Effects of Modified Glucosamine on the Chondrogenic Potential of Circulating Stem Cells under Experimental Inflammation
Abstract
1. Introduction
2. Results
2.1. In Vitro Model
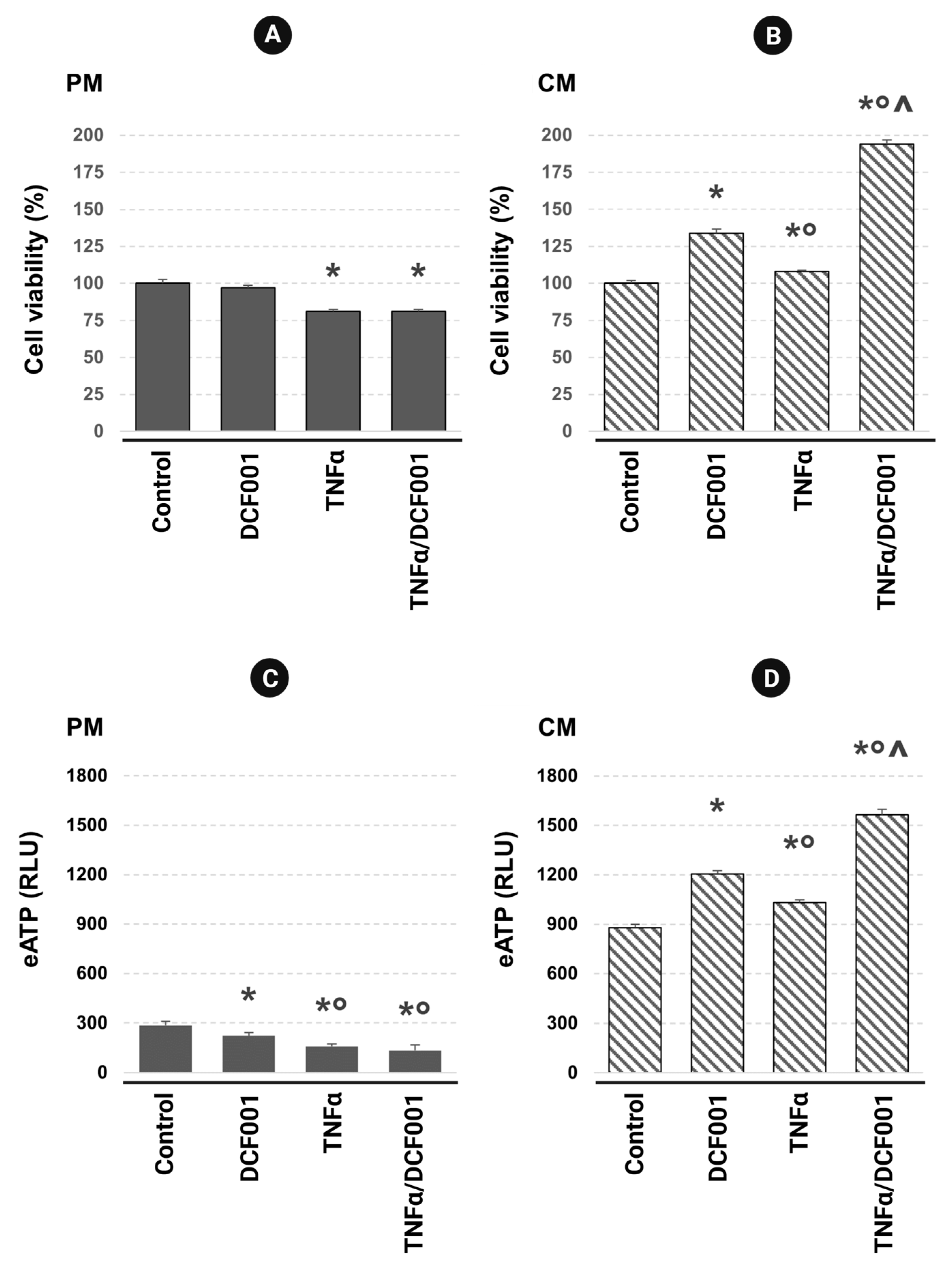
2.2. Cell Viability and ATP Release
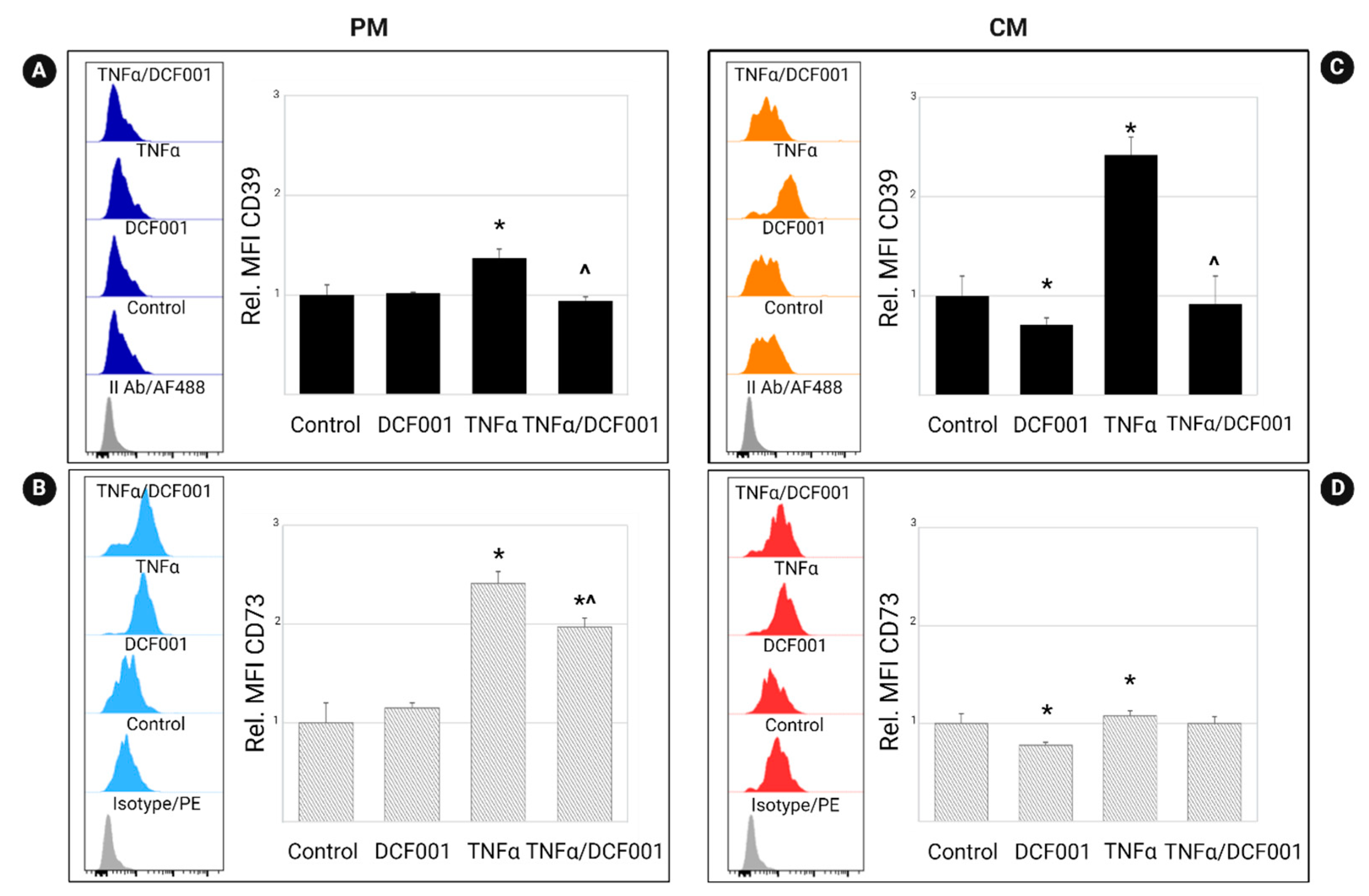
2.3. Effect of DCF001 on CD39 and CD73 Expression
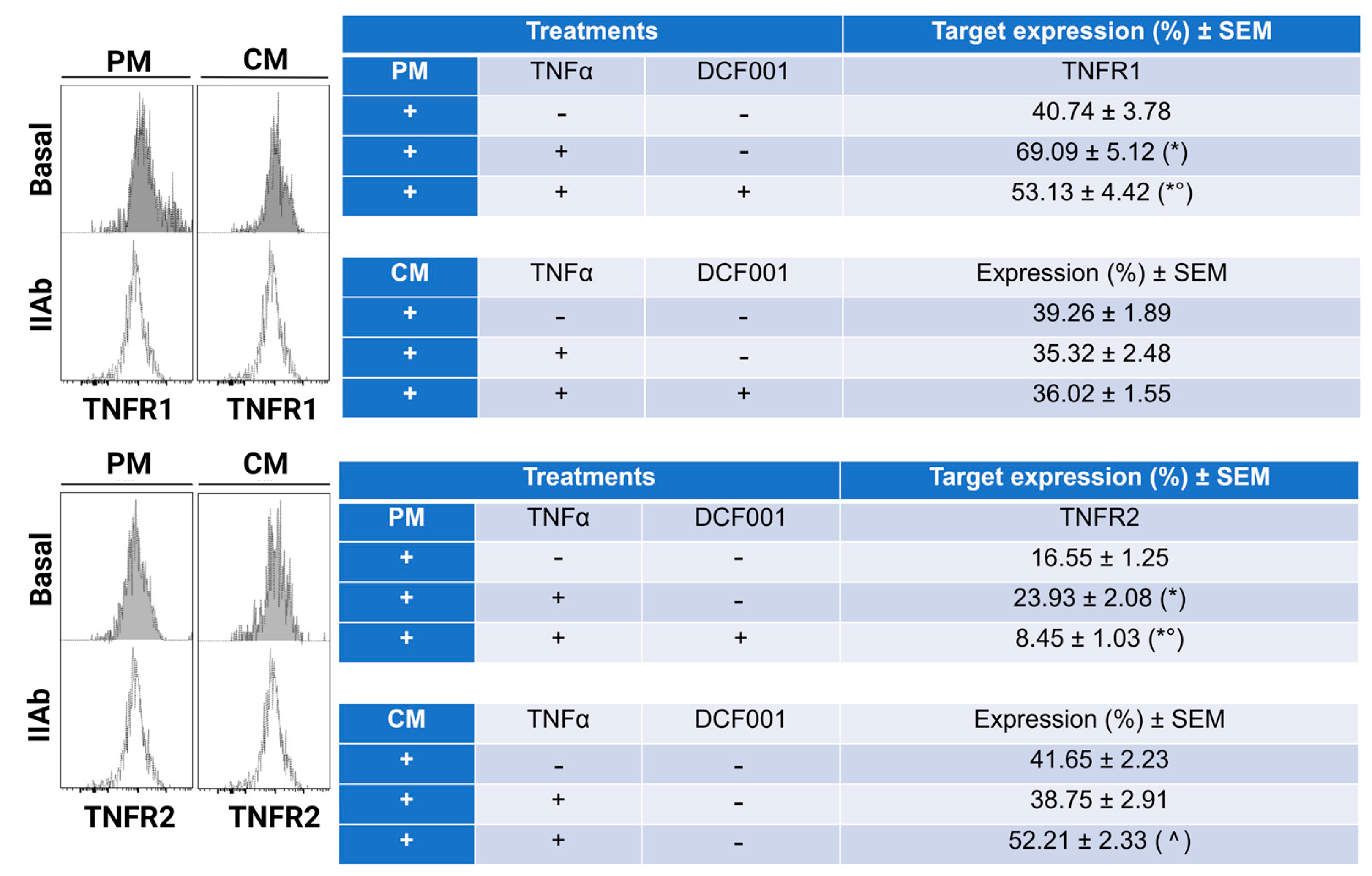
2.4. Effect of DCF001 on TNFα Receptors
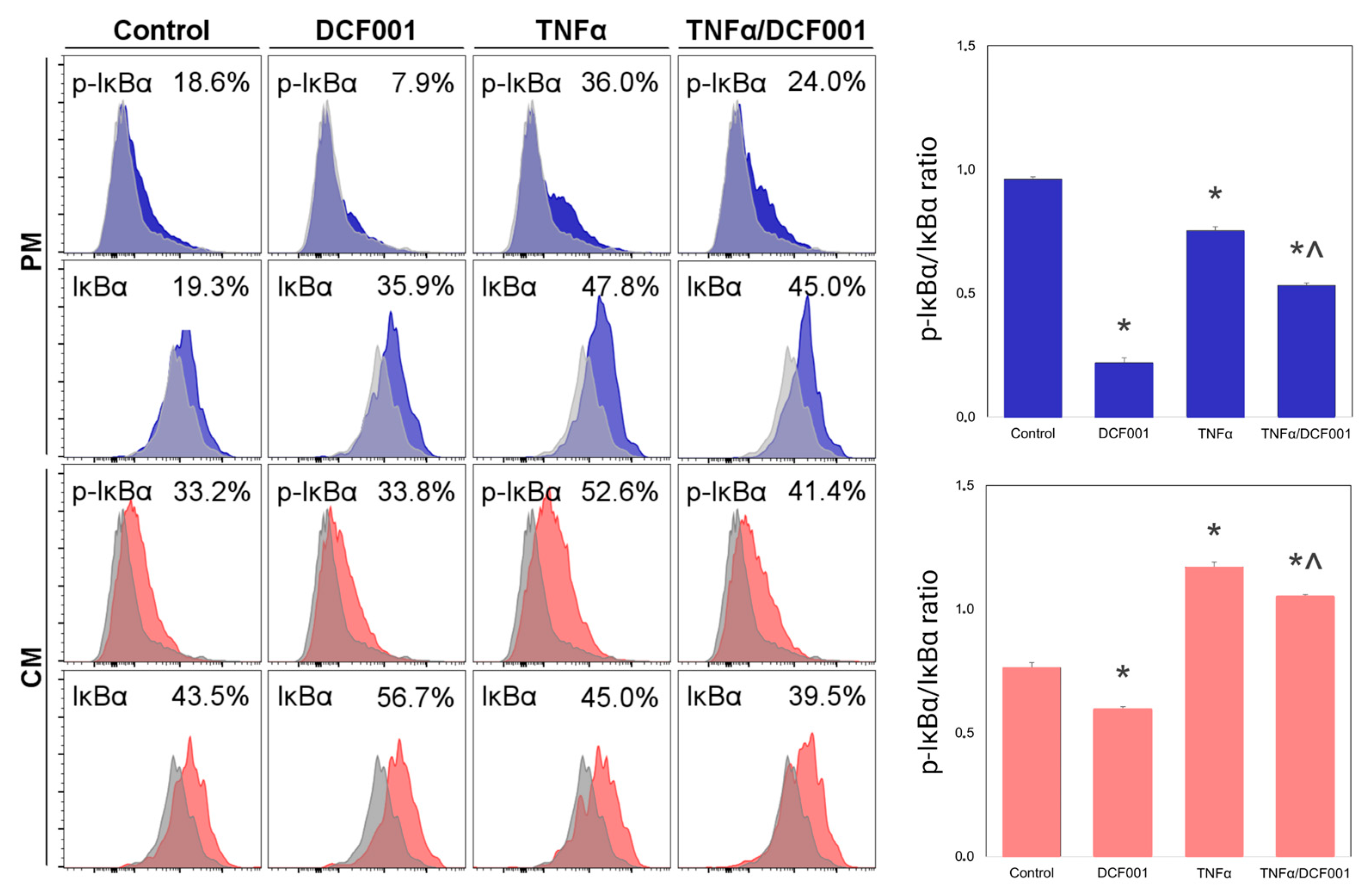
2.5. Effect of DCF001 on IκBα
2.6. Gene Expression
3. Discussion
4. Materials and Methods
4.1. In Vitro Model
4.2. Immunophenotyping
4.3. DCF001 Compound
4.4. Cell Treatments
4.5. Cell Viability Analysis
4.6. CellTiter-Glo® Assay
4.7. Flow Cytometry Analysis of CD39, CD73, and TNFα Receptors and IκBα
4.8. Gene Expression Study
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Persiani, S.; Rotini, R.; Trisolino, G.; Rovati, L.C.; Locatelli, M.; Paganini, D.; Antonioli, D.; Roda, A. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthr. Cartil. 2007, 15, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Z.; Hussain, S.M.; Cicuttini, F.M.; Wang, Y. Chapter 6—Nutrients and Dietary Supplements for Osteoarthritis. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 97–137. [Google Scholar]
- de Queiroz, R.M.; Oliveira, I.A.; Piva, B.; Bouchuid Catão, F.; da Costa Rodrigues, B.; da Costa Pascoal, A.; Diaz, B.L.; Todeschini, A.R.; Caarls, M.B.; Dias, W.B. Hexosamine Biosynthetic Pathway and Glycosylation Regulate Cell Migration in Melanoma Cells. Front. Oncol. 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Block, J.A.; Oegema, T.R.; Sandy, J.D.; Plaas, A. The effects of oral glucosamine on joint health: Is a change in research approach needed? Osteoarthr. Cartil. 2010, 18, 5–11. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, E.; Casa, B.; Bompani, R.; Scali, G.; Scali, M. Glucosamine sulphate: A controlled clinical investigation in arthrosis. Pharmatherapeutica 1981, 2, 504–508. [Google Scholar] [PubMed]
- Konopka, J.B. N-acetylglucosamine (GlcNAc) functions in cell signaling. Sci. Cairo 2012, 2012, 489208. [Google Scholar] [CrossRef]
- Grigorian, A.; Araujo, L.; Naidu, N.N.; Place, D.J.; Choudhury, B.; Demetriou, M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 2011, 286, 40133–40141. [Google Scholar] [CrossRef]
- Simon, R.R.; Marks, V.; Leeds, A.R.; Anderson, J.W. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab. Res. Rev. 2011, 27, 14–27. [Google Scholar] [CrossRef]
- Conrozier, T.; Lohse, T. Glucosamine as a Treatment for Osteoarthritis: What If It’s True? Front. Pharm. 2022, 13, 820971. [Google Scholar] [CrossRef]
- Noack, W.; Fischer, M.; Förster, K.K.; Rovati, L.C.; Setnikar, I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthr. Cartil. 1994, 2, 51–59. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Gulin, J.P.; Felson, D.T. Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. JAMA 2000, 283, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.S.; Tsourounis, C.; McCart, G.M. Glucosamine. Ann. Pharm. 