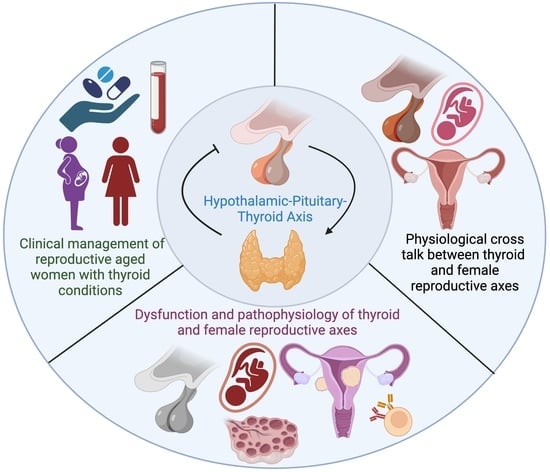
The Thyroid Hormone Axis and Female Reproduction
Abstract
:1. Introduction
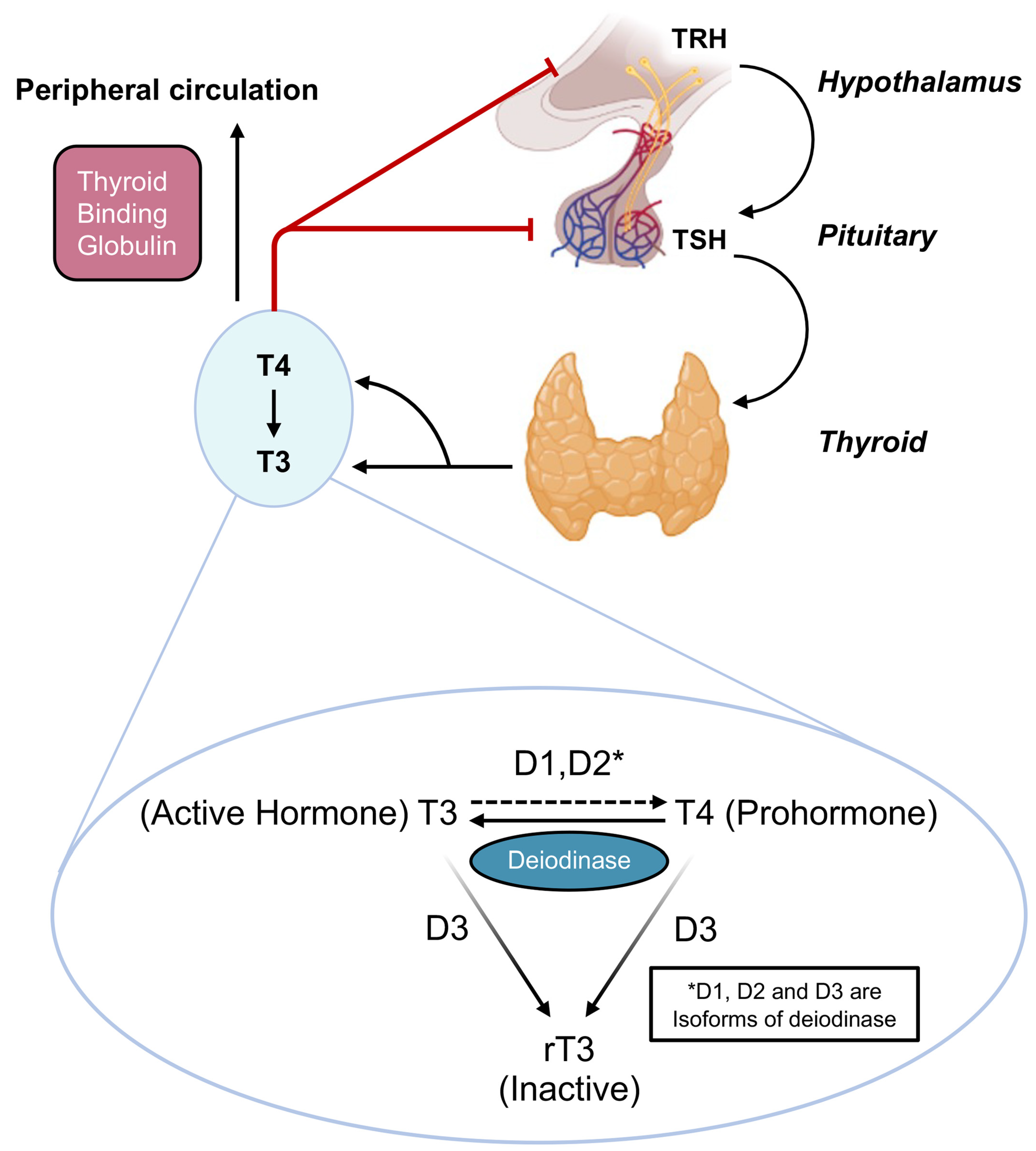
2. Overview of the Hypothalamic Pituitary Thyroid (HPT) Axis
3. Thyroid Hormones and Reproductive Physiology
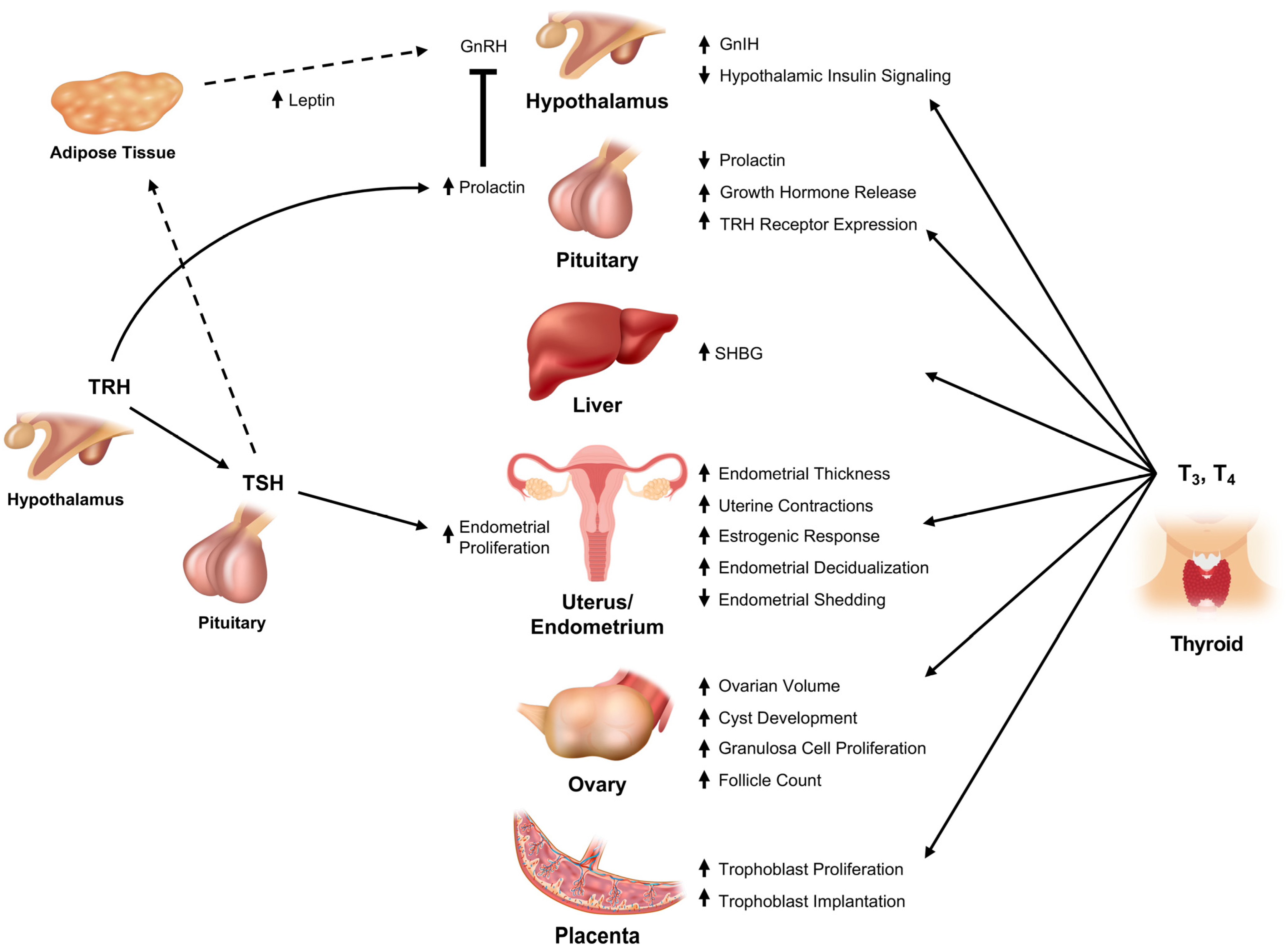
3.1. Thyroid Interface with the Hypothalamic-Pituitary Reproductive Axis
3.1.1. Thyrotropin Releasing Hormone (TRH)
3.1.2. Thyroid Stimulating Hormone
3.1.3. Triiodothyronine (T3) and Tetraiodothyronine (T4)
3.2. Lower Reproductive Axis
3.2.1. Ovary
3.2.2. Uterus and Fallopian Tubes
3.2.3. Maternal-Fetal Unit
3.3. Peripheral Transport of Gonadal Steroids
3.3.1. The Effect of Thyroid Dysfunction on Reproduction
3.3.2. Effect on Central Reproductive Control
3.3.3. Effect on the Ovary
3.3.4. Uterine and Endometrial Dysfunction
3.3.5. Pregnancy
| Study | Participants | Intervention | Outcome |
|---|---|---|---|
| Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications [204] | A total of 984 pregnant women were studied from November 2002 to October 2004; 11.7% were thyroid peroxidase antibody positive (TPOAb+). | TPOAb+ patients were divided into two groups: group A (n = 57) was treated with LT4 (1 μg/kg/day for TSH > 2.0 mU/L or TPOAb titer > 1500 kIU/L), and group B (n = 58) was not treated. The 869 TPOAb− patients (group C) served as a normal population control group. | Study suggests an association between thyroid autoimmunity and pregnancy-related adverse outcomes, particularly miscarriage and preterm delivery, and levothyroxine reduces this risk. |
| Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception (TABLET) [206] | A total of 19,585 women from 49 hospitals in the United Kingdom underwent testing for thyroid peroxidase antibodies and thyroid function | Randomly assigned 952 women to receive either LT4 (50 μg for TSH > 2.0 mU/L) or placebo (476 women in each group) before conception through the end of pregnancy. The primary outcome was live birth after at least 34 weeks of gestation. | There were no significant between-group differences in other pregnancy outcomes, including pregnancy loss or preterm birth, or in neonatal outcomes. Serious adverse events occurred in 5.9% of women in the levothyroxine group and 3.8% in the placebo group (p = 0.14). |
| Effect of Levothyroxine on Miscarriage Among Women with Normal Thyroid Function and Thyroid Autoimmunity Undergoing In Vitro Fertilization and Embryo Transfer: A Randomized Clinical Trial (POSTAL) [205] | 600 women undergoing in vitro fertilization and embryo transfer who were TPOAb+ but who had a normal thyroid function | The intervention group (n = 300) received either a 25-μg/day or 50-μg/day dose of levothyroxine at study initiation that was titrated according to the level of thyroid-stimulating hormone (>2.5 mU/L) during pregnancy. The women in the control group (n = 300) did not receive levothyroxine. All participants received the same IVF-ET and follow-up protocols | Among women in China who had intact thyroid function and TPOAb+ and were undergoing IVF-ET, treatment with levothyroxine, compared with no levothyroxine treatment, did not reduce miscarriage rates or increase live-birth rates. |
| Subclinical Hypothyroidism and Pregnancy Outcomes [215] | All women who presented to Parkland Hospital for prenatal care between 1 November 2000, and 14 April 2003. A total of 25,756 women underwent thyroid screening, 17,298 enrolled for prenatal care at 20 weeks of gestation or less, and 404 were diagnosed with subclinical hypothyroidism. | Thyroid screening using a chemiluminescent TSH assay. Women with TSH values at or above the 97.5th percentile for gestational age at screening and with free thyroxine more than 0.680 ng/dL were retrospectively identified with subclinical hypothyroidism. Pregnancy outcomes were compared with those in pregnant women with normal TSH values between the 5th and 95th percentiles. | Pregnancies in women with subclinical hypothyroidism were 3 times more likely to be complicated by placental abruption and 2 times more likely to be complicated by pre-term birth. Previously reported reduction in intelligence quotient of offspring of women with subclinical hypothyroidism may be related to the effects of prematurity. |
| Maternal Thyroid Hypofunction and Pregnancy Outcome [216] | A total of 10,990 patients had first- and second-trimester serum assayed for thyroid-stimulating hormone (TSH), free thyroxine (freeT4), and antithyroglobulin and antithyroid peroxidase antibodies. Thyroid hypofunction was defined as (1) subclinical hypothyroidism: TSH levels above the 97.5th percentile and free T4 between the 2.5th and 97.5th percentiles or (2) hypothyroxinemia: TSH between the 2.5th and 97.5th percentiles and free T4 below the 2.5th percentile. | Adverse outcomes were evaluated. Patients with thyroid hypofunction were compared with euthyroid patients (TSH and free T4 between the 2.5th and 97.5th percentiles). Patients with and without antibodies were compared. Multivariable logistic regression analysis adjusted for confounders was used. | Maternal thyroid hypofunction was not associated with any observed pattern of adverse outcomes. |
| Hypothyroxinemia and TPO-Antibody Positivity Are Risk Factors for Premature Delivery: The Generation R Study [200] | Serum TSH, free T4 (FT4), T4, and TPO antibodies (TPOAbs) were determined during early pregnancy in 5971 pregnant women from the Generation R study. | This observational study was embedded in the Generation R Study, a population-based prospective cohort from early fetal life onward in Rotterdam, Netherlands. | Hypothyroxinemia and TPOAb positivity were associated with a 2.5-fold and 1.7-fold increased risk of premature delivery respectively. The increased risk in TPOAb-positive women was found to be independent of thyroid function. |
4. Approach to Management of Thyroid-Related Reproductive Dysfunction
4.1. Non-Pregnant Women
4.1.1. Hypothyroidism
4.1.2. Hyperthyroidism
4.1.3. Thyroid Nodules
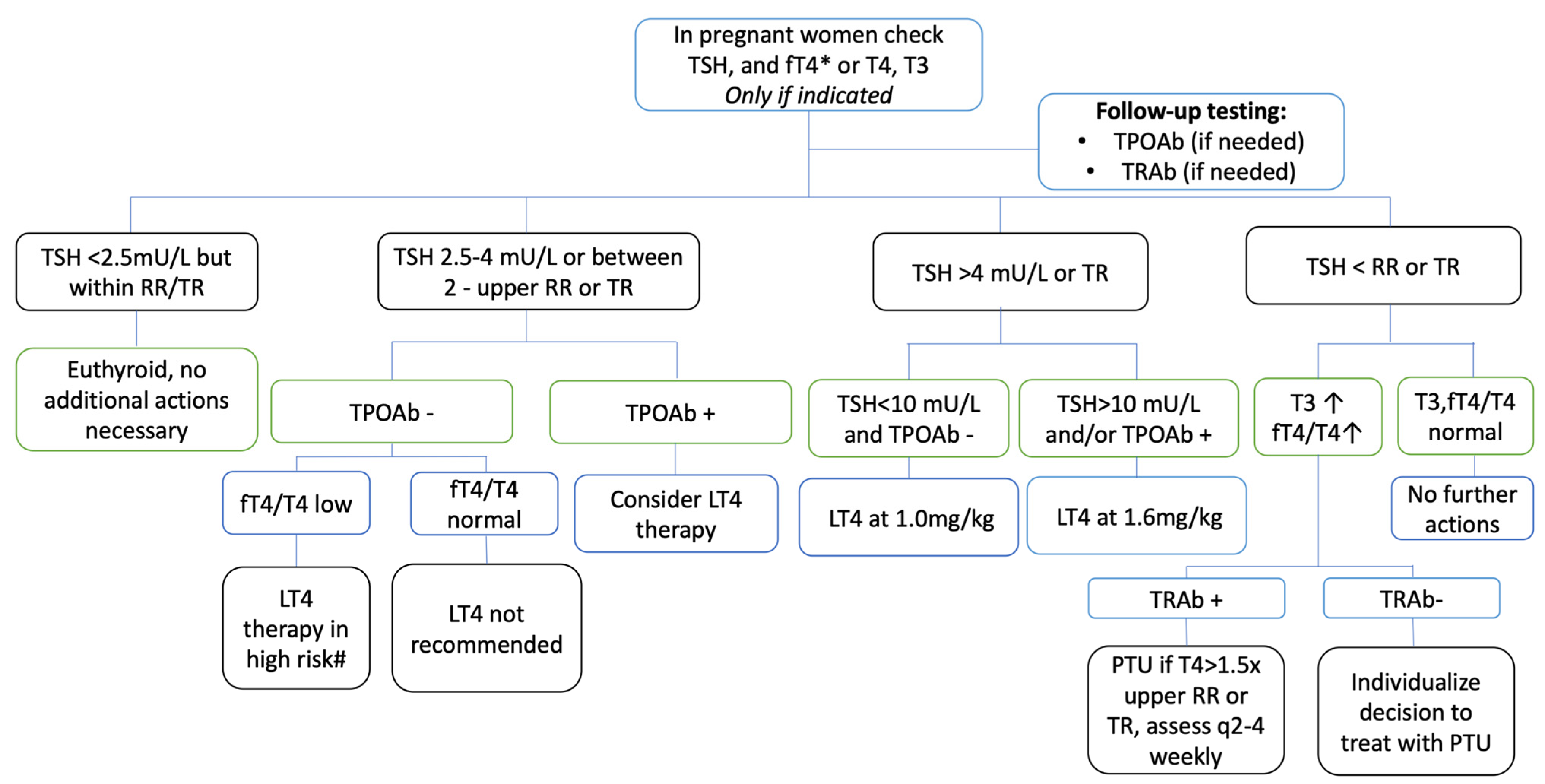
4.2. Pregnant Women
4.2.1. Hypothyroidism
4.2.2. Hyperthyroidism
4.2.3. Thyroid Nodules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TSH | Thyroid Stimulating Hormone |
| TPOAB | Anti-TPO Antibody |
| SCH | Subclinical Hypothyroidism |
| GnRH | Gonadotropin-Releasing Hormone |
| AITD | Autoimmune Thyroid Disorder |
| PCOS | Polycystic Ovary Syndrome |
| THs | Thyroid Hormones |
| HPT | Hypothalamic-Pituitary-Thyroid |
| TRH | Thyrotropin Releasing Hormone |
| T3 | Triiodothyronine |
| T4 | Tetraiodothyronine |
| LH | Luteinizing Hormone |
| FSH | Follicle Stimulating Hormone |
| LT4 | Levothyroxine |
| GnIH | Gonadotropin-Inhibiting Hormone |
| SHBG | Sex Hormone-Binding Globulin |
| GTT | Gestational Transient Thyrotoxicosis |
| POI | Premature Ovarian Insufficiency |
References
- Strikić Đula, I.; Pleić, N.; Babić Leko, M.; Gunjača, I.; Torlak, V.; Brdar, D.; Punda, A.; Polašek, O.; Hayward, C.; Zemunik, T. Epidemiology of Hypothyroidism, Hyperthyroidism and Positive Thyroid Antibodies in the Croatian Population. Biology 2022, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Stoll, K. Disparities in Thyroid Screening and Medication Use in Quebec, Canada. Health Equity 2019, 3, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, J.; Abubaker, F.; Saber, F.A. Thyroid dysfunction among adults in Bahrain: A hospital-based study. Neuro Endocrinol. Lett. 2020, 41, 1–9. [Google Scholar] [PubMed]
- Song, R.H.; Wang, B.; Yao, Q.M.; Li, Q.; Jia, X.; Zhang, J.A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef] [Green Version]
- Dhillon-Smith, R.K.; Tobias, A.; Smith, P.P.; Middleton, L.J.; Sunner, K.K.; Baker, K.; Farrell-Carver, S.; Bender-Atik, R.; Agrawal, R.; Bhatia, K.; et al. The Prevalence of Thyroid Dysfunction and Autoimmunity in Women With History of Miscarriage or Subfertility. J. Clin. Endocrinol. Metab. 2020, 105, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Hall, J.E.; Klubo-Gwiezdzinska, J. The Hypothalamic Pituitary Thyroid Axis and Sleep. Curr. Opin. Endocr. Metab. Res. 2020, 17, 8–14. [Google Scholar] [CrossRef]
