R, important downstream events are activated, such as diacylglycerol synthesis with the resultant activation of protein kinase C and promotion of the influx of extracellular Ca++ via calcium channels. Downstream events, such as inositol triphosphate production, promote Ca++ release from the endoplasmic reticulum into the cytoplasm. High calcium levels enhance proliferative and pro-survival pathways such as MAPK and extracellular signal-regulated kinases (ERK). Furthermore, calcium governs other essential processes such as cell death, migration, communication, and immune activation [80]. NK-1R antagonists promote rapid endoplasmic reticulum/mitochondria Ca++ overload and accumulation of reactive oxygen species, causing apoptosis. In conclusion, since the processes mentioned above can be deregulated and exploited by cancer cells that overexpress NK-1R, this receptor can serve as a suitable therapeutic target in cancer [50,81,82].
- Although both normal and cancer cells produce SP, lower levels of SP are detected in normal cells compared with cancer cells. More serum SP and NK-1R concentrations were found in cancer patients than healthy individuals. NK-1R overexpression has been associated with larger tumor size, higher metastatic and invasion potential tumor-node metastasis, advanced cancer stages, and poor prognosis. Thus, NK-1R overexpression and high serum SP levels could be used as predictive biomarkers for increased risk of developing cancer and cancer prognosis [57,59].
3. NK-1R Antagonists as Anticancer Drugs
4. Aprepitant Repurposing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez, M.L.; Coveñas, R. The Neurotensinergic System: A Target for Cancer Treatment. Curr. Med. Chem. 2022, 29, 3231–3260. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, Y.; Lee, M.; Nguyen, A.; Ryujin, N.T.; Huang, J.Y.; Pandit, S.K.; Chamseddine, S.; Xiao, L.; Mohamed, Y.I.; et al. Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2300706120. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.L.; Rodríguez, F.D.; Coveñas, R. Peptidergic Systems and Cancer: Focus on Tachykinin and Calcitonin/Calcitonin Gene-Related Peptide Families. Cancers 2023, 15, 1694. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.A.; Somvanshi, R.K.; Kumar, U. Comparative changes in breast cancer cell proliferation and signalling following somatostatin and cannabidiol treatment. Biochem. Biophys. Res. Commun. 2023, 643, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Huan, R.; Yue, J.; Lan, J.; Wang, J.; Cheng, Y.; Zhang, J.; Tan, Y. Hypocretin-1 suppresses malignant progression of glioblastoma cells through Notch1 signaling pathway. Brain Res. Bull. 2023, 196, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 30, 1798–1812. [Google Scholar] [CrossRef]
- García-Aranda, M.; Téllez, T.; McKenna, L.; Redondo, M. Neurokinin-1 Receptor (NK-1R) Antagonists as a New Strategy to Overcome Cancer Resistance. Cancers 2022, 14, 2255. [Google Scholar] [CrossRef]
- Beirith, I.; Renz, B.W.; Mudusetti, S.; Ring, N.S.; Kolorz, J.; Koch, D.; Bazhin, A.V.; Berger, M.; Wang, J.; Angele, M.K.; et al. Identification of the Neurokinin-1 Receptor as Targetable Stratification Factor for Drug Repurposing in Pancreatic Cancer. Cancers 2021, 13, 2703. [Google Scholar] [CrossRef]
- Rodriguez, E.; Pei, G.; Kim, S.T.; German, A.; Robinson, P. Substance P Antagonism as a Novel Therapeutic Option to Enhance Efficacy of Cisplatin in Triple Negative Breast Cancer and Protect PC12 Cells against Cisplatin-Induced Oxidative Stress and Apoptosis. Cancers 2021, 13, 3871. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P.; Esteban, F. Significance of the Overexpression of Substance P and Its Receptor NK-1R in Head and Neck Carcinogenesis: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1349. [Google Scholar] [CrossRef]
- Mehboob, R.; Kurdi, M.; Baeesa, S.; Fawzy Halawa, T.; Tanvir, I.; Maghrabi, Y.; Hakamy, S.; Saeedi, R.; Moshref, R.; Nasief, H.; et al. Immunolocalization of neurokinin 1 receptor in WHO grade 4 astrocytomas, oral squamous cell and urothelial carcinoma. Folia Neuropathol. 2022, 60, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Berisha, A.; Borniger, J.C. Neuropeptides in Cancer: Friend and Foe? Adv. Biol. 2022, 6, e2200111. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Czerwinski, G.; Tarasova, N.I.; Moody, D.L.; Michejda, C.J. The development of VIP-ellipticine conjugates. Regul. Pept. 2004, 123, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Matalińska, J.; Kosińska, K.; Halik, P.K.; Koźmiński, P.; Lipiński, P.F.J.; Gniazdowska, E.; Misicka, A. Novel NK1R-Targeted (68)Ga-/(177)Lu-Radioconjugates with Potential Application against Glioblastoma Multiforme: Preliminary Exploration of Structure-Activity Relationships. Int. J. Mol. Sci. 2022, 23, 1214. [Google Scholar] [CrossRef]