1998, 32, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Chevalier, X.; Herrero-Beaumont, G.; McAlindon, T.; Mobasheri, A.; Pavelka, K.; Schön, C.; Weinans, H.; Biesalski, H.; Participants at the Hohenheim Consensus Conference in August 29th 2011. Physiological effects of oral glucosamine on joint health: Current status and consensus on future research priorities. BMC Res. Notes 2013, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.K.; Wu, S.J.; Kim, J.M. Preparation of glucosamine by hydrolysis of chitosan with commercial α-amylase and glucoamylase. J. Zhejiang Univ. Sci. B 2011, 12, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Shin, H.D.; Chen, R.; Li, J.; Du, G.; Chen, J. Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 6149–6158. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wang, S.L. Production of Thermophilic Chitinase by Paenibacillus sp. TKU052 by Bioprocessing of Chitinous Fishery Wastes and Its Application in N-acetyl-D-glucosamine Production. Polymers 2021, 13, 3048. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, X. Categories and biomanufacturing methods of glucosamine. Appl. Microbiol. Biotechnol. 2019, 103, 7883–7889. [Google Scholar] [CrossRef]
- Tiwari, A.D.; Panda, S.S.; Girgis, A.S.; Sahu, S.; George, R.F.; Srour, A.M.; La Starza, B.; Asiri, A.M.; Hall, C.D.; Katritzky, A.R. Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers. Org. Biomol. Chem. 2014, 12, 7238–7249. [Google Scholar] [CrossRef]
- Jones, R.A.; Thillier, Y.; Panda, S.S.; Rivera Rosario, N.; Hall, C.D.; Katritzky, A.R. Synthesis and characterisation of glucosamine-NSAID bioconjugates. Org. Biomol. Chem. 2014, 12, 8325–8335. [Google Scholar] [CrossRef]
- Lin, Y. Whole-process optimization for industrial production of glucosamine sulfate sodium chloride based on QbD concept. Chin. J. Chem. Eng. 2023, 54, 153–161. [Google Scholar] [CrossRef]
- Chen, J.K.; Shen, C.R.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.D.; Severson, D.K.; Grund, A.D.; Wassink, S.L.; Burlingame, R.P.; Berry, A.; Running, J.A.; Kunesh, C.A.; Song, L.; Jerrell, T.A.; et al. Metabolic engineering of Escherichia coli for industrial production of glucosamine and N-acetylglucosamine. Metab. Eng. 2005, 7, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Nicolosi, R.J.; Borzelleca, J.F. Glucosamine effects in humans: A review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem. Toxicol. 2005, 43, 187–201. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res. Ther. 2012, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Largo, R. Glucosamine and O-GlcNAcylation: A novel immunometabolic therapeutic target for OA and chronic, low-grade systemic inflammation? Ann. Rheum. Dis. 2020, 79, 1261–1263. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, Q.; Wang, Y.; Lee, B.; Betageri, G.V.; Chow, M.S.S.; Huang, M.; Zuo, Z. Bioavailability enhancement of glucosamine hydrochloride by chitosan. Int. J. Pharm. 2013, 455, 365–373. [Google Scholar] [CrossRef]
- Gilzad Kohan, H.; Kaur, K.; Jamali, F. Synthesis and characterization of a new Peptide prodrug of glucosamine with enhanced gut permeability. PLoS ONE 2015, 10, e0126786. [Google Scholar] [CrossRef]
- Heart, E.; Choi, W.S.; Sung, C.K. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E103–E112. [Google Scholar] [CrossRef]
- Uldry, M.; Ibberson, M.; Hosokawa, M.; Thorens, B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002, 524, 199–203. [Google Scholar] [CrossRef]
