Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology
Abstract
:1. Introduction
2. Thyroid Hormones in the Development of the Inner Ear
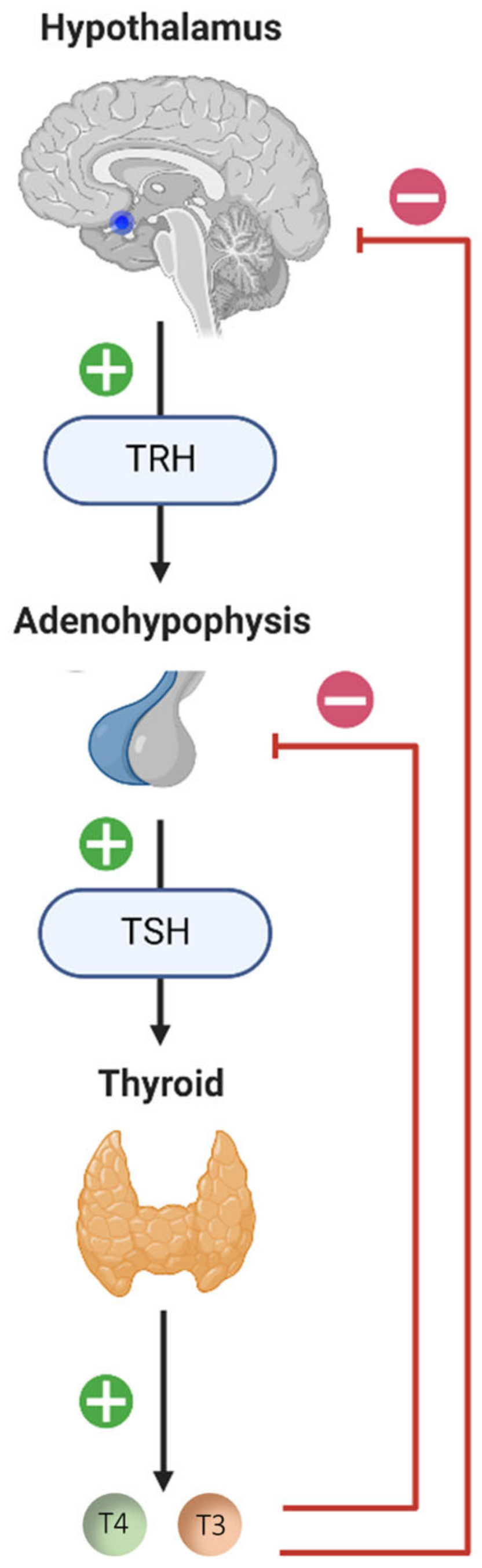
3. A Vestibular Modulation of the Thyroid Axis?
4. Thyroid Disorder and Dizziness in Humans: Is There a Link?
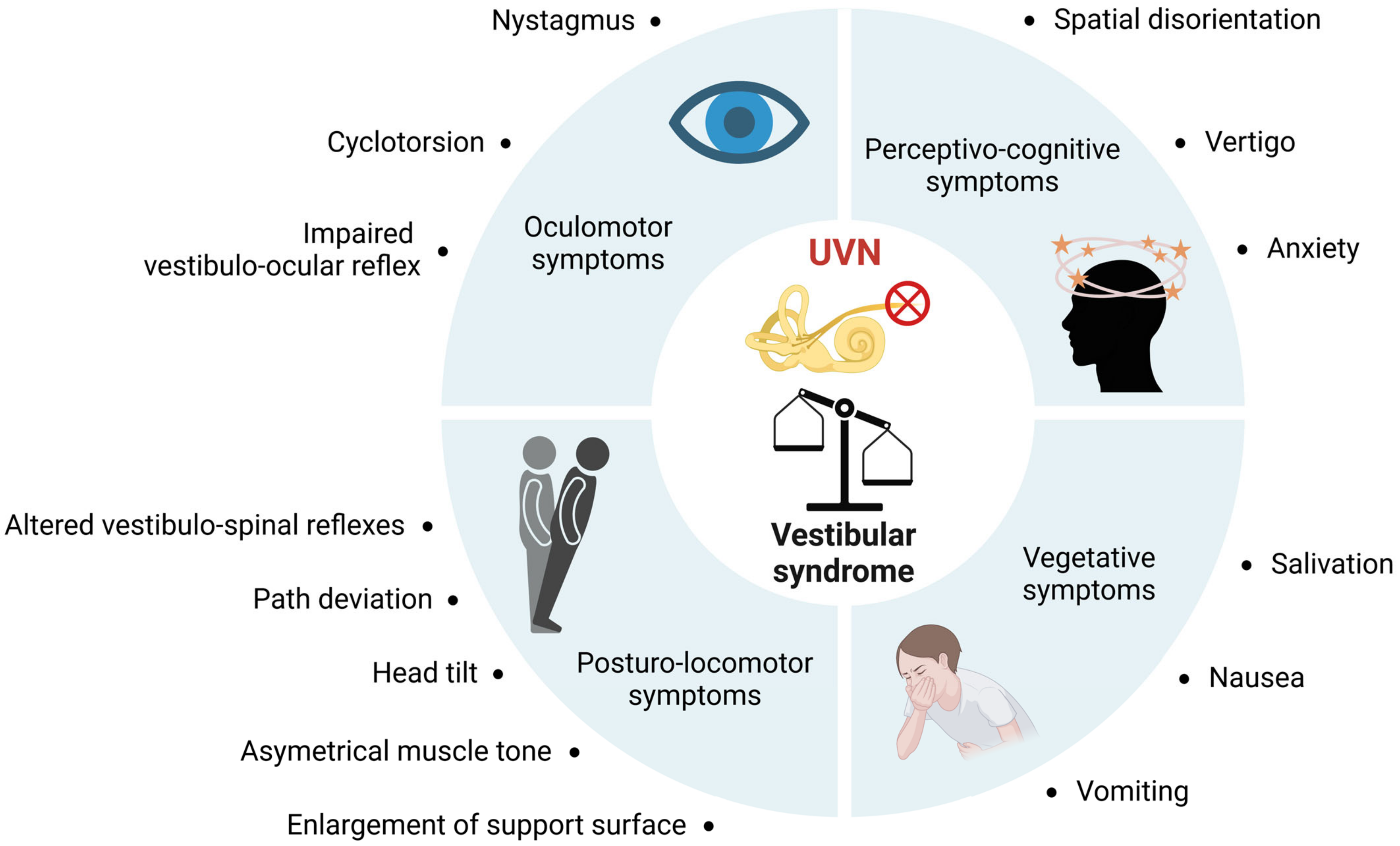
5. Thyroid Hormones and Vestibular Compensation in Animal Models of Vestibulopathy
5.1. Search Strategy
5.2. Neuronal Excitability
5.3. Energy Metabolism
5.4. Adult Neurogenesis
- (1)
- The increased probability of binding and uptake of endogenous and exogenous thyroid hormones in the deafferented vestibular nuclei, increasing the energy metabolism of neurons and glial cells.