- Halik, P.K.; Lipiński, P.F.J.; Matalińska, J.; Koźmiński, P.; Misicka, A.; Gniazdowska, E. Radiochemical Synthesis and Evaluation of Novel Radioconjugates of Neurokinin 1 Receptor Antagonist Aprepitant Dedicated for NK1R-Positive Tumors. Molecules 2020, 25, 3756. [Google Scholar] [CrossRef]
- Halik, P.K.; Koźmiński, P.; Matalińska, J.; Lipiński, P.F.J.; Misicka, A.; Gniazdowska, E. In Vitro Biological Evaluation of Aprepitant Based (177)Lu-Radioconjugates. Pharmaceutics 2022, 14, 607. [Google Scholar] [CrossRef]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. (225)Ac- and (213)Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef]
- Mander, K.; Harford-Wright, E.; Lewis, K.M.; Vink, R. Advancing drug therapy for brain tumours: A current review of the pro-inflammatory peptide Substance P and its antagonists as anti-cancer agents. Recent. Pat. CNS Drug Discov. 2014, 9, 110–121. [Google Scholar] [CrossRef]
- Song, J.; Huang, S.; Ma, P.; Zhang, B.; Jia, B.; Zhang, W. Improving NK1R-targeted gene delivery of stearyl-antimicrobial peptide CAMEL by conjugating it with substance P. Bioorg. Med. Chem. Lett. 2020, 30, 127353. [Google Scholar] [CrossRef]
- Ding, G.; Wang, T.; Han, Z.; Tian, L.; Cheng, Q.; Luo, L.; Zhao, B.; Wang, C.; Feng, S.; Wang, L.; et al. Substance P containing peptide gene delivery vectors for specifically transfecting glioma cells mediated by a neurokinin-1 receptor. J. Mater. Chem. B 2021, 9, 6347–6356. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.D.; Coveñas, R. The Neurokinin-1 Receptor: Structure Dynamics and Signaling. Receptors 2022, 1, 54–71. [Google Scholar] [CrossRef]
- Lai, J.; Lai, S.; Tuluc, F.; Tansky, M.F.; Kilpatrick, L.E.; Leeman, S.E.; Douglas, S.D. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 12605–12610. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.; Zhao, D.; Zhan, Y.; Moyer, M.P.; Pothoulakis, C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc. Natl. Acad. Sci. USA 2007, 104, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Fulenwider, H.D.; Smith, B.M.; Nichenko, A.S.; Carpenter, J.M.; Nennig, S.E.; Cheng, K.; Rice, K.C.; Schank, J.R. Cellular and behavioral effects of lipopolysaccharide treatment are dependent upon neurokinin-1 receptor activation. J. Neuroinflamm. 2018, 15, 60–64. [Google Scholar] [CrossRef]
- Walczak-Drzewiecka, A.; Ratajewski, M.; Wagner, W.; Dastych, J. HIF-1alpha is up-regulated in activated mast cells by a process that involves calcineurin and NFAT. J. Immunol. 2008, 181, 1665–1672. [Google Scholar] [CrossRef]
- Werge, T. The tachykinin tale: Molecular recognition in a historical perspective. J. Mol. Recognit. 2007, 20, 145–153. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef]
- Nässel, D.R.; Zandawala, M.; Kawada, T.; Satake, H. Tachykinins: Neuropeptides That Are Ancient, Diverse, Widespread and Functionally Pleiotropic. Front. Neurosci. 2019, 13, 1262. [Google Scholar] [CrossRef]
- Dam, T.V.; Escher, E.; Quirion, R. Evidence for the existence of three classes of neurokinin receptors in brain. Differential ontogeny of neurokinin-1, neurokinin-2 and neurokinin-3 binding sites in rat cerebral cortex. Brain Res. 1988, 453, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Tuluc, F.; Lai, J.P.; Kilpatrick, L.E.; Evans, D.L.; Douglas, S.D. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009, 30, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Weinstein, H. [19] Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar] [CrossRef]
- UniProt Database. 2023. Available online: https://www.uniprot.org/ (accessed on 21 October 2023).
- Bootman, M.D.; Chehab, T.; Bultynck, G.; Parys, J.B.; Rietdorf, K. The regulation of autophagy by calcium signals: Do we have a consensus? Cell Calcium 2018, 70, 32–46. [Google Scholar] [CrossRef]
- Javid, H.; Mohammadi, F.; Zahiri, E.; Hashemy, S.I. The emerging role of substance P/neurokinin-1 receptor signaling pathways in growth and development of tumor cells. J. Physiol. Biochem. 2019, 75, 415–421. [Google Scholar] [CrossRef]
- IUPHAR/BPS Guide to Pharmacology Database. Tachykinin Receptors (Version 2019.4). 2022. Available online: http://journals.ed.ac.uk/gtopdb-cite/article/view/3214/4264 (accessed on 21 October 2023).