- Fatima, M.; Amjad, S.; Sharaf Ali, H., Sr.; Ahmed, T.; Khan, S.; Raza, M.; Inam, M. Correlation of Subclinical Hypothyroidism With Polycystic Ovary Syndrome (PCOS). Cureus 2020, 12, e8142. [Google Scholar] [CrossRef]
- de Medeiros, S.F.; de Medeiros, M.A.S.; Ormond, C.M.; Barbosa, J.S.; Yamamoto, M.M.W. Subclinical Hypothyroidism Impact on the Characteristics of Patients with Polycystic Ovary Syndrome. A Meta-Analysis of Observational Studies. Gynecol. Obstet. Investig. 2018, 83, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Quintino-Moro, A.; Zantut-Wittmann, D.E.; Tambascia, M.; Machado, H.d.C.; Fernandes, A. High Prevalence of Infertility among Women with Graves’ Disease and Hashimoto’s Thyroiditis. Int. J. Endocrinol. 2014, 2014, 982705. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.T.; Ho, J.Y.P. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency—A nationwide Health Insurance Research Database study. Hum. Reprod. 2021, 36, 1621–1629. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Thyroid hormones and female reproduction. Biol. Reprod. 2018, 99, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Bouilly, J.; Carré, N.; Tolle, V.; Caraty, A.; Tello, J.; Simony-Conesa, F.J.; Millar, R.; Young, J.; Binart, N. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J. Clin. Investig. 2012, 122, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S.E.; Khant Aung, Z.; Phillipps, H.R.; Barad, Z.; Lein, H.J.; Boehm, U.; Szawka, R.E.; Grattan, D.R. Acute Suppression of LH Secretion by Prolactin in Female Mice Is Mediated by Kisspeptin Neurons in the Arcuate Nucleus. Endocrinology 2019, 160, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Frazão, R. Interactions between prolactin and kisspeptin to control reproduction. Arch. Endocrinol. Metab. 2016, 60, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, T.; Trompet, S.; Noordam, R.; van Klinken, J.B.; van Dijk, K.W.; Lamb, H.J.; Cobbaert, C.M.; den Heijer, M.; Jazet, I.M.; Jukema, J.W.; et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018, 107, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Petrine, J.C.P.; Franci, C.R.; Del Bianco-Borges, B. Leptin actions through the nitrergic system to modulate the hypothalamic expression of the kiss1 mRNA in the female rat. Brain Res. 2020, 1728, 146574. [Google Scholar] [CrossRef]
- Tashjian, A.H.; Barowsky, N.J.; Jensen, D.K. Thyrotropin releasing hormone: Direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem. Biophys. Res. Commun. 1971, 43, 516–523. [Google Scholar] [CrossRef]
- Onishi, T.; Miyai, K.; Kumahara, Y. Serum prolactin response to thyrotropin-releasing hormone in normal subjects and in patients with thyroid diseases (author’s transl). Nihon Naibunpi Gakkai Zasshi 1975, 51, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Selva, D.M.; Hammond, G.L. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4α. J. Mol. Endocrinol. 2009, 43, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Mukku, V.R.; Kirkland, J.L.; Hardy, M.; Stancel, G.M. Evidence for thyroid hormone receptors in uterine nuclei. Metabolism 1983, 32, 142–145. [Google Scholar] [CrossRef]
- Zhang, S.S.; Carrillo, A.J.; Darling, D.S. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol. Hum. Reprod. 1997, 3, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Wakim, A.N.; Polizotto, S.L.; Buffo, M.J.; Marrero, M.A.; Burholt, D.R. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil. Steril. 1993, 59, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Stavreus-Evers, A.; Lindeberg, M.; Landgren, B.M.; Sparre, L.S.; Hovatta, O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil. Steril. 2011, 95, 230–237.e2. [Google Scholar] [CrossRef] [PubMed]
- Skjöldebrand, L.; Brundin, J.; Carlström, A.; Pettersson, T. Thyroid associated components in serum during normal pregnancy. Acta Endocrinol. 1982, 100, 504–511. [Google Scholar] [CrossRef]
- Glinoer, D.; de Nayer, P.; Bourdoux, P.; Lemone, M.; Robyn, C.; van Steirteghem, A.; Kinthaert, J.; Lejeune, B. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab. 1990, 71, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Balucan, F.S.; Morshed, S.A.; Davies, T.F. Thyroid autoantibodies in pregnancy: Their role, regulation and clinical relevance. J. Thyroid Res. 2013, 2013, 182472. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Meima, M.E.; Peeters, R.P.; Visser, W.E. Thyroid Hormone Transporters in Pregnancy and Fetal Development. Int. J. Mol. Sci. 2022, 23, 15113. [Google Scholar] [CrossRef]
- Weetman, A.P. An update on the pathogenesis of Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Poppe, K. Management of Endocrine Disease: Thyroid and female infertility: More questions than answers? Eur. J. Endocrinol. 2021, 184, R123–R135. [Google Scholar] [CrossRef] [PubMed]
- Nillni, E.A. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front. Neuroendocrinol. 2010, 31, 134–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.; Schweizer, U. Thyroid Hormone Transport and Transporters. Vitam. Horm. 2018, 106, 19–44. [Google Scholar] [CrossRef]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193 Pt 1, 59–79. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef]
- Sharma, L.K.; Sharma, N.; Gadpayle, A.K.; Dutta, D. Prevalence and predictors of hyperprolactinemia in subclinical hypothyroidism. Eur. J. Intern. Med. 2016, 35, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Miyake, A.; Aono, T.; Sakumoto, T.; Ohmichi, M.; Yamaguchi, M.; Tanizawa, O. Effect of prolactin on the secretion of hypothalamic GnRH and pituitary gonadotropins. Horm. Res. 1991, 35 (Suppl. 1), 5–12. [Google Scholar] [CrossRef]
- Liu, X.; Herbison, A.E. Dopamine Regulation of Gonadotropin-Releasing Hormone Neuron Excitability in Male and Female Mice. Endocrinology 2013, 154, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Goodman, R.L.; Maltby, M.J.; Millar, R.P.; Hileman, S.M.; Nestor, C.C.; Whited, B.; Tseng, A.S.; Coolen, L.M.; Lehman, M.N. Evidence That Dopamine Acts via Kisspeptin to Hold GnRH Pulse Frequency in Check in Anestrous Ewes. Endocrinology 2012, 153, 5918–5927. [Google Scholar] [CrossRef] [Green Version]
- Dairaghi, L.; Constantin, S.; Oh, A.; Shostak, D.; Wray, S. The Dopamine D4 Receptor Regulates Gonadotropin-Releasing Hormone Neuron Excitability in Male Mice. eNeuro 2022, 9, ENEURO.0461-21. [Google Scholar] [CrossRef] [PubMed]
- Hapon, M.B.; Motta, A.B.; Ezquer, M.; Bonafede, M.; Jahn, G.A. Hypothyroidism prolongs corpus luteum function in the pregnant rat. Reproduction 2007, 133, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Anasti, J.N.; Flack, M.R.; Froehlich, J.; Nelson, L.M.; Nisula, B.C. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J. Clin. Endocrinol. Metab. 1995, 80, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Dhole, B.; Gupta, S.; Shekhar, S.; Kumar, A. A Novel Antigonadotropic Role of Thyroid Stimulating Hormone on Leydig Cell-Derived Mouse Leydig Tumor Cells-1 Line. Ann. Natl. Acad. Med. Sci. 2020, 56, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agard, J.A.; Duffy, D.M.; Jacot, T.; Archer, D.F. Thyroid stimulating hormone (TSH) receptor on granulosa cells. Fertil. Steril. 2011, 96, S118. [Google Scholar] [CrossRef]
- Peyneau, M.; Kavian, N.; Chouzenoux, S.; Nicco, C.; Jeljeli, M.; Toullec, L.; Reboul-Marty, J.; Chenevier-Gobeaux, C.; Reis, F.M.; Santulli, P.; et al. Role of thyroid dysimmunity and thyroid hormones in endometriosis. Proc. Natl. Acad. Sci. USA 2019, 116, 11894–11899. [Google Scholar] [CrossRef] [Green Version]
- Davies, T.; Marians, R.; Latif, R. The TSH receptor reveals itself. J. Clin. Investig. 2002, 110, 161–164. [Google Scholar] [CrossRef]
- Menendez, C.; Baldelli, R.; Camiña, J.P.; Escudero, B.; Peino, R.; Dieguez, C.; Casanueva, F.F. TSH stimulates leptin secretion by a direct effect on adipocytes. J. Endocrinol. 2003, 176, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Santini, F.; Galli, G.; Maffei, M.; Fierabracci, P.; Pelosini, C.; Marsili, A.; Giannetti, M.; Castagna, M.G.; Checchi, S.; Molinaro, E.; et al. Acute exogenous TSH administration stimulates leptin secretion in vivo. Eur. J. Endocrinol. 2010, 163, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P.F.S.; Cabral, M.D.; Silva, N.A.; Soares, D.V.; Braulio, V.B.; Couto, A.P.C.B.; Henriques, J.L.M.; Costa, A.J.L.; Buescu, A.; Vaisman, M. Serum Leptin in Overt and Subclinical Hypothyroidism: Effect of Levothyroxine Treatment and Relationship to Menopausal Status and Body Composition. Thyroid 2009, 19, 443–450. [Google Scholar] [CrossRef]