- (2)
5.5. Potentiation of T4 to T3 Conversion by the Glial Response Produced by Vestibular Neurectomy
5.6. Myelination
5.7. Angiogenesis
5.8. Muscle Tone
5.9. Interaction between Histamine and the Thyroid Axis
5.10. Thyroid Hormones, Emotional Component and Vestibular Compensation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5HT | Serotonin |
| AraC | Antimitotic Cytosine-beta-D-arabino-furanoside |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| BPPV | Benign Paroxysmal Positional Vertigo |
| CH | Congenital Hypothyroidism |
| D2 | Iodothyronine Deiodase type 2 |
| D3 | Iodothyronine Deiodase type 3 |
| HPT | Hypothalamic-Pituitary-Thyroid |
| MCT8 | Monocarboxylate Transporter-8 |
| MCT10 | Monocarboxylate Transporter-10 |
| NSC | Neural Stem Cells |
| OATP1 | Organic Anion Transporter Protein-1 |
| PVN | Paraventricular Nucleus |
| rT3 | Reverse T3 |
| SVZ | Subventricular Zone |
| T2 | Diiodothyronine |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TRH | Thyrotropin-Releasing Hormone |
| TRs | Thyroid Hormone Receptors |
| TSH | Thyroid-Stimulating Hormone |
| TTK | Transtympanic Administration of Kainic Acid |
| UVN | Unilateral Vestibular Neurectomy |
| VN | Vestibular Nuclei |
References
- Refetoff, S. Thyroid Hormone Serum Transport Proteins. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Kapoor, R.; Fanibunda, S.E.; Desouza, L.A.; Guha, S.K.; Vaidya, V.A. Perspectives on Thyroid Hormone Action in Adult Neurogenesis. J. Neurochem. 2015, 133, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Visser, W.E.; Friesema, E.C.H.; Jansen, J.; Visser, T.J. Thyroid Hormone Transport in and out of Cells. Trends. Endocrinol. Metab. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Schweizer, U.; Köhrle, J. Transport of Thyroid Hormone in Brain. Front. Endocrinol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadano-Ferraz, A.; Obregon, M.J.; Germain, D.L.S.; Bernal, J. The Type 2 Iodothyronine Deiodinase Is Expressed Primarily in Glial Cells in the Neonatal Rat Brain. Proc. Natl. Acad. Sci. USA 1997, 94, 10391–10396. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Andrews, M.H.; Lingas, R.; McCabe, C.J.; Franklyn, J.A.; Kilby, M.D.; Matthews, S.G. Maternal Nutrient Deprivation Induces Sex-Specific Changes in Thyroid Hormone Receptor and Deiodinase Expression in the Fetal Guinea Pig Brain: TRs and Deiodinases in Fetal Brains. J. Physiol. 2005, 566, 467–480. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Brent, G.A. Thyroid Hormone and the Brain: Mechanisms of Action in Development and Role in Protection and Promotion of Recovery after Brain Injury. Pharmacol. Ther. 2018, 186, 176–185. [Google Scholar] [CrossRef]
- Morte, B.; Bernal, J. Thyroid Hormone Action: Astrocyte-Neuron Communication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.C.; Privalsky, M.L. Thyroid Hormones, T3 and T4, in the Brain. Front. Endocrinol. 2014, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Lemkine, G.F.; Raji, A.; Alfama, G.; Turque, N.; Hassani, Z.; Alegria-Prévot, O.; Samarut, J.; Levi, G.; Demeneix, B.A. Adult Neural Stem Cell Cycling in Vivo Requires Thyroid Hormone and Its Alpha Receptor. FASEB J. 2005, 19, 1–17. [Google Scholar] [CrossRef] [Green Version]
- López-Juárez, A.; Remaud, S.; Hassani, Z.; Jolivet, P.; Pierre Simons, J.; Sontag, T.; Yoshikawa, K.; Price, J.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid Hormone Signaling Acts as a Neurogenic Switch by Repressing Sox2 in the Adult Neural Stem Cell Niche. Cell Stem. Cell 2012, 10, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, R.; Desouza, L.A.; Nanavaty, I.N.; Kernie, S.G.; Vaidya, V.A. Thyroid Hormone Accelerates the Differentiation of Adult Hippocampal Progenitors. J. Neuroendocrinol. 2012, 24, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.J. Differential Expression of a and 1, Thyroid Hormone Receptor Genes in Rat Brain and Pituitary. Proc. Natl. Acad. Sci. USA 1989, 86, 7250–7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, K.; Dudazy, S.; van Hogerlinden, M.; Nordström, K.; Mittag, J.; Vennström, B. The Thyroid Hormone Receptor Alpha1 Protein Is Expressed in Embryonic Postmitotic Neurons and Persists in Most Adult Neurons. Mol. Endocrinol. 2010, 24, 1904–1916. [Google Scholar] [CrossRef] [Green Version]
- Caria, M.A.; Dratman, M.B.; Kow, L.-M.; Mameli, O.; Pavlides, C. Thyroid Hormone Action: Nongenomic Modulation of Neuronal Excitability in the Hippocampus. J. Neuroendocrinol. 2009, 21, 98–107. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic Actions of Thyroid Hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Davis, P.J.; Leonard, J.L.; Davis, F.B. Mechanisms of Nongenomic Actions of Thyroid Hormone. Front. Neuroendocrinol. 2008, 29, 211–218. [Google Scholar] [CrossRef]
- Hiroi, Y.; Kim, H.-H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.-H.; Scanlan, T.S.; et al. Rapid Nongenomic Actions of Thyroid Hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Tao, L.; Liu, Z.; Jiang, Y.; Deng, X. Identification of Neural Stem Cells from Postnatal Mouse Auditory Cortex In Vitro. Stem. Cells Dev. 2019, 28, 860–870. [Google Scholar] [CrossRef]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef]