- Muñoz, M.; Coveñas, R. Substance P. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; Volume 1, pp. 571–578. [Google Scholar] [CrossRef]
- Goldsmith, L.E.; Kwatra, M.M. Tachykinin/substance P/neurokinin-1 receptors. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Oxford, UK, 2013; pp. 360–365. [Google Scholar]
- Muñoz, M.; Coveñas, R. Neurokinin/Tachykin receptors. In Encyclopedia of Molecular Pharmacology, 3rd ed.; Offermanns, S., Rosenthal, W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1093–1103. [Google Scholar] [CrossRef]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.; Bours, V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef]
- Roush, E.D.; Kwatra, M.M. Human substance P receptor expressed in Chinese hamster ovary cells directly activates G(alpha q/11), G(alpha s), G(alpha o). FEBS Lett. 1998, 428, 291–294. [Google Scholar] [CrossRef]
- Mitsuhashi, M.; Ohashi, Y.; Shichijo, S.; Christian, C.; Sudduth-Klinger, J.; Harrowe, G.; Payan, D.G. Multiple intracellular signaling pathways of the neuropeptide substance P receptor. J. Neurosci. Res. 1992, 32, 437–443. [Google Scholar] [CrossRef]
- Sagan, S.; Chassaing, G.; Pradier, L.; Lavielle, S. Tachykinin peptides affect differently the second messenger pathways after binding to CHO-expressed human NK-1 receptors. J. Pharmacol. Exp. Ther. 1996, 276, 1039–1048. [Google Scholar]
- Takeda, Y.; Blount, P.; Sachais, B.S.; Hershey, A.D.; Raddatz, R.; Krause, J.E. Ligand binding kinetics of substance P and neurokinin A receptors stably expressed in Chinese hamster ovary cells and evidence for differential stimulation of inositol 1,4,5-trisphosphate and cyclic AMP second messenger responses. J. Neurochem. 1992, 59, 740–745. [Google Scholar] [CrossRef]
- Fong, T.M.; Anderson, S.A.; Yu, H.; Huang, R.R.; Strader, C.D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992, 41, 24–30. [Google Scholar]
- Thom, C.; Ehrenmann, J.; Vacca, S.; Waltenspühl, Y.; Schöppe, J.; Medalia, O.; Plückthun, A. Structures of neurokinin 1 receptor in complex with G(q) and G(s) proteins reveal substance P binding mode and unique activation features. Sci. Adv. 2021, 7, eabk2872. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant, a New Drug for the Treatment of Hematological Malignancies: Focus on Acute Myeloid Leukemia. J. Clin. Med. 2020, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Schöppe, J.; Ehrenmann, J.; Klenk, C.; Rucktooa, P.; Schütz, M.; Doré, A.S.; Plückthun, A. Crystal structures of the human neurokinin 1 receptor in complex with clinically used antagonists. Nat. Commun. 2019, 10, 17–18. [Google Scholar] [CrossRef]
- Harris, J.A.; Faust, B.; Gondin, A.B.; Dämgen, M.A.; Suomivuori, C.; Veldhuis, N.A.; Cheng, Y.; Dror, R.O.; Thal, D.M.; Manglik, A. Selective G protein signaling driven by substance P-neurokinin receptor dynamics. Nat. Chem. Biol. 2022, 18, 109–115. [Google Scholar] [CrossRef]
- Bremer, A.A.; Leeman, S.E.; Boyd, N.D. The common C-terminal sequences of substance P and neurokinin A contact the same region of the NK-1 receptor. FEBS Lett. 2000, 486, 43–48. [Google Scholar] [CrossRef]
- Davoudmanesh, S.; Mosaabadi, J.M. Investigation of the effect of homocysteinylation of substance P on its binding to the NK1 receptor using molecular dynamics simulation. J. Mol. Model. 2018, 24, 177. [Google Scholar] [CrossRef]
- Singh, D.; Joshi, D.D.; Hameed, M.; Qian, J.; Gascón, P.; Maloof, P.B.; Mosenthal, A.; Rameshwar, P. Increased expression of preprotachykinin-I and neurokinin receptors in human breast cancer cells: Implications for bone marrow metastasis. Proc. Natl. Acad. Sci. USA 2000, 97, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, M.; Boroumand, N.; Mehrabi Bahar, M.; Jafarian, A.H.; Asadi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in breast cancer. Mol. Biol. Rep. 2019, 46, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M.; Robles-Frias, M.J.; Salinas-Martín, M.V.; Rosso, R.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab. Investig. 2010, 90, 1259–1269. [Google Scholar] [CrossRef]
- Castro, T.A.; Cohen, M.C.; Rameshwar, P. The expression of neurokinin-1 and preprotachykinin-1 in breast cancer cells depends on the relative degree of invasive and metastatic potential. Clin. Exp. Metastasis 2005, 22, 621–628. [Google Scholar] [CrossRef]
- Al-Keilani, M.S.; Elstaty, R.I.; Alqudah, M.A.; Alkhateeb, A.M. Immunohistochemical expression of substance P in breast cancer and its association with prognostic parameters and Ki-67 index. PLoS ONE 2021, 16, e0252616. [Google Scholar] [CrossRef]
- Ge, C.; Huang, H.; Huang, F.; Yang, T.; Zhang, T.; Wu, H.; Zhou, H.; Chen, Q.; Shi, Y.; Sun, Y.; et al. Neurokinin-1 receptor is an effective target for treating leukemia by inducing oxidative stress through mitochondrial calcium overload. Proc. Natl. Acad. Sci. USA 2019, 116, 19635–19645. [Google Scholar] [CrossRef]
- Akazawa, T.; Kwatra, S.G.; Goldsmith, L.E.; Richardson, M.D.; Cox, E.A.; Sampson, J.H.; Kwatra, M.M. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J. Neurochem. 2009, 109, 1079–1086. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. BioMed Res. Int. 2022, 2022, 6291504. [Google Scholar] [CrossRef]