- Riegger, J.; Baumert, J.; Zaucke, F.; Brenner, R.E. The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model. Int. J. Mol. Sci. 2021, 22, 7247. [Google Scholar] [CrossRef] [PubMed]
- Brimble, S.; Wollaston-Hayden, E.E.; Teo, C.F.; Morris, A.C.; Wells, L. The Role of the O-GlcNAc Modification in Regulating Eukaryotic Gene Expression. Curr. Signal Transduct. Ther. 2010, 5, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Merrild, N.G.; Holzmann, V.; Ariosa-Morejon, Y.; Faull, P.A.; Coleman, J.; Barrell, W.B.; Young, G.; Fischer, R.; Kelly, D.J.; Addison, O.; et al. Local depletion of proteoglycans mediates cartilage tissue repair in an ex vivo integration model. Acta Biomater. 2022, 149, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Yamada, S. Congenital Disorders of Deficiency in Glycosaminoglycan Biosynthesis. Front. Genet. 2021, 12, 717535. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Jura-Półtorak, A.; Olczyk, K.; Komosinska-Vassev, K. The Diagnostic Usefulness of Circulating Profile of Extracellular Matrix Components: Sulfated Glycosaminoglycans (sGAG), Hyaluronan (HA) and Extracellular Part of Syndecan-1 (sCD138) in Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Med. 2021, 10, 1722. [Google Scholar] [CrossRef]
- Varghese, S.; Theprungsirikul, P.; Sahani, S.; Hwang, N.; Yarema, K.J.; Elisseeff, J.H. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthr. Cartil. 2007, 15, 59–68. [Google Scholar] [CrossRef]
- Derfoul, A.; Miyoshi, A.D.; Freeman, D.E.; Tuan, R.S. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthr. Cartil. 2007, 15, 646–655. [Google Scholar] [CrossRef]
- Veronesi, F.; Giavaresi, G.; Maglio, M.; Scotto d’Abusco, A.; Politi, L.; Scandurra, R.; Olivotto, E.; Grigolo, B.; Borzì, R.M.; Fini, M. Chondroprotective activity of N-acetyl phenylalanine glucosamine derivative on knee joint structure and inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 589–599. [Google Scholar] [CrossRef]
- Naito, K.; Watari, T.; Furuhata, A.; Yomogida, S.; Sakamoto, K.; Kurosawa, H.; Kaneko, K.; Nagaoka, I. Evaluation of the effect of glucosamine on an experimental rat osteoarthritis model. Life Sci. 2010, 86, 538–543. [Google Scholar] [CrossRef]
- Leatherwood, J.L.; Gehl, K.L.; Coverdale, J.A.; Arnold, C.E.; Dabareiner, R.A.; Walter, K.N.; Lamprecht, E.D. Influence of oral glucosamine supplementation in young horses challenged with intra-articular lipopolysaccharide. J. Anim. Sci. 2016, 94, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Scotto d’Abusco, A.; Politi, L.; Giordano, C.; Scandurra, R. A peptidyl-glucosamine derivative affects IKKalpha kinase activity in human chondrocytes. Arthritis Res. Ther. 2010, 12, R18. [Google Scholar] [CrossRef] [PubMed]
- Scotto d’Abusco, A.; Cicione, C.; Calamia, V.; Negri, R.; Giordano, C.; Grigolo, B.; Politi, L.; Scandurra, R. Glucosamine and its N-acetyl-phenylalanine derivative prevent TNF-alpha-induced transcriptional activation in human chondrocytes. Clin. Exp. Rheumatol. 2007, 25, 847–852. [Google Scholar]
- Nakamura, H.; Shibakawa, A.; Tanaka, M.; Kato, T.; Nishioka, K. Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clin. Exp. Rheumatol. 2004, 22, 293–299. [Google Scholar]