- De Andrade, C.L.O.; Machado, G.C.; Fernandes, L.D.C.; de Albuquerque, J.M.; Casais-e-Silva, L.L.; Ramos, H.E.; Alves, C.D.A.D.; de Andrade, C.L.O.; Machado, G.C.; Fernandes, L.D.C.; et al. Mechanisms Involved in Hearing Disorders of Thyroid Ontogeny: A Literature Review. Arch. Endocrinol. Metab. 2017, 61, 501–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, A.A.; Lenz, D.R.; Shivatzki, S.; Cohen, K.; Ashur-Fabian, O.; Avraham, K.B. Atrophic Thyroid Follicles and Inner Ear Defects Reminiscent of Cochlear Hypothyroidism in Slc26a4-Related Deafness. Mamm. Genome 2014, 25, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uziel, A.; Marot, M.; Rabie, A. Corrective Effects of Thyroxine on Cochlear Abnormalities Induced by Congenital Hypothyroidism in the Rat. II. Electrophysiological Study. Brain Res. 1985, 351, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Dememes, D.; Dechesne, C.; Legrand, C.; Sans, A. Effects of Hypothyroidism on Postnatal Development in the Peripheral Vestibular System. Dev. Brain Res. 1986, 25, 147–152. [Google Scholar] [CrossRef]
- Meza, G.; Acuña, D.; Escobar, C. Development of Vestibular and Auditory Function: Effects of Hypothyroidism and Thyroxine Replacement Therapy on Nystagmus and Auditory Evoked Potentials in the Pigmented Rat. Int. J. Dev. Neurosci. 1996, 14, 515–522. [Google Scholar] [CrossRef]
- Meza, G.; Acuña, D.; Peñaloza, Y.; Poblano, A. Congenital Hypothyroidism. Vestibular and Auditory Damage in the Pigmented Rat. Ann. N. Y. Acad. Sci. 1991, 630, 274–276. [Google Scholar] [CrossRef]
- Albi, E.; Krüger, M.; Hemmersbach, R.; Lazzarini, A.; Cataldi, S.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Impact of Gravity on Thyroid Cells. Int. J. Mol. Sci. 2017, 18, 972. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Pickenhahn, H.; Bauer, J.; Paul, M.; Cogoli, A. Effects of Simulated Microgravity on Thyroid Carcinoma Cells. J. Gravit. Physiol. 2002, 9, P253–P256. [Google Scholar]
- Leach, C.S. An Overview of the Endocrine and Metabolic Changes in Manned Space Flight. Acta Astronaut. 1981, 8, 977–986. [Google Scholar] [CrossRef]
- Rambaut, P.C.; Leach, C.S.; Leonard, J.I. Observations in Energy Balance in Man during Spaceflight. Am. J. Physiol. 1977, 233, R208–R212. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Moldawer, L.L. Endocrine Relationships during Human Spaceflight. Am. J. Physiol. 1999, 276, E155–E162. [Google Scholar] [CrossRef] [PubMed]
- Strollo, F. Hormonal Changes in Humans during Spaceflight. Adv. Space Biol. Med. 1999, 7, 99–129. [Google Scholar] [CrossRef] [PubMed]
- Loginov, V.I. The inhibition of the thyroid and calcitonin-producing functions of the rat thyroid gland in weightlessness. Aviakosm. Ekol. Med. 1999, 33, 12–16. [Google Scholar]
- Plakhuta-Plakutina, G.I.; Kabitskiĭ, E.N.; Dmitrieva, N.P.; Amirkhanian, E.A. Studies of the morphology of the thyroid gland and thyroid hormone levels in the blood of rats in experiments on “Kosmos-1667” and “Kosmos-1887”. Kosm. Biol. Aviakosm. Med. 1990, 24, 25–27. [Google Scholar] [PubMed]
- Krasnov, I.B.; Alekseev, E.I.; Loginov, V.I. Role of the endocrine glands in divergence of plastic processes and energy metabolism in rats after extended exposure to hypergravity: Cytologic investigation. Aviakosm. Ekol. Med. 2006, 40, 29–34. [Google Scholar]
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Physiology of a Microgravity Environment Invited Review: Microgravity and Skeletal Muscle. J. Appl. Physiol. 2000, 89, 823–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiozzo, V.J.; Baker, M.J.; Herrick, R.E.; Tao, M.; Baldwin, K.M. Effect of Spaceflight on Skeletal Muscle: Mechanical Properties and Myosin Isoform Content of a Slow Muscle. J. Appl. Physiol. 1994, 76, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Desplanches, D.; Mayet, M.H.; Ilyina-Kakueva, E.I.; Sempore, B.; Flandrois, R. Skeletal Muscle Adaptation in Rats Flown on Cosmos 1667. J. Appl. Physiol. 1990, 68, 48–52. [Google Scholar] [CrossRef]
- Ilyina-Kakueva, E.I.; Portugalov, V.V.; Krivenkova, N.P. Space Flight Effects on the Skeletal Muscles of Rats. Aviat. Space Env. Med. 1976, 47, 700–703. [Google Scholar]
- Ohira, Y.; Jiang, B.; Roy, R.R.; Oganov, V.; Ilyina-Kakueva, E.; Marini, J.F.; Edgerton, V.R. Rat Soleus Muscle Fiber Responses to 14 Days of Spaceflight and Hindlimb Suspension. J. Appl. Physiol. 1992, 73, 51S–57S. [Google Scholar] [CrossRef]
- Sandonà, D.; Desaphy, J.-F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli-Betto, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of Mouse Skeletal Muscle to Long-Term Microgravity in the MDS Mission. PLoS ONE 2012, 7, e33232. [Google Scholar] [CrossRef] [Green Version]