- Harford-Wright, E.; Lewis, K.M.; Vink, R.; Ghabriel, M.N. Evaluating the role of substance P in the growth of brain tumors. Neuroscience 2014, 261, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Tong, Y.; Liu, X.; Zhang, L.; Dong, D.; Shao, J.; Zhou, Y. miR-206 Promotes Cancer Progression by Targeting Full-Length Neurokinin-1 Receptor in Breast Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819875168. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, S.; Liu, J.; Feng, F.; Guo, Y.; Zhang, W.; Zheng, G.; Wang, Q.; Cai, L.; Guo, M.; et al. SP promotes cell proliferation in esophageal squamous cell carcinoma through the NK1R/Hes1 axis. Biochem. Biophys. Res. Commun. 2019, 514, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feng, F.; Xu, G.; Zhang, H.; Hong, L.; Yang, J. Elevated SP/NK-1R in esophageal carcinoma promotes esophageal carcinoma cell proliferation and migration. Gene 2015, 560, 205–210. [Google Scholar] [CrossRef]
- Deng, X.; Tang, S.; Wu, P.; Li, Q.; Ge, X.; Xu, B.; Wang, H.; Miao, L. SP/NK-1R promotes gallbladder cancer cell proliferation and migration. J. Cell Mol. Med. 2019, 23, 7961–7973. [Google Scholar] [CrossRef]
- Gutierrez, S.; Boada, M.D. Neuropeptide-induced modulation of carcinogenesis in a metastatic breast cancer cell line (MDA-MB-231(LUC+)). Cancer Cell Int. 2018, 18, 216–218. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Fuster, G.; Fernandez-Nogueira, P.; Pastor-Arroyo, E.M.; Park, S.Y.; Mayordomo, C.; Ametller, E.; Mancino, M.; Gonzalez-Farre, X.; Russnes, H.G.; et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013, 73, 6424–6434. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Xiong, T.; Chen, X.; Zhang, Y.; Yu, M.; Yang, J.; Yao, Z. Roles of full-length and truncated neurokinin-1 receptors on tumor progression and distant metastasis in human breast cancer. Breast Cancer Res. Treat. 2013, 140, 49–61. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. BioMed Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef]
- Spitsin, S.; Pappa, V.; Douglas, S.D. Truncation of neurokinin-1 receptor-Negative regulation of substance P signaling. J. Leukoc. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Yang, J.; Tong, L.; Yuan, S.; Tian, Y.; Hong, L.; Wang, W.; Zhang, H. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol. Int. 2011, 35, 623–629. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M.; Carranza, A.; Coveñas, R. Increased nuclear localization of substance P in human gastric tumor cells. Acta Histochem. 2017, 119, 337–342. [Google Scholar] [CrossRef]
- Xu, M.; Seas, A.; Kiyani, M.; Ji, K.S.Y.; Bell, H.N. A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis. Cell Biosci. 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Safety of neurokinin-1 receptor antagonists. Expert. Opin. Drug Saf. 2013, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Rosso, M.; Coveñas, R. Neurokinin-1 receptor antagonists in lung cancer therapy. Lett. Drug Des. Discov. 2017, 14, 1465–1476. [Google Scholar] [CrossRef]
- Mayordomo, C.; García-Recio, S.; Ametller, E.; Fernández-Nogueira, P.; Pastor-Arroyo, E.M.; Vinyals, L.; Casas, I.; Gascón, P.; Almendro, V. Targeting of substance P induces cancer cell death and decreases the steady state of EGFR and Her2. J. Cell Physiol. 2012, 227, 1358–1366. [Google Scholar] [CrossRef]
- Covenas, R.; Munoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Golding, J.F.; Sanger, G.J. An assessment of the effects of neurokinin(1) receptor antagonism against nausea and vomiting: Relative efficacy, sites of action and lessons for future drug development. Br. J. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Jiang, S.; Fu, Y.; Avraham, S.; Avraham, H.K. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int. J. Cancer 2014, 134, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; McCloskey, M.; Staples, S. Prolonged use of Aprepitant in metastatic breast cancer and a reduction in CA153 tumour marker levels. Int. J. Cancer Clin. Res. 2016, 3. [Google Scholar] [CrossRef]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Ebrahimi, S.; Darban, R.A.; Hashemy, S.I. Potential in vitro therapeutic effects of targeting SP/NK1R system in cervical cancer. Mol. Biol. Rep. 2022, 49, 1067–1076. [Google Scholar] [CrossRef]
- Kolorz, J.; Demir, S.; Gottschlich, A.; Beirith, I.; Ilmer, M.; Lüthy, D.; Walz, C.; Dorostkar, M.M.; Magg, T.; Hauck, F.; et al. The Neurokinin-1 Receptor Is a Target in Pediatric Rhabdoid Tumors. Curr. Oncol. 2021, 29, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, M.A.; Ebrahimi, S.; Alalikhan, A.; Hashemi, S.F.; Hashemy, S.I. The Potential In Vitro Inhibitory Effects of Neurokinin-1 Receptor (NK-1R) Antagonist, Aprepitant, in Osteosarcoma Cell Migration and Metastasis. BioMed Res. Int. 2022, 2022, 8082608. [Google Scholar] [CrossRef]