- Seoane, L.M.; Carro, E.; Tovar, S.; Casanueva, F.F.; Dieguez, C. Regulation of in vivo TSH secretion by leptin. Regul. Pept. 2000, 92, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Radwanska, P.; Kosior-Korzecka, U. Effect of leptin on thyroid-stimulating hormone secretion and nitric oxide release from pituitary cells of ewe lambs in vitro. J. Physiol. Pharmacol. 2014, 65, 145–151. [Google Scholar] [PubMed]
- Nillni, E.A.; Vaslet, C.; Harris, M.; Hollenberg, A.; Bjørbak, C.; Flier, J.S. Leptin regulates prothyrotropin-releasing hormone biosynthesis. Evidence for direct and indirect pathways. J. Biol. Chem. 2000, 275, 36124–36133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantzoros, C.S.; Ozata, M.; Negrao, A.B.; Suchard, M.A.; Ziotopoulou, M.; Caglayan, S.; Elashoff, R.M.; Cogswell, R.J.; Negro, P.; Liberty, V.; et al. Synchronicity of Frequently Sampled Thyrotropin (TSH) and Leptin Concentrations in Healthy Adults and Leptin-Deficient Subjects: Evidence for Possible Partial TSH Regulation by Leptin in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 3284–3291. [Google Scholar] [CrossRef]
- Henson, J.R.; Carter, S.N.; Freeman, D.A. Exogenous T3 elicits long day-like alterations in testis size and the RFamides Kisspeptin and gonadotropin-inhibitory hormone in short-day Siberian hamsters. J. Biol. Rhythm. 2013, 28, 193–200. [Google Scholar] [CrossRef]
- Ogawa, S.; Ng, K.W.; Xue, X.; Ramadasan, P.N.; Sivalingam, M.; Li, S.; Levavi-Sivan, B.; Lin, H.; Liu, X.; Parhar, I.S. Thyroid Hormone Upregulates Hypothalamic kiss2 Gene in the Male Nile Tilapia, Oreochromis niloticus. Front. Endocrinol. 2013, 4, 184. [Google Scholar] [CrossRef] [Green Version]
- Tomori, Y.; Takumi, K.; Iijima, N.; Takai, S.; Ozawa, H. Kisspeptin expression is decreased in the arcuate nucleus of hypothyroid female rats with irregular estrus cycles. Neurosci. Res. 2017, 117, 35–41. [Google Scholar] [CrossRef]
- Kiyohara, M.; Son, Y.L.; Tsutsui, K. Involvement of gonadotropin-inhibitory hormone in pubertal disorders induced by thyroid status. Sci. Rep. 2017, 7, 1042. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.H.; Howards, P.P.; Darrow, L.A.; Meadows, J.W.; Kesner, J.S.; Spencer, J.B.; Terrell, M.L.; Marcus, M. Thyroid hormones and menstrual cycle function in a longitudinal cohort of premenopausal women. Paediatr. Perinat. Epidemiol. 2018, 32, 225–234. [Google Scholar] [CrossRef]
- Xu, K.; Tian, Y.; Weng, X.; Hu, X.; Heng, D.; Xia, G.; Zhang, C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2020, 379, 291–300. [Google Scholar] [CrossRef]
- Llévenes, P.; Balfagón, G.; Blanco-Rivero, J. Thyroid hormones affect nitrergic innervation function in rat mesenteric artery: Role of the PI3K/AKT pathway. Vascul. Pharmacol. 2018, 108, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.T.; Lubbers, L.S.; Macchia, E.; DeGroot, L.J.; Lehman, M.N. Thyroid Hormone Receptor (α) Distribution in Hamster and Sheep Brain: Colocalization in Gonadotropin-Releasing Hormone and Other Identified Neurons. Endocrinology 1997, 138, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Dufourny, L.; Gennetay, D.; Martinet, S.; Lomet, D.; Caraty, A. The Content of Thyroid Hormone Receptor α in Ewe Kisspeptin Neurones is not Season-Dependent. J. Neuroendocrinol. 2016, 28, 12344. [Google Scholar] [CrossRef] [PubMed]
- Pernasetti, F.; Caccavelli, L.; Van de Weerdt, C.; Martial, J.A.; Muller, M. Thyroid hormone inhibits the human prolactin gene promoter by interfering with activating protein-1 and estrogen stimulations. Mol. Endocrinol. 1997, 11, 986–996. [Google Scholar] [CrossRef]
- Salvi, R.; Castillo, E.; Voirol, M.-J.; Glauser, M.; Rey, J.-P.; Gaillard, R.C.; Vollenweider, P.; Pralong, F.P. Gonadotropin-Releasing Hormone-Expressing Neurons Immortalized Conditionally Are Activated by Insulin: Implication of the Mitogen-Activated Protein Kinase Pathway. Endocrinology 2006, 147, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Maejima, Y.; Kohno, D.; Iwasaki, Y.; Yada, T. Insulin suppresses ghrelin-induced calcium signaling in neuropeptide Y neurons of the hypothalamic arcuate nucleus. Aging 2011, 3, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Burcelin, R.m.; Thorens, B.; Glauser, M.; Gaillard, R.C.; Pralong, F.o.P. Gonadotropin-Releasing Hormone Secretion from Hypothalamic Neurons: Stimulation by Insulin and Potentiation by Leptin. Endocrinology 2003, 144, 4484–4491. [Google Scholar] [CrossRef]
- Herwig, A.; Campbell, G.; Mayer, C.D.; Boelen, A.; Anderson, R.A.; Ross, A.W.; Mercer, J.G.; Barrett, P. A thyroid hormone challenge in hypothyroid rats identifies T3 regulated genes in the hypothalamus and in models with altered energy balance and glucose homeostasis. Thyroid 2014, 24, 1575–1593. [Google Scholar] [CrossRef] [Green Version]
- Godini, A.; Ghasemi, A.; Zahediasl, S. The Possible Mechanisms of the Impaired Insulin Secretion in Hypothyroid Rats. PLoS ONE 2015, 10, e0131198. [Google Scholar] [CrossRef] [Green Version]
- Fekete, C.; Kelly, J.; Mihály, E.; Sarkar, S.; Rand, W.M.; Légrádi, G.; Emerson, C.H.; Lechan, R.M. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 2001, 142, 2606–2613. [Google Scholar] [CrossRef]
- Vella, K.R.; Ramadoss, P.; Lam, F.S.; Harris, J.C.; Ye, F.D.; Same, P.D.; O’Neill, N.F.; Maratos-Flier, E.; Hollenberg, A.N. NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab. 2011, 14, 780–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.; Kamegai, J.; Tamura, H.; Shimizu, T.; Sugihara, H.; Oikawa, S. Hypothalamic Neuropeptide Y/Y1 Receptor Pathway Activated by a Reduction in Circulating Leptin, but Not by an Increase in Circulating Ghrelin, Contributes to Hyperphagia Associated with Triiodothyronine-Induced Thyrotoxicosis. Neuroendocrinology 2003, 78, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Brabant, A.; Brabant, G.; Schuermeyer, T.; Ranft, U.; Schmidt, F.W.; Hesch, R.D.; von zur Mühlen, A. The role of glucocorticoids in the regulation of thyrotropin. Acta Endocrinol. 1989, 121, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkemade, A.; Unmehopa, U.A.; Wiersinga, W.M.; Swaab, D.F.; Fliers, E. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. J. Clin. Endocrinol. Metab. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Nicoloff, J.T.; Fisher, D.A.; Appleman, M.D., Jr. The role of glucocorticoids in the regulation of thyroid function in man. J. Clin. Investig. 1970, 49, 1922–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyma, P.; Larkins, R.G. Glucocorticoids decrease in conversion of thyroxine into 3, 5, 3′-tri-iodothyronine by isolated rat renal tubules. Clin. Sci. 1982, 62, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; McGlotten, R.; Auh, S.; Rother, K.I.; Nieman, L.K. The Hypothalamic-Pituitary-Thyroid Axis in Cushing Syndrome Before and After Curative Surgery. J. Clin. Endocrinol. Metab. 2021, 106, e1316–e1331. [Google Scholar] [CrossRef]
- Shi, Z.X.; Levy, A.; Lightman, S.L. Thyroid hormone-mediated regulation of corticotropin-releasing hormone messenger ribonucleic acid in the rat. Endocrinology 1994, 134, 1577–1580. [Google Scholar] [CrossRef]
- Johnson, E.O.; Calogero, A.E.; Konstandi, M.; Kamilaris, T.C.; La Vignera, S.; Chrousos, G.P. Effects of short- and long-duration hypothyroidism on hypothalamic-pituitary-adrenal axis function in rats: In vitro and in situ studies. Endocrine 2012, 42, 684–693. [Google Scholar] [CrossRef]
- Kumar, A.; Dewan, R.; Suri, J.; Kohli, S.; Shekhar, S.; Dhole, B.; Chaturvedi, P.K. Abolition of endocrine dimorphism in hyperthyroid males? An argument for the positive feedback effect of hyperoestrogenaemia on LH secretion. Andrologia 2012, 44, 217–225. [Google Scholar] [CrossRef]