- Riley, D.A.; Ellis, S. Research on the Adaptation of Skeletal Muscle to Hypogravity: Past and Future Directions. Adv. Space Res. 1983, 3, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Costill, D.; Gallagher, P.; Creer, A.; Peters, J.R.; Evans, H.; Riley, D.A.; Fitts, R.H. Exercise in Space: Human Skeletal Muscle after 6 Months Aboard the International Space Station. J. Appl. Physiol. 2009, 106, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.D.; Schmidt, E.D.; van der Gaag, R.; Ganpat, R.; Broersma, L.; de Boer, P.A.; Moorman, A.F.; Lamers, W.H.; Wiersinga, W.M.; Koornneef, L. Distribution of the Nuclear Thyroid-Hormone Receptor in Extraocular and Skeletal Muscles. J. Endocrinol. 1992, 133, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kádár, A.; Sánchez, E.; Wittmann, G.; Singru, P.S.; Füzesi, T.; Marsili, A.; Larsen, P.R.; Liposits, Z.; Lechan, R.M.; Fekete, C. Distribution of Hypophysiotropic Thyrotropin-Releasing Hormone (TRH)-Synthesizing Neurons in the Hypothalamic Paraventricular Nucleus of the Mouse. J. Comp. Neurol. 2010, 518, 3948–3961. [Google Scholar] [CrossRef]
- Azzena, G.B.; Melis, F.; Caria, M.A.; Teatini, G.P.; Bozzo, G. Vestibular Projections to Hypothalamic Supraoptic and Paraventricular Nuclei. Arch. Ital. Biol. 1993, 131, 127–136. [Google Scholar]
- Liu, F.; Inokuchi, A.; Komiyama, S. Neuronal Responses to Vestibular Stimulation in the Guinea Pig Hypothalamic Paraventricular Nucleus. Eur. Arch. Otorhinolaryngol. 1997, 254, 95–100. [Google Scholar] [CrossRef]
- Markia, B.; Kovács, Z.I.; Palkovits, M. Projections from the Vestibular Nuclei to the Hypothalamic Paraventricular Nucleus: Morphological Evidence for the Existence of a Vestibular Stress Pathway in the Rat Brain. Brain Struct. Funct. 2008, 213, 239–245. [Google Scholar] [CrossRef]
- Horowitz, S.S.; Blanchard, J.; Morin, L.P. Medial Vestibular Connections with the Hypocretin (Orexin) System. J. Comp. Neurol. 2005, 487, 127–146. [Google Scholar] [CrossRef]
- Saman, Y.; Bamiou, D.E.; Gleeson, M.; Dutia, M.B. Interactions between Stress and Vestibular Compensation—A Review. Front. Neurol. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Saman, Y.; Arshad, Q.; Dutia, M.; Rea, P. Stress and the Vestibular System. Int. Rev. Neurobiol. 2020, 152, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Gliddon, C.M.; Darlington, C.L.; Smith, P.F. Activation of the Hypothalamic-Pituitary-Adrenal Axis Following Vestibular Deafferentation in Pigmented Guinea Pig. Brain Res. 2003, 964, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Manrique, C.; Lacour, M. Stress Axis Plasticity during Vestibular Compensation in the Adult Cat. Neuroscience 2009, 160, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Trottier, S.; Mourre, C.; Lacour, M. Changes in the Histaminergic System during Vestibular Compensation in the Cat: Histamine and Vestibular Compensation. J. Physiol. 2006, 573, 723–739. [Google Scholar] [CrossRef]
- Cureoglu, S.; da Costa Monsanto, R.; Paparella, M.M. Histopathology of Meniere’s Disease. Oper. Tech. Otolayngol. Head Neck Surg. 2016, 27, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, G.; Lopez, I.A.; Sepahdari, A.R.; Ishiyama, A. Meniere’s Disease: Histopathology, Cytochemistry, and Imaging. Ann. N. Y. Acad. Sci. 2015, 1343, 49–57. [Google Scholar] [CrossRef]
- Oberman, B.S.; Patel, V.A.; Cureoglu, S.; Isildak, H. The Aetiopathologies of Ménière’s Disease: A Contemporary Review. Acta Otorhinolaryngol. Ital. 2017, 37, 250–263. [Google Scholar] [CrossRef]
- Powers, W.H. Metabolic Aspects of Ménière’s Disease. Laryngoscope 1978, 88, 122–129. [Google Scholar] [CrossRef]
- Lin, W.-L.; Chen, C.-Y.; Hsu, T.-Y.; Chen, W.-K.; Lin, C.-L.; Chen, H.-C. Hypothyroidism Is an Independent Risk Factor for Menière’s Disease: A Population-Based Cohort Study. Medicine 2019, 98, e15166. [Google Scholar] [CrossRef]
- Bhatia, P.L.; Gupta, O.P.; Agrawal, M.K.; Mishr, S.K. Audiological and Vestibular Function Tests in Hypothyroidism. Laryngoscope 1977, 87, 2082–2089. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Choi, H.G. Association between Ménière’s Disease and Thyroid Diseases: A Nested Case-Control Study. Sci. Rep. 2020, 10, 18224. [Google Scholar] [CrossRef]
- Santosh, U.P.; Rao, M.S.S. Incidence of Hypothyroidism in Meniere’s Disease. J. Clin. Diagn. Res. 2016, 10, MC01–MC03. [Google Scholar] [CrossRef]
- Miśkiewicz-Orczyk, K.A.; Lisowska, G.; Kajdaniuk, D.; Wojtulek, M. Can Hashimoto’s Thyroiditis Cause Vertigo? Endokrynol. Pol. 2020, 70, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, J.Y.; Lee, H.J.; Gi, M.; Kim, B.G.; Choi, J.Y. Autoimmunity as a Candidate for the Etiopathogenesis of Meniere’s Disease: Detection of Autoimmune Reactions and Diagnostic Biomarker Candidate. PLoS ONE 2014, 9, e111039. [Google Scholar] [CrossRef] [Green Version]
- Fattori, B.; Nacci, A.; Dardano, A.; Dallan, I.; Grosso, M.; Traino, C.; Mancini, V.; Ursino, F.; Monzani, F. Possible Association between Thyroid Autoimmunity and Menière’s Disease. Clin. Exp. Immunol. 2008, 152, 28–32. [Google Scholar] [CrossRef]
- Chiarella, G.; Russo, D.; Monzani, F.; Petrolo, C.; Fattori, B.; Pasqualetti, G.; Cassandro, E.; Costante, G. Hashimoto Thyroiditis and Vestibular Dysfunction. Endocr. Pract. 2017, 23, 863–868. [Google Scholar] [CrossRef]
- Chiarella, G.; Tognini, S.; Nacci, A.; Sieli, R.; Costante, G.; Petrolo, C.; Mancini, V.; Guzzi, P.H.; Pasqualetti, G.; Cassandro, E.; et al. Vestibular Disorders in Euthyroid Patients with Hashimoto’s Thyroiditis: Role of Thyroid Autoimmunity. Clin. Endocrinol. 2014, 81, 600–605. [Google Scholar] [CrossRef]