- Momen Razmgah, M.; Ghahremanloo, A.; Javid, H.; AlAlikhan, A.; Afshari, A.; Hashemy, S.I. The effect of substance P and its specific antagonist (aprepitant) on the expression of MMP-2, MMP-9, VEGF, and VEGFR in ovarian cancer cells. Mol. Biol. Rep. 2022, 49, 9307–9314. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Mirzavi, F.; Aghaee-Bakhtiari, S.H.; Hashemy, S.I. SP/NK1R system regulates carcinogenesis in prostate cancer: Shedding light on the antitumoral function of aprepitant. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119221. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Hu, W.; Hu, M.; Tao, Y.; Hu, H.; Miao, X.; Yang, W.; Zhu, Q.; Mou, L. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis. 2022, 13, 41. [Google Scholar] [CrossRef]
- Javid, H.; Afshari, A.R.; Zahedi Avval, F.; Asadi, J.; Hashemy, S.I. Aprepitant Promotes Caspase-Dependent Apoptotic Cell Death and G2/M Arrest through PI3K/Akt/NF-κB Axis in Cancer Stem-Like Esophageal Squamous Cell Carcinoma Spheres. BioMed Res. Int. 2021, 2021, 8808214. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Meng, Y.; Ma, J.; Zhang, Q.; Shao, G.; Wang, L.; Cheng, X.; Hong, X.; Wang, Y.; et al. A Novel Mechanism of Endoplasmic Reticulum Stress- and c-Myc-Degradation-Mediated Therapeutic Benefits of Antineurokinin-1 Receptor Drugs in Colorectal Cancer. Adv. Sci. 2021, 8, e2101936. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanloo, A.; Javid, H.; Afshari, A.R.; Hashemy, S.I. Investigation of the Role of Neurokinin-1 Receptor Inhibition Using Aprepitant in the Apoptotic Cell Death through PI3K/Akt/NF-κB Signal Transduction Pathways in Colon Cancer Cells. BioMed Res. Int. 2021, 2021, 1383878. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumaravel, S.; Dhole, S.; Roy, S.; Pavan, V.; Chakraborty, S. Neuropeptide Substance P Enhances Inflammation-Mediated Tumor Signaling Pathways and Migration and Proliferation of Head and Neck Cancers. Indian J. Surg. Oncol. 2021, 12, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Javid, H.; Afshari, A.R.; Mashkani, B.; Hashemy, S.I. Substance P accelerates the progression of human esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and VEGFR1 overexpression. Mol. Biol. Rep. 2020, 47, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Matalińska, J.; Świć, A.; Lipiński, P.; Misicka, A. Antiproliferative effects of [D-Pro2, D-Trp7,9]-Substance P and aprepitant on several cancer cell lines and their selectivity in comparison to normal cells. Folia Neuropathol. 2020, 58, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bayati, S.; Razani, E.; Bashash, D.; Safaroghli-Azar, A.; Safa, M.; Ghaffari, S.H. Antileukemic effects of neurokinin-1 receptor inhibition on hematologic malignant cells: A novel therapeutic potential for aprepitant. Anticancer Drugs 2018, 29, 243–252. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef]
- Nizam, E.; Erin, N. Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma cells; Effects independent of Substance P. Biomed. Pharmacother. 2018, 108, 263–270. [Google Scholar] [CrossRef]
- Dikmen, M.; Gökhaner, G.; Cantürk, Z. Evaluation of the antileukemic effects of neurokinin-1 receptor antagonists, aprepitant, and L-733,060, in chronic and acute myeloid leukemic cells. Anticancer Drugs 2019, 30, e0769. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, X.; Huang, F.; Shao, G.; Meng, Y.; Wang, L.; Wang, T.; Jia, X.; Yang, T.; Wang, X.; et al. Aprepitant Sensitizes Acute Myeloid Leukemia Cells to the Cytotoxic Effects of Cytosine Arabinoside in vitro and in vivo. Drug Des. Devel Ther. 2020, 14, 2413–2422. [Google Scholar] [CrossRef]
- Nowicki, M.; Ostalska-Nowicka, D.; Kondraciuk, B.; Miskowiak, B. The significance of substance P in physiological and malignant haematopoiesis. J. Clin. Pathol. 2007, 60, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Nizam, E.; Köksoy, S.; Erin, N. NK1R antagonist decreases inflammation and metastasis of breast carcinoma cells metastasized to liver but not to brain; phenotype-dependent therapeutic and toxic consequences. Cancer Immunol. Immunother. 2020, 69, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yuan, S.; Ma, J.; Liu, H.; Huang, L.; Zhang, F. Neurokinin-1 receptor is highly expressed in cervical cancer and its antagonist induces cervical cancer cell apoptosis. Eur. J. Histochem. 2023, 67, 3570. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; DeMorrow, S.; Venter, J.; Frampton, G.; Han, Y.; Francis, H.; Standeford, H.; Avila, S.; McDaniel, K.; McMillin, M.; et al. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, 759. [Google Scholar] [CrossRef]
- Lorestani, S.; Ghahremanloo, A.; Jangjoo, A.; Abedi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in colorectal cancer. Mol. Biol. Rep. 2020, 47, 3469–3474. [Google Scholar] [CrossRef]
- Golestaneh, M.; Firoozrai, M.; Javid, H.; Hashemy, S.I. The substance P/ neurokinin-1 receptor signaling pathway mediates metastasis in human colorectal SW480 cancer cells. Mol. Biol. Rep. 2022, 49, 4893–4900. [Google Scholar] [CrossRef]
- Javid, H.; Asadi, J.; Zahedi Avval, F.; Afshari, A.R.; Hashemy, S.I. The role of substance P/neurokinin 1 receptor in the pathogenesis of esophageal squamous cell carcinoma through constitutively active PI3K/Akt/NF-κB signal transduction pathways. Mol. Biol. Rep. 2020, 47, 2253–2263. [Google Scholar] [CrossRef]
- Afshari, A.R.; Motamed-Sanaye, A.; Sabri, H.; Soltani, A.; Karkon-Shayan, S.; Radvar, S.; Javid, H.; Mollazadeh, H.; Sathyapalan, T.; Sahebkar, A. Neurokinin-1 Receptor (NK-1R) Antagonists: Potential Targets in the Treatment of Glioblastoma Multiforme. Curr. Med. Chem. 2021, 28, 4877–4892. [Google Scholar] [CrossRef]