- Gojska, N.M.; Belsham, D.D. Glucocorticoid receptor-mediated regulation of Rfrp (GnIH) and Gpr147 (GnIH-R) synthesis in immortalized hypothalamic neurons. Mol. Cell. Endocrinol. 2014, 384, 23–31. [Google Scholar] [CrossRef]
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA 2009, 106, 11324–11329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poling, M.C.; Quennell, J.H.; Anderson, G.M.; Kauffman, A.S. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J. Neuroendocrinol. 2013, 25, 876–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.T.; Hendrikse, M.; Veldhuis, J.D.; Clarke, I.J.; Anderson, R.A.; Millar, R.P. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin. Endocrinol. 2017, 86, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulut, F.; Kacar, E.; Bilgin, B.; Hekim, M.G.; Keleştemur, M.M.; Sahin, Z.; Ayar, A.; Ozcan, M. Crosstalk between kisspeptin and gonadotropin-inhibitory hormone in the silence of puberty: Preclinical evidence from a calcium signaling study. J. Recept. Signal Transduct. Res. 2022, 42, 608–613. [Google Scholar] [CrossRef]
- Zubair, H.; Saqib, M.; Khan, M.N.; Shamas, S.; Irfan, S.; Shahab, M. Variation in Hypothalamic GnIH Expression and Its Association with GnRH and Kiss1 during Pubertal Progression in Male Rhesus Monkeys (Macaca mulatta). Animals 2022, 12, 3533. [Google Scholar] [CrossRef]
- Ubuka, T.; Morgan, K.; Pawson, A.J.; Osugi, T.; Chowdhury, V.S.; Minakata, H.; Tsutsui, K.; Millar, R.P.; Bentley, G.E. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE 2009, 4, e8400. [Google Scholar] [CrossRef] [Green Version]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhang, C.; Guo, L.; Zhu, B.; Feng, Y.; Yu, S.; An, N.; Wang, X. Effects of 3, 5, 3′-triiodothyronine (t3) and follicle stimulating hormone on apoptosis and proliferation of rat ovarian granulosa cells. Chin. J. Physiol. 2013, 56, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Maruo, T.; Hiramatsu, S.; Otani, T.; Hayashi, M.; Mochizuki, M. Increase in the expression of thyroid hormone receptors in porcine granulosa cells early in follicular maturation. Acta Endocrinol. 1992, 127, 152–160. [Google Scholar] [CrossRef]
- Kobayashi, N.; Orisaka, M.; Cao, M.; Kotsuji, F.; Leader, A.; Sakuragi, N.; Tsang, B.K. Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 2009, 150, 5566–5574. [Google Scholar] [CrossRef]
- Saha, S.K.; Ghosh, P.; Konar, A.; Bhattacharya, S.; Roy, S.S. Differential expression of procollagen lysine 2-oxoglutarate 5-deoxygenase and matrix metalloproteinase isoforms in hypothyroid rat ovary and disintegration of extracellular matrix. Endocrinology 2005, 146, 2963–2975. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Rijntjes, E.; Swarts, H.; Bunschoten, A.; van der Stelt, I.; Keijer, J.; Teerds, K. Dietary-Induced Chronic Hypothyroidism Negatively Affects Rat Follicular Development and Ovulation Rate and Is Associated with Oxidative Stress. Biol. Reprod. 2016, 94, 90. [Google Scholar] [CrossRef]
- Rodríguez-Castelán, J.; Méndez-Tepepa, M.; Carrillo-Portillo, Y.; Anaya-Hernández, A.; Rodríguez-Antolín, J.; Zambrano, E.; Castelán, F.; Cuevas-Romero, E. Hypothyroidism Reduces the Size of Ovarian Follicles and Promotes Hypertrophy of Periovarian Fat with Infiltration of Macrophages in Adult Rabbits. BioMed Res. Int. 2017, 2017, 3795950. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, T.; Khan, Q.U.; Saad, S. The Interplay Between Hyperthyroidism and Ovarian Cytoarchitecture in Albino Rats. Cureus 2021, 13, e14517. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Hayashi, M.; Matsuo, H.; Yamamoto, T.; Okada, H.; Mochizuki, M. The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology 1987, 121, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.L.; Skalka, M. Thyroid hormone as a regulator of basal and human chorionic gonadotrophin-stimulated steroidogenesis by cultured porcine theca and granulosa cells isolated at different stages of the follicular phase. Reprod. Fertil. Dev. 1996, 8, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Mukku, V.; Hardy, M.; Young, R. Evidence for triiodothyronine receptors in human endometrium and myometrium. Am. J. Obstet. Gynecol. 1983, 146, 380–383. [Google Scholar] [CrossRef]
- Bagheripuor, F.; Ghanbari, M.; Piryaei, A.; Ghasemi, A. Effects of fetal hypothyroidism on uterine smooth muscle contraction and structure of offspring rats. Exp. Physiol. 2018, 103, 683–692. [Google Scholar] [CrossRef]
- Steinsapir, J.; Rojas, A.M.; Mena, M.; Tchernitchin, A.N. Effects of Thyroid Hormone on Some Uterine Responses to Estrogen. Endocrinology 1982, 110, 1773–1779. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Gardner, R.M.; Mukku, V.R.; Akhtar, M.; Stancel, G.M. Hormonal control of uterine growth: The effect of hypothyroidism on estrogen-stimulated cell division. Endocrinology 1981, 108, 2346–2351. [Google Scholar] [CrossRef]
- Gardner, R.M.; Kirkland, J.L.; Ireland, J.S.; Stancel, G.M. Regulation of the uterine response to estrogen by thyroid hormone. Endocrinology 1978, 103, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castelán, J.; Del Moral-Morales, A.; Piña-Medina, A.G.; Zepeda-Pérez, D.; Castillo-Romano, M.; Méndez-Tepepa, M.; Espindola-Lozano, M.; Camacho-Arroyo, I.; Cuevas-Romero, E. Hypothyroidism induces uterine hyperplasia and inflammation related to sex hormone receptors expression in virgin rabbits. Life Sci. 2019, 230, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Inuwa, I.; Williams, M.A. Morphometric study on the uterine horn and thyroid gland in hypothyroid, and thyroxine treated hypothyroid rats. J. Anat. 1996, 188 Pt 2, 383–393. [Google Scholar] [PubMed]
- Erbaş, E.; Gedikli, S. Investigation of the endometrial receptivity status in experimental hypothyroid-induced female rats. Iran. J. Basic Med. Sci. 2022, 25, 1077–1083. [Google Scholar] [CrossRef]
- Kowalczyk-Zieba, I.; Staszkiewicz-Chodor, J.; Boruszewska, D.; Lukaszuk, K.; Jaworska, J.; Woclawek-Potocka, I. Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling. Animals 2021, 11, 2636. [Google Scholar] [CrossRef]
- Tanaka, T.; Umesaki, N. Leptin regulates the proliferation and apoptosis of human endometrial epithelial cells. Int. J. Mol. Med. 2008, 22, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Bläuer, M.; Heinonen, P.K.; Martikainen, P.M.; Tomás, E.; Ylikomi, T. A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum. Reprod. 2005, 20, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Buchanan, D.S.; Hafez, S.A.; Grazul-Bilska, A.T.; Redmer, D.A. Uteroplacental vascular development and placental function: An update. Int. J. Dev. Biol. 2010, 54, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Galton, V.A.; Martinez, E.; Hernandez, A.; St Germain, E.A.; Bates, J.M.; St Germain, D.L. The type 2 iodothyronine deiodinase is expressed in the rat uterus and induced during pregnancy. Endocrinology 2001, 142, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Silva, J.; Silva, C.; Ocarino, N.; Serakides, R. Thyroid hormones affect decidualization and angiogenesis in the decidua and metrial gland of rats. Pesqui. Veterinária Bras. 2017, 37, 1002–1014. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.A.; Ocarino, N.M.; Silva, J.F.; Boeloni, J.N.; Nascimento, E.F.; Silva, I.J.; Castro, R.D.; Moreira, L.P.; Almeida, F.R.; Chiarini-Garcia, H.; et al. Administration of thyroxine affects the morphometric parameters and VEGF expression in the uterus and placenta and the uterine vascularization but does not affect reproductive parameters in gilts during early gestation. Reprod. Domest. Anim. 2011, 46, e7–e16. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.G.; Seliktar, J.; Li, X.; Hershman, J.M.; Braunstein, G.D.; Melmed, S. In vivo and in vitro regulation of thyroid leukemia inhibitory factor (LIF): Marker of hypothyroidism. J. Clin. Endocrinol. Metab. 1999, 84, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Kakita-Kobayashi, M.; Murata, H.; Nishigaki, A.; Hashimoto, Y.; Komiya, S.; Tsubokura, H.; Kido, T.; Kida, N.; Tsuzuki-Nakao, T.; Matsuo, Y.; et al. Thyroid Hormone Facilitates in vitro Decidualization of Human Endometrial Stromal Cells via Thyroid Hormone Receptors. Endocrinology 2020, 161, bqaa049. [Google Scholar] [CrossRef]