- Papi, G.; Guidetti, G.; Corsello, S.M.; Di Donato, C.; Pontecorvi, A. The Association between Benign Paroxysmal Positional Vertigo and Autoimmune Chronic Thyroiditis Is Not Related to Thyroid Status. Thyroid 2010, 20, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Mccabe, B.F.; Ryu, J.H. Experiments on Vestibular Compensation. Laryngoscope 1969, 79, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Precht, W.; Shimazu, H.; Markham, C.H. A Mechanism of Central Compensation of Vestibular Function Following Hemilabyrinthectomy. J. Neurophysiol. 1966, 29, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.E.; Cullen, K.E. Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacour, M.; Tighilet, B. Plastic Events in the Vestibular Nuclei during Vestibular Compensation: The Brain Orchestration of a “Deafferentation” Code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Helmchen, C.; Vidal, P.-P. Vestibular Compensation: The Neuro-Otologist’s Best Friend. J. Neurol. 2016, 263, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.F.; Curthoys, I.S. Mechanisms of Recovery Following Unilateral Labyrinthectomy: A Review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef]
- Curthoys, I.S. Vestibular Compensation and Substitution. Curr. Opin. Neurol. 2000, 13, 27. [Google Scholar] [CrossRef]
- Darlington, C.L.; Dutia, M.B.; Smith, P.F. The Contribution of the Intrinsic Excitability of Vestibular Nucleus Neurons to Recovery from Vestibular Damage: Recovery from Vestibular Damage: Role of Vestibular Nucleus. Eur. J. Neurosci. 2002, 15, 1719–1727. [Google Scholar] [CrossRef]
- Darlington, C.L.; Smith, P.F. Molecular Mechanisms of Recovery from Vestibular Damage in Mammals: Recent Advances. Prog. Neurobiol. 2000, 62, 313–325. [Google Scholar] [CrossRef]
- Ris, L.; de Waele, C.; Serafin, M.; Vidal, P.P.; Godaux, E. Neuronal Activity in the Ipsilateral Vestibular Nucleus Following Unilateral Labyrinthectomy in the Alert Guinea Pig. J. Neurophysiol. 1995, 74, 2087–2099. [Google Scholar] [CrossRef]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF Signaling Promotes Vestibular Compensation by Increasing Neurogenesis and Remodeling the Expression of Potassium-Chloride Cotransporter KCC2 and GABAA Receptor in the Vestibular Nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef] [Green Version]
- Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. [Google Scholar] [CrossRef]
- Tighilet, B.; Leonard, J.; Mourre, C.; Chabbert, C. Apamin Treatment Accelerates Equilibrium Recovery and Gaze Stabilization in Unilateral Vestibular Neurectomized Cats: Cellular and Behavioral Aspects. Neuropharmacology 2019, 144, 133–142. [Google Scholar] [CrossRef]
- Tighilet, B.; Bourdet, A.; Péricat, D.; Timon-David, E.; Rastoldo, G.; Chabbert, C. SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals 2021, 14, 1226. [Google Scholar] [CrossRef]
- Tighilet, B.; Brezun, J.M.; Sylvie, G.D.D.; Gaubert, C.; Lacour, M. New Neurons in the Vestibular Nuclei Complex after Unilateral Vestibular Neurectomy in the Adult Cat. Eur. J. Neurosci. 2007, 25, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Chabbert, C. Adult Neurogenesis Promotes Balance Recovery after Vestibular Loss. Prog. Neurobiol. 2019, 174, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Brezun, J.M.; Leonard, J.; Lacour, M.; Tighilet, B. Neurogenesis and Astrogenesis Contribution to Recovery of Vestibular Functions in the Adult Cat Following Unilateral Vestibular Neurectomy: Cellular and Behavioral Evidence. Neuroscience 2009, 164, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.; Blaesse, A.; Kysenius, K.; Huttunen, H.J.; Tanhuanpää, K.; Saarma, M.; Rivera, C. Thyroxin Regulates BDNF Expression to Promote Survival of Injured Neurons. Mol. Cell. Neurosci. 2009, 42, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Fanibunda, S.E.; Desouza, L.A.; Kapoor, R.; Vaidya, R.A.; Vaidya, V.A. Thyroid Hormone Regulation of Adult Neurogenesis. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2018; Volume 106, pp. 211–251. ISBN 978-0-12-814116-8. [Google Scholar]
- Gothié, J.-D.; Demeneix, B.; Remaud, S. Comparative Approaches to Understanding Thyroid Hormone Regulation of Neurogenesis. Mol. Cell. Endocrinol. 2017, 459, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Remaud, S.; Gothié, J.-D.; Morvan-Dubois, G.; Demeneix, B.A. Thyroid Hormone Signaling and Adult Neurogenesis in Mammals. Front. Endocrinol. 2014, 5, 62. [Google Scholar] [CrossRef]
- Hoffmann, G.; Dietzel, I.D. Thyroid Hormone Regulates Excitability in Central Neurons from Postnatal Rats. Neuroscience 2004, 125, 369–379. [Google Scholar] [CrossRef]
- Makii, E.A.; Nerush, P.A.; Rodinskii, A.G.; Myakoushko, V.A. Evoked Activity of Afferent and Efferent Fibers of the Sciatic Nerve in Rats under Conditions of Experimental Hyperthyroidism. Neurophysiology 2002, 34, 44–51. [Google Scholar] [CrossRef]