- Rezaei, S.; Assaran Darban, R.; Javid, H.; Hashemy, S.I. The Therapeutic Potential of Aprepitant in Glioblastoma Cancer Cells through Redox Modification. BioMed Res. Int. 2022, 2022, 8540403. [Google Scholar] [CrossRef]
- Muñoz, M.; Rosso, M.; Coveñas, R. Neurokinin-1 Receptor Antagonists against Hepatoblastoma. Cancers 2019, 11, 1258. [Google Scholar] [CrossRef]
- Hongfeng, Z.; Andong, J.; Liwen, S.; Mingping, B.; Xiaowei, Y.; Mingyong, L.; Aimin, Y. lncRNA RMRP knockdown suppress hepatocellular carcinoma biological activities via regulation miRNA-206/TACR1. J. Cell Biochem. 2020, 121, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Ilmer, M.; Garnier, A.; Vykoukal, J.; Alt, E.; von Schweinitz, D.; Kappler, R.; Berger, M. Targeting the Neurokinin-1 Receptor Compromises Canonical Wnt Signaling in Hepatoblastoma. Mol. Cancer Ther. 2015, 14, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Esteban, F.; Ramos-García, P.; Muñoz, M.; González-Moles, M.Á. Substance P and Neurokinin 1 Receptor in Chronic Inflammation and Cancer of the Head and Neck: A Review of the Literature. Int. J. Environ. Res. Public. Health 2021, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Borrego, J.F.; Huelsmeyer, M.K.; Pinkerton, M.E.; Muszynski, J.L.; Miller, S.A.K.; Kurzman, I.D.; Vail, D.M. Neurokinin-1 receptor expression and antagonism by the NK-1R antagonist maropitant in canine melanoma cell lines and primary tumour tissues. Vet. Comp. Oncol. 2016, 14, 210–224. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Li, L.; Hu, W.; Tao, Y.; Gao, W.; Ye, Z.; Jia, H.; Wang, J.; Miao, X.; et al. Neurokinin-1 receptor drives PKCɑ-AURKA/N-Myc signaling to facilitate the neuroendocrine progression of prostate cancer. Cell Death Dis. 2023, 14, 384. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, K.; Ping, F.; Gao, Y.; Liu, L.; Feng, B. Cross-talk between 5-hydroxytryptamine and substance P in the melanogensis and apoptosis of B16F10 melanoma cells. Eur. J. Pharmacol. 2016, 775, 106–112. [Google Scholar] [CrossRef]
- Berger, M.; VON Schweinitz, D. Therapeutic Innovations for Targeting Childhood Neuroblastoma: Implications of the Neurokinin-1 Receptor System. Anticancer Res. 2017, 37, 5911–5918. [Google Scholar] [CrossRef][Green Version]
- Henssen, A.G.; Odersky, A.; Szymansky, A.; Seiler, M.; Althoff, K.; Beckers, A.; Speleman, F.; Schäfers, S.; De Preter, K.; Astrahanseff, K.; et al. Targeting tachykinin receptors in neuroblastoma. Oncotarget 2017, 8, 430–443. [Google Scholar] [CrossRef]
- Pohl, A.; Kappler, R.; Mühling, J.; VON Schweinitz, D.; Berger, M. Expression of Truncated Neurokinin-1 Receptor in Childhood Neuroblastoma is Independent of Tumor Biology and Stage. Anticancer Res. 2017, 37, 6079–6085. [Google Scholar] [CrossRef]
- Pan, B.; Liu, D.; Yang, L.; Wüthrich, K. GPCR large-amplitude dynamics by (19)F-NMR of aprepitant bound to the neurokinin 1 receptor. Proc. Natl. Acad. Sci. USA 2022, 119, e2122682119. [Google Scholar] [CrossRef]
- AlAlikhan, A.; Ghahremanloo, A.; Javid, H.; Ebrahimi, S.; Hashemy, S.I. The Effect of Blocking Neurokinin-1 Receptor by Aprepitant on the Inflammatory and Apoptosis Pathways in Human Ovarian Cancer Cells. Cell Biochem. Biophys. 2022, 80, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, Y.; Guo, Y.; Zhang, Z.; Lian, G.; Chen, Y.; Li, J.; Su, Y.; Li, J.; Yang, K.; et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics 2018, 8, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Ma, K.; Wu, H.; Cao, T. A Substance P (SP)/Neurokinin-1 Receptor Axis Promotes Perineural Invasion of Pancreatic Cancer and Is Affected by lncRNA LOC389641. J. Immunol. Res. 2022, 2022, 5582811. [Google Scholar] [CrossRef] [PubMed]
- González-Santana, A.; Marrero-Hernández, S.; Dorta, I.; Hernández, M.; Pinto, F.M.; Báez, D.; Bello, A.R.; Candenas, L.; Almeida, T.A. Altered expression of the tachykinins substance P/neurokinin A/hemokinin-1 and their preferred neurokinin 1/neurokinin 2 receptors in uterine leiomyomata. Fertil. Steril. 2016, 106, 1521–1529. [Google Scholar] [CrossRef]
- Fackler, O.T.; Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008, 181, 879–884. [Google Scholar] [CrossRef]
- Meshki, J.; Douglas, S.D.; Lai, J.; Schwartz, L.; Kilpatrick, L.E.; Tuluc, F. Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J. Biol. Chem. 2009, 284, 9280–9289. [Google Scholar] [CrossRef]
- Coveñas, R.; Rodríguez, F.D.; Muñoz, M. The Neurokinin-1 Receptor: A Promising Antitumor Target. Receptors 2022, 1, 72–97. [Google Scholar] [CrossRef]
- Niu, X.; Hou, J.; Li, J. The NK1 receptor antagonist NKP608 inhibits proliferation of human colorectal cancer cells via Wnt signaling pathway. Biol. Res. 2018, 51, 14. [Google Scholar] [CrossRef]
- Zhou, J.; Ling, J.; Song, H.; Lv, B.; Wang, L.; Shang, J.; Wang, Y.; Chang, C.; Ping, F.; Qian, J. Neurokinin-1 receptor is a novel positive regulator of Wnt/ β-catenin signaling in melanogenesis. Oncotarget 2016, 7, 81268–81280. [Google Scholar] [CrossRef]