- Gouze, J.-N.; Gouze, E.; Popp, M.P.; Bush, M.L.; Dacanay, E.A.; Kay, J.D.; Levings, P.P.; Patel, K.R.; Saran, J.-P.S.; Watson, R.S.; et al. Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1β. Arthritis Res. Ther. 2006, 8, R173. [Google Scholar] [CrossRef]
- Someya, A.; Ikegami, T.; Sakamoto, K.; Nagaoka, I. Glucosamine Downregulates the IL-1β-Induced Expression of Proinflammatory Cytokine Genes in Human Synovial MH7A Cells by O-GlcNAc Modification-Dependent and -Independent Mechanisms. PLoS ONE 2016, 11, e0165158. [Google Scholar] [CrossRef]
- Drovanti, A.; Bignamini, A.A.; Rovati, A.L. Therapeutic activity of oral glucosamine sulfate in osteoarthrosis: A placebo-controlled double-blind investigation. Clin. Ther. 1980, 3, 260–272. [Google Scholar]
- Müller-Fassbender, H.; Bach, G.L.; Haase, W.; Rovati, L.C.; Setnikar, I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthr. Cartil. 1994, 2, 61–69. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Smith, L.; Reginster, J.Y.; Bruyère, O.; Beaudart, C.; Honvo, G.; Maggi, S. Glucosamine sulphate: An umbrella review of health outcomes. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x20975927. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., 3rd; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Marczyński, W.; Tłustochowicz, W.; Tomaszewski, W.; Białecki, J. Literature Analysis Regarding the Combination of Substances: Glucosamine + Chondroitin in the Treatment of Osteoarthritis. Ortop. Traumatol. Rehabil. 2022, 24, 407–416. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Gong, C. Repairing effects of glucosamine sulfate in combination with etoricoxib on articular cartilages of patients with knee osteoarthritis. J. Orthop. Surg. Res. 2020, 15, 150. [Google Scholar] [CrossRef]
- Bassleer, C.; Rovati, L.; Franchimont, P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthr. Cartil. 1998, 6, 427–434. [Google Scholar] [CrossRef]
- Ando, M.; Magi, S.; Seki, M.; Suzuki, Y.; Kasukawa, T.; Lefaudeux, D.; Hoffmann, A.; Okada, M. IκBα is required for full transcriptional induction of some NFκB-regulated genes in response to TNF in MCF-7 cells. NPJ Syst. Biol. Appl. 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Mathes, E.; O’Dea, E.L.; Hoffmann, A.; Ghosh, G. NF-kappaB dictates the degradation pathway of IkappaBalpha. Embo J. 2008, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, C.; Greiner, J.F.W.; Kaltschmidt, B. The Transcription Factor NF-κB in Stem Cells and Development. Cells 2021, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Lu, A.; Dorronsoro, A.; Scibetta, A.; Robbins, P.D.; Niedernhofer, L.J.; Huard, J. Inhibition of NF-κB improves the stress resistance and myogenic differentiation of MDSPCs isolated from naturally aged mice. PLoS ONE 2017, 12, e0179270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, Y.; Chen, G.; Yang, Y.; Li, Z.; Chen, T.; Sun, W.; Yu, M.; Pan, K.; Guo, W.; Tian, W. Effect of canonical NF-κB signaling pathway on the differentiation of rat dental epithelial stem cells. Stem Cell Res. Ther. 2019, 10, 139. [Google Scholar] [CrossRef]
- Chang, J.; Liu, F.; Lee, M.; Wu, B.; Ting, K.; Zara, J.N.; Soo, C.; Al Hezaimi, K.; Zou, W.; Chen, X.; et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 9469–9474. [Google Scholar] [CrossRef]