- Oshima, K.; Gorbman, A. Influence of Thyroxine and Steroid Hormones on Spontaneous and Evoked Unitary Activity in the Olfactory Bulb of Goldfish. Gen. Comp. Endocrinol. 1966, 7, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Sarkaki, A.; Ali Mard, S.; Ahangarpour, A.; Khorsandi, L.; Farbood, Y. Central and Peripheral Administrations of Levothyroxine Improved Memory Performance and Amplified Brain Electrical Activity in the Rat Model of Alzheimer’s Disease. Neuropeptides 2016, 59, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.; Rivera, C. Interplay between Thyroxin, BDNF and GABA in Injured Neurons. Neuroscience 2013, 239, 241–252. [Google Scholar] [CrossRef]
- Horn, E.; Rayer, B. A Hormonal Component in Central Vestibular Compensation. Z. Naturforsch. C Biosci. 1980, 35, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Igarashi, M. Effect of Thyrotropin-Releasing Hormone on Vestibular Compensation in Primates. Am. J. Otolaryngol. 1986, 7, 177–180. [Google Scholar] [CrossRef]
- Calzà, L.; Giardino, L.; Zanni, M.; Galetti, G. Muscarinic and Gamma-Aminobutyric Acid-Ergic Receptor Changes during Vestibular Compensation. Eur. Arch. Oto-Rhino-Laryngol. 1992, 249, 34–39. [Google Scholar] [CrossRef]
- Zanni, M.; Giardino, L.; Toschi, L.; Galetti, G.; Calzà, L. Distribution of Neurotransmitters, Neuropeptides, and Receptors in the Vestibular Nuclei Complex of the Rat: An Immunocytochemical, in Situ Hybridization and Quantitative Receptor Autoradiographic Study. Brain Res. Bull. 1995, 36, 443–452. [Google Scholar] [CrossRef]
- Yu, D.; Zhou, H.; Yang, Y.; Jiang, Y.; Wang, T.; Lv, L.; Zhou, Q.; Yang, Y.; Dong, X.; He, J.; et al. The Bidirectional Effects of Hypothyroidism and Hyperthyroidism on Anxiety- and Depression-like Behaviors in Rats. Horm. Behav. 2015, 69, 106–115. [Google Scholar] [CrossRef]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 505. [Google Scholar] [CrossRef]
- Tighilet, B.; Péricat, D.; Frelat, A.; Cazals, Y.; Rastoldo, G.; Boyer, F.; Dumas, O.; Chabbert, C. Adjustment of the Dynamic Weight Distribution as a Sensitive Parameter for Diagnosis of Postural Alteration in a Rodent Model of Vestibular Deficit. PLoS ONE 2017, 12, e0187472. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.V.; Williams, D.B.; Fitzgerald, R.M.; Im, H.K.; Vonvoigtlander, P.F. Thyroid Hormonal Modulation of the Binding and Activity of the GABAA Receptor Complex of Brain. Neuroscience 1996, 73, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Puia, G.; Losi, G. Thyroid Hormones Modulate GABAA Receptor-Mediated Currents in Hippocampal Neurons. Neuropharmacology 2011, 60, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Yonkers, M.A.; Ribera, A.B. Molecular Components Underlying Nongenomic Thyroid Hormone Signaling in Embryonic Zebrafish Neurons. Neural Dev. 2009, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Yonkers, M.A.; Ribera, A.B. Sensory Neuron Sodium Current Requires Nongenomic Actions of Thyroid Hormone During Development. J. Neurophysiol. 2008, 100, 2719–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidoff, R.A.; Ruskin, H.M. The Effects of Microelectrophoretically Applied Thyroid Hormone on Single Cat Central Nervous System Neurons. Neurology 1972, 22, 467–472. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic Effects of the Intracellular Regulation of Thyroid Hormone: Old Players, New Concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Kaila, K.; Price, T.J.; Payne, J.A.; Puskarjov, M.; Voipio, J. Cation-Chloride Cotransporters in Neuronal Development, Plasticity and Disease. Nat. Rev. Neurosci. 2014, 15, 637–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desouza, L.A.; Ladiwala, U.; Daniel, S.M.; Agashe, S.; Vaidya, R.A.; Vaidya, V.A. Thyroid Hormone Regulates Hippocampal Neurogenesis in the Adult Rat Brain. Mol. Cell. Neurosci. 2005, 29, 414–426. [Google Scholar] [CrossRef]
- Weitzel, J.M.; Alexander Iwen, K. Coordination of Mitochondrial Biogenesis by Thyroid Hormone. Mol. Cell. Endocrinol. 2011, 342, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid Hormone Action in Mitochondria. J. Mol. Endocrinol. 2001, 26, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging New Sites for Adult Neurogenesis in the Mammalian Brain: A Comparative Study between the Hypothalamus and the Classical Neurogenic Zones: Neurogenesis in Adult Hypothalamus. Eur. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Escoffier, G.; Gharbi, A.; Watabe, I.; Tighilet, B. GABAA Receptor Agonist and Antagonist Alter Vestibular Compensation and Different Steps of Reactive Neurogenesis in Deafferented Vestibular Nuclei of Adult Cats. J. Neurosci. 2013, 33, 15555–15566. [Google Scholar] [CrossRef] [Green Version]
- Remaud, S.; Ortiz, F.C.; Perret-Jeanneret, M.; Aigrot, M.-S.; Gothié, J.-D.; Fekete, C.; Kvárta-Papp, Z.; Gereben, B.; Langui, D.; Lubetzki, C.; et al. Transient Hypothyroidism Favors Oligodendrocyte Generation Providing Functional Remyelination in the Adult Mouse Brain. Elife 2017, 6, e29996. [Google Scholar] [CrossRef]
- Rastoldo, G.; El Mahmoudi, N.; Marouane, E.; Pericat, D.; Watabe, I.; Toneto, A.; López-Juárez, A.; Chabbert, C.; Tighilet, B. Adult and Endemic Neurogenesis in the Vestibular Nuclei after Unilateral Vestibular Neurectomy. Prog. Neurobiol. 2021, 196, 101899. [Google Scholar] [CrossRef] [PubMed]