- Godwin, P.; Baird, A.M.; Heavey, S.; Barr, M.P.; O’Byrne, K.J.; Gately, K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front. Oncol. 2013, 3, 120. [Google Scholar] [CrossRef]
- Ständer, S.; Siepmann, D.; Herrgott, I.; Sunderkötter, C.; Luger, T.A. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef] [PubMed]
- Roila, F.; Rolski, J.; Ramlau, R.; Dediu, M.; Russo, M.W.; Bandekar, R.R.; Grunberg, S.M. Randomized, double-blind, dose-ranging trial of the oral neurokinin-1 receptor antagonist casopitant mesylate for the prevention of cisplatin-induced nausea and vomiting. Ann. Oncol. 2009, 20, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Harle, A.; Dockry, R.; Holt, K.; Russell, P.; Molassiotis, A.; Yorke, J.; Robinson, R.; Birrell, M.A.; Belvisi, M.G.; et al. Aprepitant for Cough in Lung Cancer. A Randomized Placebo-controlled Trial and Mechanistic Insights. Am. J. Respir. Crit. Care Med. 2021, 203, 737–745. [Google Scholar] [CrossRef]
- Noronha, V.; Bhattacharjee, A.; Patil, V.M.; Joshi, A.; Menon, N.; Shah, S.; Kannan, S.; Mukadam, S.A.; Maske, K.; Ishi, S.; et al. Aprepitant for Cough Suppression in Advanced Lung Cancer: A Randomized Trial. Chest 2020, 157, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Suzuki, Y.; Shibata, K.; Kawakami, J. Simple Liquid Chromatography-Tandem Mass Spectrometry Method for Quantitation of Total and Free Aprepitant and Its Active N-Dealkylated Metabolites in Human Plasma. Ther. Drug Monit. 2021, 43, 422–428. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Neurokinin-1 Receptor Antagonists as Anticancer Drugs. Lett. Drug Des. Discov. 2019, 16, 1110–1129. [Google Scholar] [CrossRef]
- Garnier, A.; Ilmer, M.; Kappler, R.; Berger, M. Therapeutic Innovations for Targeting Hepatoblastoma. Anticancer Res. 2016, 36, 5577–5592. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Hu, J.; Yang, W.; Ren, H.; Ding, D.; Zhang, L.; Liu, X. Neurokinin-1 activation affects EGFR related signal transduction in triple negative breast cancer. Cell Signal 2015, 27, 1315–1324. [Google Scholar] [CrossRef]
- Robinson, P.; Kasembeli, M.; Bharadwaj, U.; Engineer, N.; Eckols, K.T.; Tweardy, D.J. Substance P Receptor Signaling Mediates Doxorubicin-Induced Cardiomyocyte Apoptosis and Triple-Negative Breast Cancer Chemoresistance. BioMed Res. Int. 2016, 2016, 1959270. [Google Scholar] [CrossRef]
- Eblen, S.T.; Slack, J.K.; Weber, M.J.; Catling, A.D. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell Biol. 2002, 22, 6023–6033. [Google Scholar] [CrossRef]
- Al-Keilani, M.; Bdeir, R.; Elstaty, R.I.; Alqudah, M.A. Expression of substance P, neurokinin 1 receptor, Ki-67 and pyruvate kinase M2 in hormone receptor negative breast cancer and evaluation of impact on overall survival. BMC Cancer 2023, 23, 158. [Google Scholar] [CrossRef]
- Kitchens, C.A.; McDonald, P.R.; Pollack, I.F.; Wipf, P.; Lazo, J.S. Synergy between microtubule destabilizing agents and neurokinin 1 receptor antagonists identified by an siRNA synthetic lethal screen. FASEB J. 2009, 23, 756.13. [Google Scholar] [CrossRef]
- Alfieri, A.B.; Cubeddu, L.X. Efectos de los antagonistas de los receptores NK1 y de la dexametasona sobre la inflamación neurogénica inducida por ciclofosfamida y por radiación X, en la rata. Arch. Venez. Farmacol. Ter. 2004, 23, 61–66. [Google Scholar]
- Alfieri, A.B.; Cubeddu, L.X. Role of NK1 receptors on cisplatin-induced nephrotoxicity in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Quintana, A.; Trujillo-Hacha, P.; Piruat, J.I.; Bejarano-García, J.A.; García-Guerrero, E.; Pérez-Simón, J.A.; Muñoz, M. Human acute myeloid leukemia cells express Neurokinin-1 receptor, which is involved in the antileukemic effect of Neurokinin-1 receptor antagonists. Investig. New Drugs 2019, 37, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Crespo, J.C.; Crespo, J.P.; Coveñas, R. Neurokinin-1 receptor antagonist aprepitant and radiotherapy, a successful combination therapy in a patient with lung cancer: A case report. Mol. Clin. Oncol. 2019, 11, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martin, M.V.; de Agustin Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Munoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Kramer, M.S. NK1 receptor antagonists for depression: Why a validated concept was abandoned. J. Affect. Disord. 2017, 223, 121–125. [Google Scholar] [CrossRef]
- Ratti, E.; Bettica, P.; Alexander, R.; Archer, G.; Carpenter, D.; Evoniuk, G.; Gomeni, R.; Lawson, E.; Lopez, M.; Millns, H.; et al. Full central neurokinin-1 receptor blockade is required for efficacy in depression: Evidence from orvepitant clinical studies. J. Psychopharmacol. 2013, 27, 424–434. [Google Scholar] [CrossRef]
- Okumura, L.M.; da Silva Ries, S.A.; Meneses, C.F.; Michalowski, M.B.; Ferreira, M.A.P.; Moreira, L.B. Adverse events associated with aprepitant pediatric bone cancer patients. J. Oncol. Pharm. Pract. 2019, 25, 735–738. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Bashash, D.; Safaroghli-Azar, A.; Bayati, S.; Razani, E.; Pourbagheri-Sigaroodi, A.; Gharehbaghian, A.; Momeny, M.; Sanjadi, M.; Rezaie-Tavirani, M.; Ghaffari, S.H. Neurokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-κB axis: Shedding new light on resistance to Aprepitant. Int. J. Biochem. Cell Biol. 2018, 103, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Mirzavi, F.; Hashemy, S.I.; Khaleghi Ghadiri, M.; Stummer, W.; Gorji, A. The in vitro anti-cancer synergy of neurokinin-1 receptor antagonist, aprepitant, and 5-aminolevulinic acid in glioblastoma. Biofactors 2023, 49, 900–911. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Zhou, W.; Wang, Y.; Wang, X.; Ge, X.; Wang, F.; Zhou, F.; Deng, X.; Miao, L. Aprepitant inhibits the development and metastasis of gallbladder cancer via ROS and MAPK activation. BMC Cancer 2023, 23, 471–478. [Google Scholar] [CrossRef]