- Mattheij, J.A.M.; Swarts, J.J.M.; Lokerse, P.; van Kampen, J.T.; Van der Heide, D. Effect of hypothyroidism on the pituitary-gonadal axis in the adult female rat. J. Endocrinol. 1995, 146, 87–94. [Google Scholar] [CrossRef]
- Silva, C.M.; Serakides, R.; Oliveira, T.S.; Ocarino, N.M.; Nascimento, E.F.; Nunes, V.A. Histomorfometria e histoquímica dos ovários, tubas e útero de ratas hipotireóideas em metaestro-diestro. Arq. Bras. Med. Vet. Zootec. 2004, 56, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Wartofsky, L. Levothyroxine treatment in pregnancy: Indications, efficacy, and therapeutic regimen. J. Thyroid Res. 2011, 2011, 843591. [Google Scholar] [CrossRef] [Green Version]
- Glinoer, D. The regulation of thyroid function during normal pregnancy: Importance of the iodine nutrition status. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 133–152. [Google Scholar] [CrossRef]
- Smallridge, R.C.; Glinoer, D.; Hollowell, J.G.; Brent, G. Thyroid function inside and outside of pregnancy: What do we know and what don’t we know? Thyroid 2005, 15, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, H.; Maruo, T.; Hayashi, M.; Mochizuki, M. Modification of endocrine function of trophoblasts by thyroid hormone. Nihon Sanka Fujinka Gakkai Zasshi 1991, 43, 1533–1538. [Google Scholar] [PubMed]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. In vitro effects of triiodothyronine on gene expression in mouse trophoblast cells. Placenta 2015, 36, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.S.; Leite, E.D.; Souza, C.A.; Ocarino, N.M.; Ferreira, E.; Cassali, G.D.; Gomes, M.G.; Serakides, R. Histomorphometry and expression of Cdc47 and caspase-3 in hyperthyroid rat uteri and placentas during gestation and postpartum associated with fetal development. Reprod. Fertil. Dev. 2007, 19, 498–509. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Placental angiogenic and hormonal factors are affected by thyroid hormones in rats. Pathol. Res. Pract. 2015, 211, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rassie, K.; Giri, R.; Joham, A.E.; Teede, H.; Mousa, A. Human Placental Lactogen in Relation to Maternal Metabolic Health and Fetal Outcomes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15621. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Madsen, M.H.; Rasmussen, N.G.; Hegedüs, L.; Hornnes, P. Anti-Thyroid Peroxidase Antibodies During Pregnancy and Postpartum. Relation to Postpartum Thyroiditis. Autoimmunity 1990, 6, 211–214. [Google Scholar] [CrossRef]
- Dumoulin, S.C.; Perret, B.P.; Bennet, A.P.; Caron, P.J. Opposite effects of thyroid hormones on binding proteins for steroid hormones (sex hormone-binding globulin and corticosteroid-binding globulin) in humans. Eur. J. Endocrinol. 1995, 132, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Molteni, A.; Warpeha, R.L.; Brizio-Molteni, L.; Fors, E.M. Estradiol receptor-binding protein in head and neck neoplastic and normal tissue. Arch. Surg. 1981, 116, 207–210. [Google Scholar] [CrossRef]
- Ben-Rafael, Z.; Struass, J.F., 3rd; Arendash-Durand, B.; Mastroianni, L., Jr.; Flickinger, G.L. Changes in thyroid function tests and sex hormone binding globulin associated with treatment by gonadotropin. Fertil. Steril. 1987, 48, 318–320. [Google Scholar] [CrossRef]
- Arafah, B.M. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N. Engl. J. Med. 2001, 344, 1743–1749. [Google Scholar] [CrossRef]
- Glinoer, D.; Gershengorn, M.C.; Dubois, A.; Robbins, J. Stimulation of thyroxine-binding globulin synthesis by isolated rhesus monkey hepatocytes after in vivo beta-estradiol administration. Endocrinology 1977, 100, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Junior, C.M.; Pavesi, H.; Drobrzenski, B.; Amaral, G.M.D. Effects of oral versus transdermal estradiol plus micronized progesterone on thyroid hormones, hepatic proteins, lipids, and quality of life in menopausal women with hypothyroidism: A clinical trial. Menopause 2021, 28, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [Green Version]
- Thoyyib, M.; Garg, S.; Gupta, N.; Aggarwal, S.; Pandit, S. Study on Coagulation Factor VIII and Fibrinogen Levels in Patients with Thyroid Disorders. Indian J. Endocrinol. Metab. 2018, 22, 479–484. [Google Scholar] [CrossRef]
- Davis, L.E.; Leveno, K.J.; Cunningham, F.G. Hypothyroidism complicating pregnancy. Obstet. Gynecol. 1988, 72, 108–112. [Google Scholar]
- Zhang, Y.; Li, Y.; Shan, Z.; Xu, Y.; Li, C.; Xie, X.; Li, Y.; Wang, W.; Mao, J.; Teng, W. Association of Overt and Subclinical Hyperthyroidism During Weeks 4-8 with Adverse Pregnancy Outcomes. J. Womens Health 2019, 28, 842–848. [Google Scholar] [CrossRef]
- Derakhshan, A.; Peeters, R.P.; Taylor, P.N.; Bliddal, S.; Carty, D.M.; Meems, M.; Vaidya, B.; Chen, L.; Knight, B.A.; Ghafoor, F.; et al. Association of maternal thyroid function with birthweight: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2020, 8, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Wang, X.; Ouyang, L.; Li, Y. Relationship Between Subclinical Hypothyroidism in Pregnancy and Hypertensive Disorder of Pregnancy: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 823710. [Google Scholar] [CrossRef]
- Hizkiyahu, R.; Badeghiesh, A.; Baghlaf, H.; Dahan, M.H. Associations between hyperthyroidism and adverse obstetric and neonatal outcomes: A study of a population database including almost 17,000 women with hyperthyroidism. Clin. Endocrinol. 2022, 97, 347–354. [Google Scholar] [CrossRef]
- Turunen, S.; Vääräsmäki, M.; Lahesmaa-Korpinen, A.M.; Leinonen, M.K.; Gissler, M.; Männistö, T.; Suvanto, E. Maternal hyperthyroidism and pregnancy outcomes: A population-based cohort study. Clin. Endocrinol. 2020, 93, 721–728. [Google Scholar] [CrossRef]
- Alves Junior, J.M.; Bernardo, W.M.; Ward, L.S.; Villagelin, D. Effect of Hyperthyroidism Control During Pregnancy on Maternal and Fetal Outcome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 800257. [Google Scholar] [CrossRef]
- Saran, S.; Gupta, B.S.; Philip, R.; Singh, K.S.; Bende, S.A.; Agroiya, P.; Agrawal, P. Effect of hypothyroidism on female reproductive hormones. Indian J. Endocrinol. Metab. 2016, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Hu, X.; Weng, X.; Tian, Y.; Xu, K.; Heng, D.; Liu, W.; Ding, Y.; Yang, Y.; et al. Effects of Thyroid Dysfunction on Reproductive Hormones in Female Rats. Chin. J. Physiol. 2018, 61, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Pauli, S.A.; Berga, S.L. Athletic amenorrhea: Energy deficit or psychogenic challenge? Ann. N. Y. Acad. Sci. 2010, 1205, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Bachimanchi, B.; Vaikkakara, S.; Sachan, A.; Praveen Kumar, G.; Venkatanarasu, A.; Sai Krishna Chaitanya, P.; Sreedivya, B.; Poojari, R. Effect of Adequate Thyroid Hormone Replacement on the Hypothalamo-Pituitary-Gonadal Axis in Premenopausal Women with Primary Hypothyroidism. Eur. Thyroid J. 2019, 8, 152–158. [Google Scholar] [CrossRef]
- Ajmani, N.S.; Sarbhai, V.; Yadav, N.; Paul, M.; Ahmad, A.; Ajmani, A.K. Role of Thyroid Dysfunction in Patients with Menstrual Disorders in Tertiary Care Center of Walled City of Delhi. J. Obstet. Gynaecol. India 2016, 66, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez, E.M.; Arata, G.B. Effects of Thyroid Status on Pituitary Gonadotropin and Testicular Reserve in Men. Arch. Androl. 1997, 38, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, M.E.; LaRochelle, F.T., Jr.; Moore, R.B. Effect of thyroid status on spontaneous and induced surges of luteinizing hormone. Endocrinology 1976, 99, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shekhar, S.; Dhole, B. Thyroid and male reproduction. Indian J. Endocrinol. Metab. 2014, 18, 23–31. [Google Scholar] [CrossRef]
- Koutras, D.A. Disturbances of Menstruation in Thyroid Disease. Ann. N. Y. Acad. Sci. 1997, 816, 280–284. [Google Scholar] [CrossRef]