- Marouane, E.; El Mahmoudi, N.; Rastoldo, G.; Péricat, D.; Watabe, I.; Lapôtre, A.; Tonetto, A.; Xavier, F.; Dumas, O.; Chabbert, C.; et al. Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei. Cells 2021, 10, 3377. [Google Scholar] [CrossRef] [PubMed]
- Campos-Torres, A.; Touret, M.; Vidal, P.P.; Barnum, S.; de Waele, C. The Differential Response of Astrocytes within the Vestibular and Cochlear Nuclei Following Unilateral Labyrinthectomy or Vestibular Afferent Activity Blockade by Transtympanic Tetrodotoxin Injection in the Rat. Neuroscience 2005, 130, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Campos-Torres, A.; Vidal, P.P.; de Waele, C. Evidence for a Microglial Reaction within the Vestibular and Cochlear Nuclei Following Inner Ear Lesion in the Rat. Neuroscience 1999, 92, 1475–1490. [Google Scholar] [CrossRef]
- De Waele, C.; Campos Torres, A.; Josset, P.; Vidal, P.P. Evidence for Reactive Astrocytes in Rat Vestibular and Cochlear Nuclei Following Unilateral Inner Ear Lesion. Eur. J. Neurosci. 1996, 8, 2006–2018. [Google Scholar] [CrossRef]
- Dutheil, S.; Lacour, M.; Tighilet, B. Neurogenic Potential of the Vestibular Nuclei and Behavioural Recovery Time Course in the Adult Cat Are Governed by the Nature of the Vestibular Damage. PLoS ONE 2011, 6, e22262. [Google Scholar] [CrossRef] [Green Version]
- Bringuier, C.M.; Hatat, B.; Boularand, R.; Chabbert, C.; Tighilet, B. Characterization of Thyroid Hormones Antivertigo Effects in a Rat Model of Excitotoxically-Induced Vestibulopathy. Front. Neurol. 2022, 13, 877319. [Google Scholar] [CrossRef]
- Calzà, L.; Fernández, M.; Giardino, L. Role of the Thyroid System in Myelination and Neural Connectivity. Compr. Physiol. 2015, 5, 1405–1421. [Google Scholar] [CrossRef]
- Emery, B. Regulation of Oligodendrocyte Differentiation and Myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, M.; Giuliani, A.; Pirondi, S.; D’Intino, G.; Giardino, L.; Aloe, L.; Levi-Montalcini, R.; Calzà, L. Thyroid Hormone Administration Enhances Remyelination in Chronic Demyelinating Inflammatory Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 16363–16368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohácsik, P.; Zeöld, A.; Bianco, A.C.; Gereben, B. Thyroid Hormone and the Neuroglia: Both Source and Target. J. Thyroid Res. 2011, 2011, 215718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartley, M.D.; Banerji, T.; Tagge, I.J.; Kirkemo, L.L.; Chaudhary, P.; Calkins, E.; Galipeau, D.; Shokat, M.D.; DeBell, M.J.; Van Leuven, S.; et al. Myelin Repair Stimulated by CNS-Selective Thyroid Hormone Action. JCI Insight 2019, 4, e126329. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dokas, L.A.; Godfrey, D.A.; Rubin, A.M. Remodeling of Synaptic Connections in the Deafferented Vestibular Nuclear Complex. J. Vestib. Res. 2002, 12, 167–183. [Google Scholar] [CrossRef]
- Raymond, J.; Ez-Zaher, L.; Demêmes, D.; Lacour, M. Quantification of Synaptic Density Changes in the Medial Vestibular Nucleus of the Cat Following Vestibular Neurectomy. Restor. Neurol. Neurosci. 1991, 3, 197–203. [Google Scholar] [CrossRef]
- Arai, K.; Jin, G.; Navaratna, D.; Lo, E.H. Brain Angiogenesis in Developmental and Pathological Processes: Neurovascular Injury and Angiogenic Recovery after Stroke. FEBS J. 2009, 276, 4644–4652. [Google Scholar] [CrossRef] [Green Version]
- Arenillas, J.F.; Sobrino, T.; Castillo, J.; Dávalos, A. The Role of Angiogenesis in Damage and Recovery from Ischemic Stroke. Curr. Treat. Options Cardio. Med. 2007, 9, 205–212. [Google Scholar] [CrossRef]
- Talhada, D.; Santos, C.R.A.; Gonçalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms After Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, R.J.; Zimmerman, M.B.; Suvarna, P.R.; Morkin, E.; Pennock, G.D.; Goldman, S. A Thyroid Hormone Analog Stimulates Angiogenesis in the Post-Infarcted Rat Heart. J. Mol. Cell. Cardiol. 1998, 30, 923–932. [Google Scholar] [CrossRef]
- Schlenker, E.H.; Hora, M.; Liu, Y.; Redetzke, R.A.; Morkin, E.; Gerdes, A.M. Effects of Thyroidectomy, T4, and DITPA Replacement on Brain Blood Vessel Density in Adult Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1504–R1509. [Google Scholar] [CrossRef] [Green Version]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of Thyroid Hormone in Skeletal Muscle Physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid Hormones and Skeletal Muscle—New Insights and Potential Implications. Nat. Rev. Endocrinol. 2014, 10, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Simonides, W.S.; van Hardeveld, C. Thyroid Hormone as a Determinant of Metabolic and Contractile Phenotype of Skeletal Muscle. Thyroid 2008, 18, 205–216. [Google Scholar] [CrossRef]
- Péricat, D.; Farina, A.; Agavnian-Couquiaud, E.; Chabbert, C.; Tighilet, B. Complete and Irreversible Unilateral Vestibular Loss: A Novel Rat Model of Vestibular Pathology. J. Neurosci. Methods 2017, 283, 83–91. [Google Scholar] [CrossRef]
- Zennou-Azogui, Y.; Borel, L.; Lacour, M.; Ez-Zaher, L.; Ouaknine, M. Recovery of Head Postural Control Following Unilateral Vestibular Neurectomy in the Cat: Neck Muscle Activity and Neuronal Correlates in Deiters’ Nuclei. Acta Oto-Laryngol. 1993, 113, 5–19. [Google Scholar] [CrossRef]
- Bergquist, F.; Dutia, M.B. Central Histaminergic Modulation of Vestibular Function—A Review. Sheng Li Xue Bao 2006, 58, 293–304. [Google Scholar] [PubMed]