- Chisari, E.; Yaghmour, K.M.; Khan, W.S. The effects of TNF-alpha inhibition on cartilage: A systematic review of preclinical studies. Osteoarthr. Cartil. 2020, 28, 708–718. [Google Scholar] [CrossRef]
- Markway, B.D.; Cho, H.; Anderson, D.E.; Holden, P.; Ravi, V.; Little, C.B.; Johnstone, B. Reoxygenation enhances tumour necrosis factor alpha-induced degradation of the extracellular matrix produced by chondrogenic cells. Eur. Cell Mater. 2016, 31, 425–439. [Google Scholar] [CrossRef]
- Urech, D.M.; Feige, U.; Ewert, S.; Schlosser, V.; Ottiger, M.; Polzer, K.; Schett, G.; Lichtlen, P. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNF{alpha} single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann. Rheum. Dis. 2010, 69, 443–449. [Google Scholar] [CrossRef]
- Güler-Yüksel, M.; Allaart, C.F.; Watt, I.; Goekoop-Ruiterman, Y.P.; de Vries-Bouwstra, J.K.; van Schaardenburg, D.; van Krugten, M.V.; Dijkmans, B.A.; Huizinga, T.W.; Lems, W.F.; et al. Treatment with TNF-α inhibitor infliximab might reduce hand osteoarthritis in patients with rheumatoid arthritis. Osteoarthr. Cartil. 2010, 18, 1256–1262. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, D.; Xu, R.H. Critical Role of Tumor Necrosis Factor Signaling in Mesenchymal Stem Cell-Based Therapy for Autoimmune and Inflammatory Diseases. Front. Immunol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Sitcheran, R.; Cogswell, P.C.; Baldwin, A.S., Jr. NF-kappaB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003, 17, 2368–2373. [Google Scholar] [CrossRef]
- Catrina, A.I.; Lampa, J.; Ernestam, S.; af Klint, E.; Bratt, J.; Klareskog, L.; Ulfgren, A.K. Anti-tumour necrosis factor (TNF)-alpha therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 2002, 41, 484–489. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Cruceta, J.; Graves, B.D.; Einhorn, T.A. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs 2001, 169, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Dorn, G.W., 2nd; Mann, D.L. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. J. Biol. Chem. 1997, 272, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Cardoso, R.; Pereira-Costa, F.; Pedro Faria, J.; Bandarrinha, P.; Bessa-Andrês, C.; Correia-de-Sá, P.; Bernardo Noronha-Matos, J. Adenosinergic signalling in chondrogenesis and cartilage homeostasis: Friend or foe? Biochem. Pharm. 2020, 174, 113784. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, A.I.; Beekhuizen, M.; t Hart, M.C.; Radstake, T.R.; Dhert, W.J.; Saris, D.B.; van Osch, G.J.; Creemers, L.B. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res. Ther. 2014, 16, 441. [Google Scholar] [CrossRef]
- Chu, C.Q.; Field, M.; Feldmann, M.; Maini, R.N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991, 34, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.S.; Wang, J.F.; Zhang, S.L.; Li, Z.; Pei, Z.; Guan, Z.P. Effects of Tumor Necrosis Factor Alpha on the Expression of Programmed Cell Death Factor 5 in Arthritis. Orthop. Surg. 2019, 11, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef]