- Zheng, Y.; Sang, M.; Liu, F.; Gu, L.; Li, J.; Wu, Y.; Shan, B. Aprepitant inhibits the progression of esophageal squamous cancer by blocking the truncated neurokinin-1 receptor. Oncol. Rep. 2023, 50, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, T.; Liu, Q.; Cummins, S. Copy number alteration of neuropeptides and receptors in multiple cancers. Sci. Rep. 2017, 7, 4598. [Google Scholar] [CrossRef]
- Rapoport, B.L.; Jordan, K.; Weinstein, C. Neurokinin 1 receptor antagonists in the prevention of chemotherapy-induced nausea and vomiting: Focus on fosaprepitant. Future Oncol. 2018, 14, 77–92. [Google Scholar] [CrossRef]
- Navari, R.M. Fosaprepitant: A neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert. Rev. Anticancer. Ther. 2008, 8, 1733–1742. [Google Scholar] [CrossRef]
- Kanduluru, A.K.; Srinivasarao, M.; Wayua, C.; Low, P.S. Evaluation of a Neurokinin-1 Receptor-Targeted Technetium-99m Conjugate for Neuroendocrine Cancer Imaging. Mol. Imaging Biol. 2020, 22, 377–383. [Google Scholar] [CrossRef]
- Kanduluru, A.K.; Low, P.S. Development of a Ligand-Targeted Therapeutic Agent for Neurokinin-1 Receptor Expressing Cancers. Mol. Pharm. 2017, 14, 3859–3865. [Google Scholar] [CrossRef]
- Recio, R.; Vengut-Climent, E.; Mouillac, B.; Orcel, H.; López-Lázaro, M.; Calderón-Montaño, J.M.; Álvarez, E.; Khiar, N.; Fernández, I. Design, synthesis and biological studies of a library of NK1-Receptor Ligands Based on a 5-arylthiosubstituted 2-amino-4,6-diaryl-3-cyano-4H-pyran core: Switch from antagonist to agonist effect by chemical modification. Eur. J. Med. Chem. 2017, 138, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Recio, R.; Lerena, P.; Pozo, E.; Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M.; Valdivia, V.; Pernia Leal, M.; Mouillac, B.; Organero, J.Á.; et al. Carbohydrate-Based NK1R Antagonists with Broad-Spectrum Anticancer Activity. J. Med. Chem. 2021, 64, 10350–10370. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety of PVT-1 Treatment in Patients with Advanced Non-Small Cell Lung Cancer. Available online: https://www.clinicaltrials.gov/study/NCT04840004 (accessed on 24 October 2023).


| Cancer | Aprepitant | L-732,138 | L-733,060 | Others | References |
|---|---|---|---|---|---|
| Acute lymphoblastic leukemia | + | + | + | [51,105,106] | |
| Acute myeloid leukemia | + | + | + | CP-96,345 (+) | [104,105] |
| Breast cancer | + | + | + | CP-96,345 (+); RP-67,580 (+); SR-140,333 (+) | [102,103,107] |
| Cervical cancer | + | ns | ns | [89,108] | |
| Chronic myeloid leukemia | + | ns | + | [104] | |
| Colangiocarcinoma * | ns | ns | +↓ | [109] | |
| Colorectal carcinoma * | +,↓ | + | + | NKP-608 (+); SR-140,333 (+) | [96,97,110,111] |
| Esophageal carcinoma | + | ns | ns | [95,99,112] | |
| Gallbladder cancer * | ns | ns | ns | L-703,606 (+, ↓) | [70] |
| Gastric carcinoma | + | + | + | [22] | |
| Glioblastoma multiforme | + | + | + | [15,64,113,114] | |
| Head and neck cancer | ns | ns | ns | L-703,606 (+) | [98] |
| Hepatoblastoma * | +, ↓ | + | + | [115,116,117] | |
| Larynx carcinoma | + | + | + | [118] | |
| Lung cancer | + | + | + | [94] | |
| Melanoma | + | + | + | [119,120,121] | |
| Neuroblastoma | + | + | + | [122,123,124,125] | |
| Oral squamous cell carcinoma | ns | ns | ns | ||
| Osteosarcoma * | + | + | + | [88,91] | |
| Ovarian cancer | + | ns | ns | [92,126] | |
| Pancreatic carcinoma | + | + | + | [127,128] | |
| Prostate cancer * | +, ↓ | ns | ns | [93,120] | |
| Retinoblastoma | + | + | + | [22] | |
| Rhabdoid tumors | + | ns | ns | [90] | |
| Urinary bladder carcinoma | + | ns | ns | [100] | |
| Uterine leiomyomata | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coveñas, R.; Rodríguez, F.D.; Robinson, P.; Muñoz, M. The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant. Int. J. Mol. Sci. 2023, 24, 15936. https://doi.org/10.3390/ijms242115936
Coveñas R, Rodríguez FD, Robinson P, Muñoz M. The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant. International Journal of Molecular Sciences. 2023; 24(21):15936. https://doi.org/10.3390/ijms242115936
Chicago/Turabian StyleCoveñas, Rafael, Francisco D. Rodríguez, Prema Robinson, and Miguel Muñoz. 2023. "The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant" International Journal of Molecular Sciences 24, no. 21: 15936. https://doi.org/10.3390/ijms242115936
APA StyleCoveñas, R., Rodríguez, F. D., Robinson, P., & Muñoz, M. (2023). The Repurposing of Non-Peptide Neurokinin-1 Receptor Antagonists as Antitumor Drugs: An Urgent Challenge for Aprepitant. International Journal of Molecular Sciences, 24(21), 15936. https://doi.org/10.3390/ijms242115936