- Chen, Z.-P.; Zhang, X.-Y.; Peng, S.-Y.; Yang, Z.-Q.; Wang, Y.-B.; Zhang, Y.-X.; Chen, X.; Wang, J.-J.; Zhu, J.-N. Histamine H1 Receptor Contributes to Vestibular Compensation. J. Neurosci. 2019, 39, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Lozada, A.F.; Aarnisalo, A.A.; Karlstedt, K.; Stark, H.; Panula, P. Plasticity of Histamine H3 Receptor Expression and Binding in the Vestibular Nuclei after Labyrinthectomy in Rat. BMC Neurosci 2004, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacour, M.; Tighilet, B. Vestibular Compensation in the Cat: The Role of the Histaminergic System. Acta Otolaryngol. Suppl. 2000, 544, 15–18. [Google Scholar] [CrossRef]
- Tighilet, B.; Léonard, J.; Watabe, I.; Bernard-Demanze, L.; Lacour, M. Betahistine Treatment in a Cat Model of Vestibular Pathology: Pharmacokinetic and Pharmacodynamic Approaches. Front. Neurol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Tighilet, B.; Mourre, C.; Trottier, S.; Lacour, M. Histaminergic Ligands Improve Vestibular Compensation in the Cat: Behavioural, Neurochemical and Molecular Evidence. Eur. J. Pharmacol. 2007, 568, 149–163. [Google Scholar] [CrossRef]
- Tighilet, B.; Trottier, S.; Mourre, C.; Chotard, C.; Lacour, M. Betahistine Dihydrochloride Interaction with the Histaminergic System in the Cat: Neurochemical and Molecular Mechanisms. Eur. J. Pharmacol. 2002, 446, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Leonard, J.; Lacour, M. Betahistine Dihydrochloride Treatment Facilitates Vestibular Compensation in the Cat. J. Vestib. Res. 1995, 5, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Lacour, M. Histamine Immunoreactivity Changes in Vestibular-Lesioned and Histaminergic-Treated Cats. Eur. J. Pharmacol. 1997, 330, 65–77. [Google Scholar] [CrossRef]
- Roberts, F.; Calcutt, C.R. Histamine and the Hypothalamus. Neuroscience 1983, 9, 721–739. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Laurino, A.; Cinci, L.; Gencarelli, M.; Raimondi, L. Thyroid Hormone, Thyroid Hormone Metabolites and Mast Cells: A Less Explored Issue. Front. Cell Neurosci. 2019, 13, 79. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Agrawal, J.K.; Dubey, G.P. Effect of L-Thyroxine and Carbimazole on Blood Levels of Biogenic Amines in Rat. Exp. Clin. Endocrinol. Diabetes 1993, 101, 307–310. [Google Scholar] [CrossRef]
- Horii, A.; Uno, A.; Kitahara, T.; Mitani, K.; Masumura, C.; Kizawa, K.; Kubo, T. Effects of Fluvoxamine on Anxiety, Depression, and Subjective Handicaps of Chronic Dizziness Patients with or without Neuro-Otologic Diseases. J. Vestib. Res. 2007, 17, 1–8. [Google Scholar] [CrossRef]
- Horner, K.C.; Cazals, Y. Stress Hormones in Ménière’s Disease and Acoustic Neuroma. Brain Res. Bull. 2005, 66, 1–8. [Google Scholar] [CrossRef]
- Van Cruijsen, N.; Dullaart, R.P.F.; Wit, H.P.; Albers, F.W.J. Analysis of Cortisol and Other Stress-Related Hormones in Patients with Ménière’s Disease. Otol. Neurotol. 2005, 26, 1214–1219. [Google Scholar] [CrossRef]
- Hersey, M.; Reneaux, M.; Berger, S.N.; Mena, S.; Buchanan, A.M.; Ou, Y.; Tavakoli, N.; Reagan, L.P.; Clopath, C.; Hashemi, P. A Tale of Two Transmitters: Serotonin and Histamine as in Vivo Biomarkers of Chronic Stress in Mice. J. Neuroinflamm. 2022, 19, 167. [Google Scholar] [CrossRef]
- Puglisi-Allegra, S.; Andolina, D. Serotonin and Stress Coping. Behav. Brain Res. 2015, 277, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, R.; Kjær, J.N.; Østergaard, S.D.; Madsen, M.M. Thyroid Hormone Treatment in the Management of Treatment-Resistant Unipolar Depression: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2020, 141, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Heinz, A.; Whybrow, P.C. Thyroid Hormones, Serotonin and Mood: Of Synergy and Significance in the Adult Brain. Mol. Psychiatry 2002, 7, 140–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.H.; Ali, M.Y. The Relationships between Thyroid Hormones and the Brain Serotonin (5-HT) System and Mood: Of Synergy and Significance in the Adult Brain- A Review. Faridpur. Med. Coll. J. 2014, 9, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Straka, H.; Zwergal, A.; Cullen, K.E. Vestibular Animal Models: Contributions to Understanding Physiology and Disease. J. Neurol. 2016, 263 (Suppl. 1), S10–S23. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastoldo, G.; Tighilet, B. Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology. Int. J. Mol. Sci. 2023, 24, 9826. https://doi.org/10.3390/ijms24129826
Rastoldo G, Tighilet B. Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology. International Journal of Molecular Sciences. 2023; 24(12):9826. https://doi.org/10.3390/ijms24129826
Chicago/Turabian StyleRastoldo, Guillaume, and Brahim Tighilet. 2023. "Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology" International Journal of Molecular Sciences 24, no. 12: 9826. https://doi.org/10.3390/ijms24129826
APA StyleRastoldo, G., & Tighilet, B. (2023). Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology. International Journal of Molecular Sciences, 24(12), 9826. https://doi.org/10.3390/ijms24129826