- Murakami, S.; Lefebvre, V.; de Crombrugghe, B. Potent Inhibition of the Master Chondrogenic FactorSox9 Gene by Interleukin-1 and Tumor Necrosis Factor-α*. J. Biol. Chem. 2000, 275, 3687–3692. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Chen, S.; Tan, Z.; Xiong, K.; Li, Y.; Ye, Y.; Luo, Z.-P.; He, F.; Gong, Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic. Biol. Med. 2014, 68, 234–246. [Google Scholar] [CrossRef]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Müller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB–dependent pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef]
- Stanish, W.D.; McCormack, R.; Forriol, F.; Mohtadi, N.; Pelet, S.; Desnoyers, J.; Restrepo, A.; Shive, M.S. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Jt. Surg. Am. 2013, 95, 1640–1650. [Google Scholar] [CrossRef]
- Chung, J.Y.; Lee, D.H.; Kim, T.H.; Kwack, K.S.; Yoon, K.H.; Min, B.H. Cartilage extra-cellular matrix biomembrane for the enhancement of microfractured defects. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 1249–1259. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Sun, C.; Wang, Q.; Yang, W.; Zhang, Z.; Xia, Z.; Shao, Z.; Wang, B. Endogenous Repair and Regeneration of Injured Articular Cartilage: A Challenging but Promising Therapeutic Strategy. Aging Dis. 2021, 12, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Voskamp, C.; Koevoet, W.J.L.M.; Somoza, R.A.; Caplan, A.I.; Lefebvre, V.; van Osch, G.J.V.M.; Narcisi, R. Enhanced Chondrogenic Capacity of Mesenchymal Stem Cells after TNFα Pre-treatment. Front. Bioeng. Biotechnol. 2020, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Suh, H.N.; Kim, M.O.; Han, H.J. Glucosamine-induced reduction of integrin β4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem Cells Dev. 2013, 22, 2975–2989. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, W.; Chen, H.; Shao, X.; Lin, P.; Liu, X.; Li, X.; Ye, H. Glucosamine promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2018, 42, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L. Extracellular ATP: Effects, sources and fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Chen, Y.F.; Cowan, P.J.; Chen, X.P. Extracellular ATP signaling and clinical relevance. Clin. Immunol. 2018, 188, 67–73. [Google Scholar] [CrossRef]
- Corciulo, C.; Lendhey, M.; Wilder, T.; Schoen, H.; Cornelissen, A.S.; Chang, G.; Kennedy, O.D.; Cronstein, B.N. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat. Commun. 2017, 8, 15019. [Google Scholar] [CrossRef]
- Corciulo, C.; Cronstein, B.N. Signaling of the Purinergic System in the Joint. Front. Pharm. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Hanley, P.J.; Musset, B.; Renigunta, V.; Limberg, S.H.; Dalpke, A.H.; Sus, R.; Heeg, K.M.; Preisig-Müller, R.; Daut, J. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc. Natl. Acad. Sci. USA 2004, 101, 9479–9484. [Google Scholar] [CrossRef]
- Dexheimer, V.; Frank, S.; Richter, W. Proliferation as a requirement for in vitro chondrogenesis of human mesenchymal stem cells. Stem Cells Dev. 2012, 21, 2160–2169. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nakamura, T.; Doyle, A.; Ishikawa, M.; de Vega, S.; Fukumoto, S.; Yamada, Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 2010, 285, 18948–18958. [Google Scholar] [CrossRef]