- Chen, C.W.; Huang, Y.L.; Tzeng, C.R.; Huang, R.L.; Chen, C.H. Idiopathic Low Ovarian Reserve Is Associated with More Frequent Positive Thyroid Peroxidase Antibodies. Thyroid 2017, 27, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kitahara, Y.; Osuka, S.; Tsukui, Y.; Kobayashi, M.; Iwase, A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod. Med. Biol. 2022, 21, e12427. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.M.; Mínguez-Alarcón, L.; Messerlian, C.; de Poortere, R.A.; Williams, P.L.; Broeren, M.A.; Hauser, R.; Souter, I.C. Association of Thyroid Function and Autoimmunity with Ovarian Reserve in Women Seeking Infertility Care. Thyroid 2018, 28, 1349–1358. [Google Scholar] [CrossRef]
- Bahri, S.; Tehrani, F.R.; Amouzgar, A.; Rahmati, M.; Tohidi, M.; Vasheghani, M.; Azizi, F. Overtime trend of thyroid hormones and thyroid autoimmunity and ovarian reserve: A longitudinal population study with a 12-year follow up. BMC Endocr. Disord. 2019, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Seungdamrong, A.; Steiner, A.Z.; Gracia, C.R.; Legro, R.S.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Casson, P.; Christman, G.M.; Robinson, R.D.; et al. Preconceptional antithyroid peroxidase antibodies, but not thyroid-stimulating hormone, are associated with decreased live birth rates in infertile women. Fertil. Steril. 2017, 108, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Konishi, S.; Mizuno, Y. Pre-Conceptional Anti-Thyroid Antibodies and Thyroid Function in Association with Natural Conception Rates. Int. J. Environ. Res. Public Health 2022, 19, 13177. [Google Scholar] [CrossRef]
- d’Assunção, V.R.N.; Montagna, E.; d’Assunção, L.E.N.; Caldas, M.M.P.; Christofolini, D.M.; Barbosa, C.P.; Negreiros, R.A.M.; Laganà, A.S.; de Oliveira, R.; Bianco, B. Effect of thyroid function on assisted reproduction outcomes in euthyroid infertile women: A single center retrospective data analysis and a systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1023635. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Kruszewska, J.; Klicka, K.; Kowalczyk, J.; Grymowicz, M.; Skórska, J.; Pięta, W.; Smolarczyk, R. Premature ovarian insufficiency—Aetiopathology, epidemiology, and diagnostic evaluation. Prz. Menopauzalny 2018, 17, 105–108. [Google Scholar] [CrossRef]
- Ayesha; Jha, V.; Goswami, D. Premature Ovarian Failure: An Association with Autoimmune Diseases. J. Clin. Diagn. Res. 2016, 10, QC10–QC12. [Google Scholar] [CrossRef]
- Košir Pogačnik, R.; Meden Vrtovec, H.; Vizjak, A.; Uršula Levičnik, A.; Slabe, N.; Ihan, A. Possible role of autoimmunity in patients with premature ovarian insufficiency. Int. J. Fertil. Steril. 2014, 7, 281–290. [Google Scholar]
- Szeliga, A.; Calik-Ksepka, A.; Maciejewska-Jeske, M.; Grymowicz, M.; Smolarczyk, K.; Kostrzak, A.; Smolarczyk, R.; Rudnicka, E.; Meczekalski, B. Autoimmune Diseases in Patients with Premature Ovarian Insufficiency-Our Current State of Knowledge. Int. J. Mol. Sci. 2021, 22, 2594. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, V.; Mangialardo, C.; Zacà, C.; Barberi, M.; Sereni, E.; Borini, A.; Centanni, M.; Coticchio, G.; Verga-Falzacappa, C.; Canipari, R. Thyroid hormones T3 and T4 regulate human luteinized granulosa cells, counteracting apoptosis and promoting cell survival. J. Endocrinol. Investig. 2020, 43, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Cuomo, D.; Giacco, A.; Mallardo, M.; De Felice, M.; Ambrosino, C. Thyroid Hormones and Functional Ovarian Reserve: Systemic vs. Peripheral Dysfunctions. J. Clin. Med. 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Rijntjes, E.; Swarts, H.J.; Keijer, J.; Teerds, K.J. Prolonged hypothyroidism severely reduces ovarian follicular reserve in adult rats. J. Ovarian Res. 2017, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Unuane, D.; Velkeniers, B.; Bravenboer, B.; Drakopoulos, P.; Tournaye, H.; Parra, J.; De Brucker, M. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum. Reprod. 2017, 32, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef]
- Ding, X.; Yang, L.; Wang, J.; Tang, R.; Chen, Q.; Pan, J.; Yang, H.; Chen, X.; Chen, Z.; Mu, L. Subclinical Hypothyroidism in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, B.S.; Bhana, S.; Raal, F.J. Dyslipidemia in South African patients with hypothyroidism. J. Clin. Transl. Endocrinol. 2022, 29, 100302. [Google Scholar] [CrossRef]
- Hussain, A.; Elmahdawi, A.M.; Elzeraidi, N.E.; Nouh, F.; Algathafi, K. The Effects of Dyslipidemia in Subclinical Hypothyroidism. Cureus 2019, 11, e6173. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhou, L.; Wu, K.; Li, Y.; Xu, J.; Jiang, D.; Gao, L. Abnormal Glucose Metabolism and Insulin Resistance Are Induced via the IRE1α/XBP-1 Pathway in Subclinical Hypothyroidism. Front. Endocrinol. 2019, 10, 303. [Google Scholar] [CrossRef]
- Rimmer, M.; Tan, B.K.; Teede, H.; Thangaratinam, S.; Wattar, B.H.A. Metabolic inflexibility in women with polycystic ovary syndrome: A systematic review. Gynecol. Endocrinol. 2020, 36, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Ornat, L.; López-Baena, M.T.; Santabárbara, J.; Savirón-Cornudella, R.; Pérez-Roncero, G.R. Circulating kisspeptin and anti-müllerian hormone levels, and insulin resistance in women with polycystic ovary syndrome: A systematic review, meta-analysis, and meta-regression. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Benetti-Pinto, C.L.; Berini Piccolo, V.R.; Garmes, H.M.; Teatin Juliato, C.R. Subclinical hypothyroidism in young women with polycystic ovary syndrome: An analysis of clinical, hormonal, and metabolic parameters. Fertil. Steril. 2013, 99, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Romitti, M.; Fabris, V.C.; Ziegelmann, P.K.; Maia, A.L.; Spritzer, P.M. Association between PCOS and autoimmune thyroid disease: A systematic review and meta-analysis. Endocr. Connect. 2018, 7, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Chen, Y.; Shen, Y.; Zhou, S.; Fei, W.; Yang, Y.; Que, H. Correlation between Hashimoto’s thyroiditis and polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1025267. [Google Scholar] [CrossRef]
- Mobeen, S.A.R. Ovarian Cyst. In StatPearls; Internet, Updated 13 June 2022; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tresa, A.; Rema, P.; Suchetha, S.; Dinesh, D.; Sivaranjith, J.; Nath, A.G. Hypothyroidism Presenting as Ovarian Cysts-a Case Series. Indian J. Surg. Oncol. 2021, 12 (Suppl. 2), 343–347. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K. Giant multicystic ovaries in a young girl. Indian J. Med. Res. 2020, 152 (Suppl. 1), S89. [Google Scholar] [CrossRef]
- Yigit, O.; Sert, T.K.; Ekinci, D.; Kirankaya, A.; Kilinc, S. The effect of subclinical hypothyroidism on ovarian volume in prepubertal girls. North Clin. Istanb. 2023, 10, 48–52. [Google Scholar] [CrossRef]
- Lee, H.J.; Jo, H.N.; Noh, H.K.; Kim, S.H.; Joo, J.K. Is there association between thyroid stimulating hormone levels and the four phenotypes in polycystic ovary syndrome? Ginekol. Pol. 2022, 94, 203–210. [Google Scholar] [CrossRef]
- Dieterich, M.; Bolz, M.; Reimer, T.; Costagliola, S.; Gerber, B. Two different entities of spontaneous ovarian hyperstimulation in a woman with FSH receptor mutation. Reprod. Biomed. Online 2010, 20, 751–758. [Google Scholar] [CrossRef]
- Smits, G.; Olatunbosun, O.; Delbaere, A.; Pierson, R.; Vassart, G.; Costagliola, S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N. Engl. J. Med. 2003, 349, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Muderris, I.I.; Boztosun, A.; Oner, G.; Bayram, F. Effect of thyroid hormone replacement therapy on ovarian volume and androgen hormones in patients with untreated primary hypothyroidism. Ann. Saudi Med. 2011, 31, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, E.S.L.; Innecco Arêas, J.V.; Rezende Campos, M.C.; Innecco Arêas, I.; Martins Resende, B.A. Spontaneous ovarian hyperstimulation syndrome in a pregnant woman with hypothyroidism: A case report. F S Rep. 2021, 2, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Talaei, A.; Chehrei, A.; Rezvanfar, M.R.; Rafei, F. The Study of the Effect of Levothyroxine on Dysfunctional Uterine Bleeding (DUB) in Euthyroid Women. Iran. South Med. J. 2017, 20, 317–325. [Google Scholar]