- Kwon, H.J. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J. Endocrinol. 2012, 214, 337–348. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Kerkelä, E.; Laitinen, A.; Räbinä, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T cells. Stem Cells 2016, 34, 781–790. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Beldi, G.; Khosravi, M.; Abdelgawad, M.E.; Salomon, B.L.; Uzan, G.; Haouas, H.; Naserian, S. TNFα/TNFR2 signaling pathway: An active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res. Ther. 2020, 11, 281. [Google Scholar] [CrossRef]
- Wei, J.; Wang, K.; Hettinghouse, A.; Liu, C. Atsttrin Promotes Cartilage Repair Primarily Through TNFR2-Akt Pathway. Front. Cell Dev. Biol. 2020, 8, 577572. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Bi, R.; Liu, X.; Mei, J.; Jiang, N.; Zhu, S. Notch Signaling Regulates MMP-13 Expression via Runx2 in Chondrocytes. Sci. Rep. 2019, 9, 15596. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in Arthritis: Cell biology of osteoarthritis. Arthritis Res. Ther. 2001, 3, 107. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Hong, Y.; Park, E.Y.; Kim, D.; Lee, H.; Jung, H.S.; Jun, H.S. Glucosamine potentiates the differentiation of adipose-derived stem cells into glucose-responsive insulin-producing cells. Ann. Transl. Med. 2020, 8, 561. [Google Scholar] [CrossRef]
- Sandell, L.J.; Morris, N.; Robbins, J.R.; Goldring, M.B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: Differential expression of the amino-propeptide. J. Cell Biol. 1991, 114, 1307–1319. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1-SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Vornehm, S.I.; Dudhia, J.; Von der Mark, K.; Aigner, T. Expression of collagen types IX and XI and other major cartilage matrix components by human fetal chondrocytes in vivo. Matrix. Biol. 1996, 15, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Vuorio, E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J. Cell Biol. 1987, 104, 1077–1084. [Google Scholar] [CrossRef]
- Müller, P.K.; Lemmen, C.; Gay, S.; Gauss, V.; Kühn, K. Immunochemical and biochemical study of collagen synthesis by chondrocytes in culture. Exp. Cell Res. 1977, 108, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, E.; Aigner, T.; von der Mark, K.; Stöss, H.; Bertling, W. In situ hybridization studies on the expression of type X collagen in fetal human cartilage. Dev. Biol. 1991, 148, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte Hypertrophy in Osteoarthritis: Mechanistic Studies and Models for the Identification of New Therapeutic Strategies. Cells 2022, 11, 4034. [Google Scholar] [CrossRef]
- Wang, M.; Sampson, E.R.; Jin, H.; Li, J.; Ke, Q.H.; Im, H.J.; Chen, D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013, 15, R5. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Bertalot, T.; Vicentini, N.; Gatti, T.; Tescari, S.; De Filippis, V.; Marega, C.; Menna, E.; Gasparella, M.; Parnigotto, P.P.; et al. Neuronal commitment of human circulating multipotent cells by carbon nanotube-polymer scaffolds and biomimetic peptides. Nanomedicine 2016, 11, 1929–1946. [Google Scholar] [CrossRef]
- Messore, A.; Madia, V.N.; Pescatori, L.; Saccoliti, F.; Tudino, V.; De Leo, A.; Bortolami, M.; De Vita, D.; Scipione, L.; Pepi, F.; et al. Novel Symmetrical Benzazolyl Derivatives Endowed with Potent Anti-Heparanase Activity. J. Med. Chem. 2018, 61, 10834–10859. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2−∆∆CT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]