- Zhang, C.Y.; Li, H.; Zhang, S.; Suharwardy, S.; Chaturvedi, U.; Fischer-Colbrie, T.; Maratta, L.A.; Onnela, J.P.; Coull, B.A.; Hauser, R.; et al. Abnormal uterine bleeding patterns determined through menstrual tracking among participants in the Apple Women’s Health Study. Am. J. Obstet. Gynecol. 2023, 228, 213.e1–213.e22. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.S.; Park, E.J.; Seo, Y.S.; Kim, H.J.; Kwon, S.Y.; Park, W.I. Graves Disease Is Associated With Endometriosis: A 3-Year Population-Based Cross-Sectional Study. Medicine 2016, 95, e2975. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Glinoer, D.; Van Steirteghem, A.; Tournaye, H.; Devroey, P.; Schiettecatte, J.; Velkeniers, B. Thyroid dysfunction and autoimmunity in infertile women. Thyroid 2002, 12, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Von Basedow, K. Exophthalmos durch Hypertrophie des Zillgewebes in der Augenhohle. Wochenschr Helik 1840, 6, 197–204. [Google Scholar]
- Kakuno, Y.; Amino, N.; Kanoh, M.; Kawai, M.; Fujiwara, M.; Kimura, M.; Kamitani, A.; Saya, K.; Shakuta, R.; Nitta, S.; et al. Menstrual disturbances in various thyroid diseases. Endocr. J. 2010, 57, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Elbers, L.P.B.; Fliers, E.; Cannegieter, S.C. The influence of thyroid function on the coagulation system and its clinical consequences. J. Thromb. Haemost. 2018, 16, 634–645. [Google Scholar] [CrossRef] [Green Version]
- Ellervik, C.; Mora, S.; Kuś, A.; Åsvold, B.; Marouli, E.; Deloukas, P.; Sterenborg, R.; Teumer, A.; Burgess, S.; Sabater-Lleal, M.; et al. Effects of Thyroid Function on Hemostasis, Coagulation, and Fibrinolysis: A Mendelian Randomization Study. Thyroid 2021, 31, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Thyroid Disease in Pregnancy: ACOG Practice Bulletin Summary, Number 223. Obstet. Gynecol. 2020, 135, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Lazarus, J.H. Hypothyroidism in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 547–556. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Derakhshan, A.; Männistö, T.; Bliddal, S.; Popova, P.V.; Carty, D.M.; Chen, L.; Taylor, P.; Mosso, L.; Oken, E.; et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Q.; Liu, J.; Wang, Y.Q.; Yang, Y.; Yan, C.H.; Hua, J. The Impact of Subclinical Hypothyroidism on Adverse Perinatal Outcomes and the Role of Thyroid Screening in Pregnancy. Front. Endocrinol. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth. Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-analysis. JAMA 2019, 322, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korevaar, T.I.M.; Schalekamp-Timmermans, S.; de Rijke, Y.B.; Visser, W.E.; Visser, W.; de Muinck Keizer-Schrama, S.M.P.F.; Hofman, A.; Ross, H.A.; Hooijkaas, H.; Tiemeier, H.; et al. Hypothyroxinemia and TPO-Antibody Positivity Are Risk Factors for Premature Delivery: The Generation R Study. J. Clin. Endocrinol. Metab. 2013, 98, 4382–4390. [Google Scholar] [CrossRef] [Green Version]
- Bliddal, S.; Feldt-Rasmussen, U.; Rasmussen, Å.K.; Kolte, A.M.; Hilsted, L.M.; Christiansen, O.B.; Nielsen, C.H.; Nielsen, H.S. Thyroid Peroxidase Antibodies and Prospective Live Birth Rate: A Cohort Study of Women with Recurrent Pregnancy Loss. Thyroid 2019, 29, 1465–1474. [Google Scholar] [CrossRef]
- Yuan, N.; Sun, J.; Li, Z.; Chai, S.; Zhang, X.; Ji, L. Relationship between anti-thyroid peroxidase antibody positivity and pregnancy-related and fetal outcomes in Euthyroid women: A single-center cohort study. BMC Pregnancy Childbirth 2020, 20, 491. [Google Scholar] [CrossRef]
- Korevaar, T.I.; de Rijke, Y.B.; Chaker, L.; Medici, M.; Jaddoe, V.W.; Steegers, E.A.; Visser, T.J.; Peeters, R.P. Stimulation of Thyroid Function by Human Chorionic Gonadotropin During Pregnancy: A Risk Factor for Thyroid Disease and a Mechanism for Known Risk Factors. Thyroid 2017, 27, 440–450. [Google Scholar] [CrossRef]
- Negro, R.; Formoso, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. Levothyroxine Treatment in Euthyroid Pregnant Women with Autoimmune Thyroid Disease: Effects on Obstetrical Complications. J. Clin. Endocrinol. Metab. 2006, 91, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, H.; Chi, H.; Zeng, L.; Xiao, W.; Wang, Y.; Li, R.; Liu, P.; Wang, C.; Tian, Q.; et al. Effect of Levothyroxine on Miscarriage Among Women With Normal Thyroid Function and Thyroid Autoimmunity Undergoing In Vitro Fertilization and Embryo Transfer: A Randomized Clinical Trial. JAMA 2017, 318, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Dhillon-Smith, R.K.; Middleton, L.J.; Sunner, K.K.; Cheed, V.; Baker, K.; Farrell-Carver, S.; Bender-Atik, R.; Agrawal, R.; Bhatia, K.; Edi-Osagie, E.; et al. Levothyroxine in Women with Thyroid Peroxidase Antibodies before Conception. N. Engl. J. Med. 2019, 380, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, Y.; Maraka, S.; Abdelouahab, N.; Huang, H.F.; Fraser, W.D.; Fan, J. Pregnancy and Neonatal Outcomes With Levothyroxine Treatment in Women With Subclinical Hypothyroidism Based on New Diagnostic Criteria: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 797423. [Google Scholar] [CrossRef]
- Rao, M.; Zeng, Z.; Zhou, F.; Wang, H.; Liu, J.; Wang, R.; Wen, Y.; Yang, Z.; Su, C.; Su, Z.; et al. Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 344–361. [Google Scholar] [CrossRef]
- Sorah, K.; Alderson, T.L. Hyperthyroidism in Pregnancy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cooper, D.S.; Laurberg, P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013, 1, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Amino, N.; Tamaki, H.; Ito, E.; Mitsuda, N.; Miyai, K.; Tanizawa, O. Gestational thyrotoxicosis and hyperemesis gravidarum: Possible role of hCG with higher stimulating activity. Clin. Endocrinol. 1993, 38, 345–350. [Google Scholar] [CrossRef]
- He, X.; Yan, Q.; Liu, C.; Wang, Z.; Liao, P.; Liu, T.; Shi, Z.; Song, Q.; Cui, X.; Wang, W.; et al. Association of maternal thyroid dysfunction and autoimmunity with adverse birth outcomes. Endocr. Connect. 2022, 11, e210599. [Google Scholar] [CrossRef]
- Zgliczynska, M.; Ostrowska, M.; Szymusik, I.; Ciebiera, M.; Kosinska-Kaczynska, K. Maternal thyroid function in multiple pregnancies—A systematic review. Front. Endocrinol. 2023, 13, 1044655. [Google Scholar] [CrossRef]
- Nazarpour, S.; Amiri, M.; Bidhendi Yarandi, R.; Azizi, F.; Ramezani Tehrani, F. Maternal Subclinical Hyperthyroidism and Adverse Pregnancy Outcomes: A Systematic Review and Meta-analysis of Observational Studies. Int. J. Endocrinol. Metab. 2022, 20, e120949. [Google Scholar] [CrossRef]
- Casey, B.M.; Dashe, J.S.; Wells, C.E.; McIntire, D.D.; Byrd, W.; Leveno, K.J.; Cunningham, F.G. Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol. 2005, 105, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Cleary-Goldman, J.; Malone, F.D.; Lambert-Messerlian, G.; Sullivan, L.; Canick, J.; Porter, T.F.; Luthy, D.; Gross, S.; Bianchi, D.W.; D’Alton, M.E. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol. 2008, 112, 85–92. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygaard, B.; Jensen, E.W.; Kvetny, J.; Jarløv, A.; Faber, J. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur. J. Endocrinol. 2009, 161, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Appelhof, B.C.; Fliers, E.; Wekking, E.M.; Schene, A.H.; Huyser, J.; Tijssen, J.G.; Endert, E.; van Weert, H.C.; Wiersinga, W.M. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: A double-blind, randomized, controlled clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 2666–2674. [Google Scholar] [CrossRef] [Green Version]
- Bunevicius, R.; Kazanavicius, G.; Zalinkevicius, R.; Prange, A.J., Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N. Engl. J. Med. 1999, 340, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panicker, V.; Saravanan, P.; Vaidya, B.; Evans, J.; Hattersley, A.T.; Frayling, T.M.; Dayan, C.M. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J. Clin. Endocrinol. Metab. 2009, 94, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- McAninch, E.A.; Bianco, A.C. The Swinging Pendulum in Treatment for Hypothyroidism: From (and Toward?) Combination Therapy. Front. Endocrinol. 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Henrichs, J.; Ghassabian, A.; Peeters, R.P.; Tiemeier, H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: How and why? Clin. Endocrinol. 2013, 79, 152–162. [Google Scholar] [CrossRef]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheetham, T. How to use thionamide anti-thyroid drug in the young—What’s new? Thyroid Res. 2021, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Andersen, S. Antithyroid drugs and birth defects. Thyroid Res. 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Effects of fetal involvement of inadvertent radioactive iodine therapy for the treatment of thyroid diseases during an unsuspected pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 53–59. [Google Scholar] [CrossRef]