| Primary Antibodies | Manufacturing Company |
| Mouse anti-human CD39 | Santa Cruz Biotecnology, Inc. (Dallas, TX, USA) |
| FITC mouse anti-human CD44 | BioLegend, Inc. (San Diego, CA, USA) |
| PE mouse anti-human CD45 | Santa Cruz Biotecnology, Inc. |
| PE mouse anti-human CD73 | BioLegend, Inc. |
| FITC mouse anti-human CD90 | Santa Cruz Biotecnology, Inc. |
| PE mouse anti-human CD105 | Santa Cruz Biotecnology, Inc. |
| PE mouse anti-human HLA DR | Santa Cruz Biotecnology, Inc. |
| Rabbit anti-human TNFRI | Immunological Sciences (Rome, Italy) |
| Rabbit anti-human TNFRII | Immunological Sciences |
| Mouse anti-human phospho-IκBα (Ser32/36) | Cell Signaling Technology (Danvers, MA, USA) |
| Rabbit anti-human IκBα | Cell Signaling Technology |
| Isotype controls | Manufacturing Company |
| FITC Isotype Control | Santa Cruz Biotechnology, Inc. |
| PE Isotype Control | BD Biosciences (San Jose, CA, USA) |
| Secondary antibodies | Manufacturing Company |
| Alexa Fluor® 488-conjugated anti-rabbit secondary antibody | Invitrogen (Waltham, MA, USA) |
| Alexa Fluor® 488-conjugated anti-mouse secondary antibody | Invitrogen |
| Target Gene | Acronym | Sequence (5′–3′) | Reference Sequence |
|---|---|---|---|
| Hypoxanthine Phosphoribosyltransferase1 | HPRT1 | F: TGGACAGGACTGAACGTCTTGCT R: TTGAGCACACAGAGGGCTACAATG | NM_000194.2 |
| Collagen type II alpha 1 chain | COL2A1 | F: CGGGCAGAGGGCAATAGCAGGTT R: CAATGATGGGGAGGCGTGAG | NM_001844.4 |
| Runt-related transcription factor | RUNX2 | F: TCCGGAATGCCTCTTGCTGTTATGA R: ACTGAGGCGGTCAGAGAACAAACT | BC108919 |
| Matrix metallopeptidase 13 | MMP13 | F: GTTGGTCCGATGTAACTCCTC R: GAAGTCGCCATGCTCCTTAAT | NM_002427 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparella, M.; Cenzi, C.; Piccione, M.; Madia, V.N.; Di Santo, R.; Tudino, V.; Artico, M.; Taurone, S.; De Ponte, C.; Costi, R.; et al. Effects of Modified Glucosamine on the Chondrogenic Potential of Circulating Stem Cells under Experimental Inflammation. Int. J. Mol. Sci. 2023, 24, 10397. https://doi.org/10.3390/ijms241210397
Gasparella M, Cenzi C, Piccione M, Madia VN, Di Santo R, Tudino V, Artico M, Taurone S, De Ponte C, Costi R, et al. Effects of Modified Glucosamine on the Chondrogenic Potential of Circulating Stem Cells under Experimental Inflammation. International Journal of Molecular Sciences. 2023; 24(12):10397. https://doi.org/10.3390/ijms241210397
Chicago/Turabian StyleGasparella, Marco, Carola Cenzi, Monica Piccione, Valentina Noemi Madia, Roberto Di Santo, Valeria Tudino, Marco Artico, Samanta Taurone, Chiara De Ponte, Roberta Costi, and et al. 2023. "Effects of Modified Glucosamine on the Chondrogenic Potential of Circulating Stem Cells under Experimental Inflammation" International Journal of Molecular Sciences 24, no. 12: 10397. https://doi.org/10.3390/ijms241210397
APA StyleGasparella, M., Cenzi, C., Piccione, M., Madia, V. N., Di Santo, R., Tudino, V., Artico, M., Taurone, S., De Ponte, C., Costi, R., & Di Liddo, R. (2023). Effects of Modified Glucosamine on the Chondrogenic Potential of Circulating Stem Cells under Experimental Inflammation. International Journal of Molecular Sciences, 24(12), 10397. https://doi.org/10.3390/ijms241210397