- Hubalewska-Dydejczyk, A.; Gietka-Czernel, M.; Trofimiuk-Müldner, M.; Zgliczyński, W.; Ruchała, M.; Lewiński, A.; Bednarczuk, T.; Syrenicz, A.; Kos-Kudła, B.; Jarząb, B.; et al. Thyroid diseases and fertility disorders—Guidelines of the Polish Society of Endocrinology. Endokrynol. Pol. 2022, 73, 645–679. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.; Abalovich, M.; Alexander, E.K.; Amino, N.; Barbour, L.; Cobin, R.H.; Eastman, C.J.; Lazarus, J.H.; Luton, D.; Mandel, S.J.; et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2543–2565. [Google Scholar] [CrossRef] [Green Version]
- Momotani, N.; Noh, J.; Oyanagi, H.; Ishikawa, N.; Ito, K. Antithyroid drug therapy for Graves’ disease during pregnancy. Optimal regimen for fetal thyroid status. N. Engl. J. Med. 1986, 315, 24–28. [Google Scholar] [CrossRef]
- Vander, J.B.; Gaston, E.A.; Dawber, T.R. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann. Intern. Med. 1968, 69, 537–540. [Google Scholar] [CrossRef]
- Reiners, C.; Wegscheider, K.; Schicha, H.; Theissen, P.; Vaupel, R.; Wrbitzky, R.; Schumm-Draeger, P.M. Prevalence of thyroid disorders in the working population of Germany: Ultrasonography screening in 96,278 unselected employees. Thyroid 2004, 14, 926–932. [Google Scholar] [CrossRef]
- Moon, J.H.; Hyun, M.K.; Lee, J.Y.; Shim, J.I.; Kim, T.H.; Choi, H.S.; Ahn, H.Y.; Kim, K.W.; Park, D.J.; Park, Y.J.; et al. Prevalence of thyroid nodules and their associated clinical parameters: A large-scale, multicenter-based health checkup study. Korean J. Intern. Med. 2018, 33, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.O.; Lee, K.; Lee, S.M.; Seo, G.H. Association Between Pregnancy Outcomes and Radioactive Iodine Treatment After Thyroidectomy Among Women With Thyroid Cancer. JAMA Intern. Med. 2020, 180, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Weeke, J.; Dybkjaer, L.; Granlie, K.; Eskjaer Jensen, S.; Kjaerulff, E.; Laurberg, P.; Magnusson, B. A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol. 1982, 101, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Roti, E.; Gardini, E.; Minelli, R.; Bianconi, L.; Flisi, M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J. Endocrinol. Investig. 1991, 14, 1–9. [Google Scholar] [CrossRef]
- Soldin, O.P.; Tractenberg, R.E.; Hollowell, J.G.; Jonklaas, J.; Janicic, N.; Soldin, S.J. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid 2004, 14, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Ross, D.S. Hypothyroidism during Pregnancy: Clinical Manifestations, Diagnosis, and Treatment; Cooper, D.S., Lockwood, C.J., Eds.; UptoDate: Waltham, MA, USA, 2023. [Google Scholar]
| Hypothyroidism | Hyperthyroidism | |
|---|---|---|
| Non-pregnant women | Weight gain, decreased fertility [135], galactorrhea, hypermenorrhea, polymenorrhea, and hypocoagulable states [136] | Weight loss, amenorrhea, oligomenorrhea, and hypercoagulable states [136] |
| Pregnant women | Placental abruption [137], postpartum hemorrhage, severe preterm delivery, preeclampsia [138], low birth weight [139], and gestational hypertension [140] | Preeclampsia [138], placental previa [141], placental rupture, preterm birth [142], gestational hypertension, preterm premature rupture, and spontaneous abortion [143] |
| Study | Author Year | Journal | Highlighted Recommendations |
|---|---|---|---|
| Thyroid Disease in Pregnancy, ACOG Practice Bulletin, Number 223 [195] | ACOG, 2020. | Obstetrics & Gynecology | Universal screening for thyroid disease in pregnancy is not recommended. Pregnant women with overt hypothyroidism should be treated with adequate thyroid hormone replacement to minimize the risk of adverse outcomes. The TSH level should be monitored in pregnant women being treated for hypothyroidism, and the dose of levothyroxine should be adjusted accordingly with a goal TSH level between the lower limit of the reference range and 2.5 mU/L. Thyroid-stimulating hormone typically is evaluated every 4–6 weeks while adjusting medications. |
| 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum [135] | Alexander, 2017 | Thyroid | Recommended pregnant women ingest 250 lg iodine daily. To achieve a total of 250 lg iodine ingestion daily, strategies may need to be varied based on country of origin. TT4 is a highly reliable substitute for FT4, when used in conjunction with a FT4 index, during the last part of pregnancy. TPOAb positive pregnant women should have serum TSH measured at time of pregnancy confirmation and every 4 weeks thereafter. |
| Thyroid Diseases and Fertility Disorders-Guidelines of the Polish Society of Endocrinology [230] | Hubalewska-Dydejczyk, 2022 | Endokrynologia Polska | Women diagnosed with fertility problems should have thyroid function evaluated and hypothyroidism treated with L-thyroxine as standard of care. Women suffering subclinical hypothyroidism are recommended treatment with L-thyroxine while undergoing fertility treatment for maintenance of TSH < 2.5 mU/L. |
| Management of Thyroid Dysfunction during Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline [231] | Groot, 2012 | The Journal of Clinical Endocrinology and Metabolism | Recommended caution in the interpretation of serum free T4 levels during pregnancy and that each laboratory establish trimester-specific reference ranges for pregnant women if using a free T4 assay. Noted positive association between the presence of thyroid antibodies and pregnancy loss. Does not recommend universal screening for antithyroid antibodies. |
| 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis [226] | Ross, 2016 | Thyroid | Recommended that if Methimazole is chosen as primary treatment for Graves’ disease medication should be continued for 12–18 months and discontinued at that point if TSH and TRAb levels have normalized. Recommended measurement of TRAb levels prior to stopping anti-thyroid drugs therapy to predict which patients can be weaned from the medication, with normal levels indicating greater chance for remission. |
| Antithyroid Drug Therapy for Graves’ Disease during Pregnancy. Optimal Regimen for Fetal Thyroid Status [232] | Noh,1986 | The New England Journal of Medicine | Indicated high free thyroxine levels and antibodies inhibiting binding of thyrotropin as useful indexes of fetal need for antithyroid treatment, and thioamide dosage which maintains maternal free thyroxine levels in a mildly thyrotoxic range as appropriate for maintaining euthyroid status in the fetus. |
| Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement [218] | Jonklaas, 2014 | Thyroid | Concluded that levothyroxine ought to remain the standard of care for treating hypothyroidism. Recommended against alternative preparations of thyroid stimulation, including levothyroxine–liothyronine combination therapy, thyroid extract therapy and others. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. https://doi.org/10.3390/ijms24129815
Brown EDL, Obeng-Gyasi B, Hall JE, Shekhar S. The Thyroid Hormone Axis and Female Reproduction. International Journal of Molecular Sciences. 2023; 24(12):9815. https://doi.org/10.3390/ijms24129815
Chicago/Turabian StyleBrown, Ethan D. L., Barnabas Obeng-Gyasi, Janet E. Hall, and Skand Shekhar. 2023. "The Thyroid Hormone Axis and Female Reproduction" International Journal of Molecular Sciences 24, no. 12: 9815. https://doi.org/10.3390/ijms24129815
APA StyleBrown, E. D. L., Obeng-Gyasi, B., Hall, J. E., & Shekhar, S. (2023). The Thyroid Hormone Axis and Female Reproduction. International Journal of Molecular Sciences, 24(12), 9815. https://doi.org/10.3390/ijms24129815