Acetylsalicylic Acid Supplementation Affects the Neurochemical Phenotyping of Porcine Duodenal Neurons
Abstract
1. Introduction
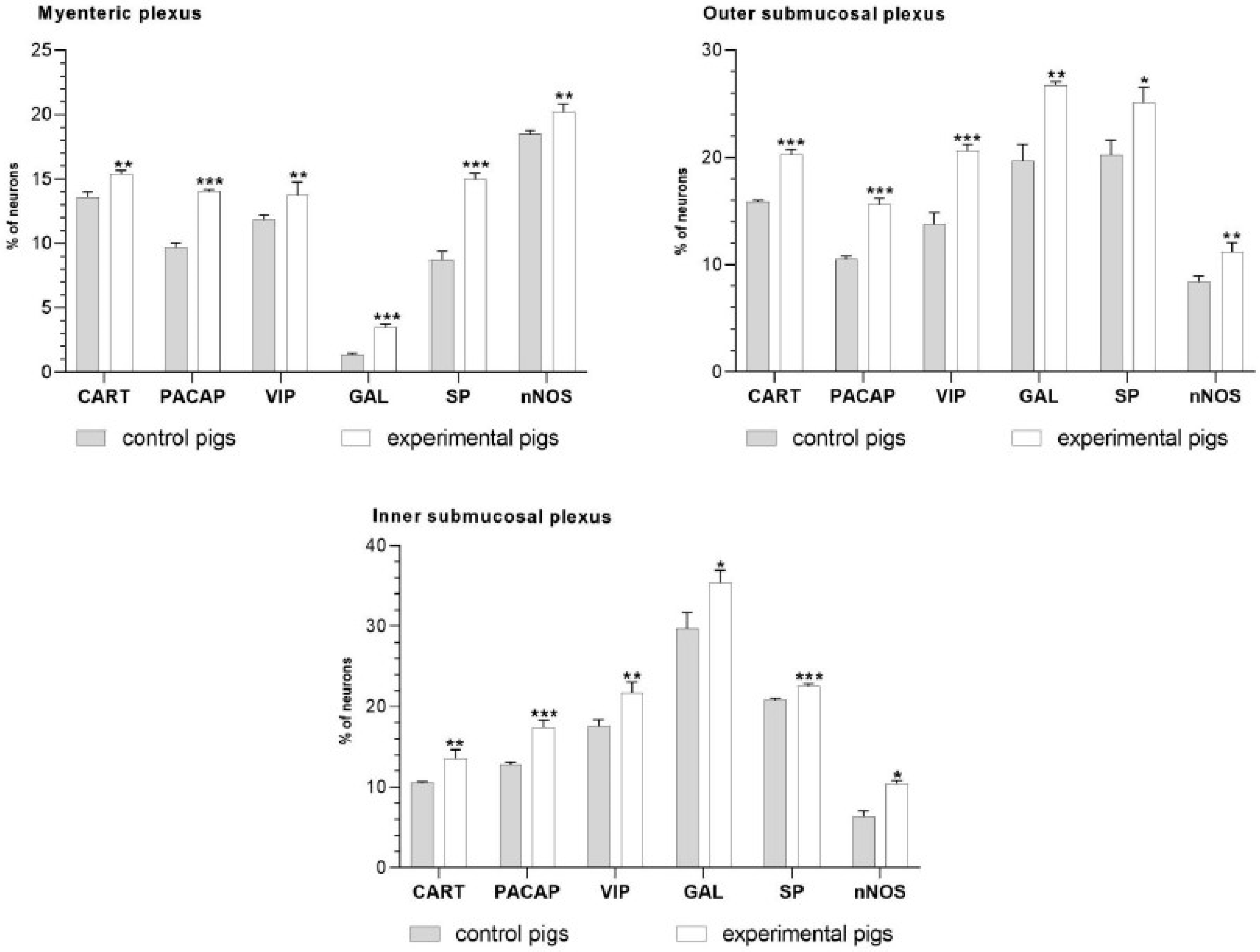
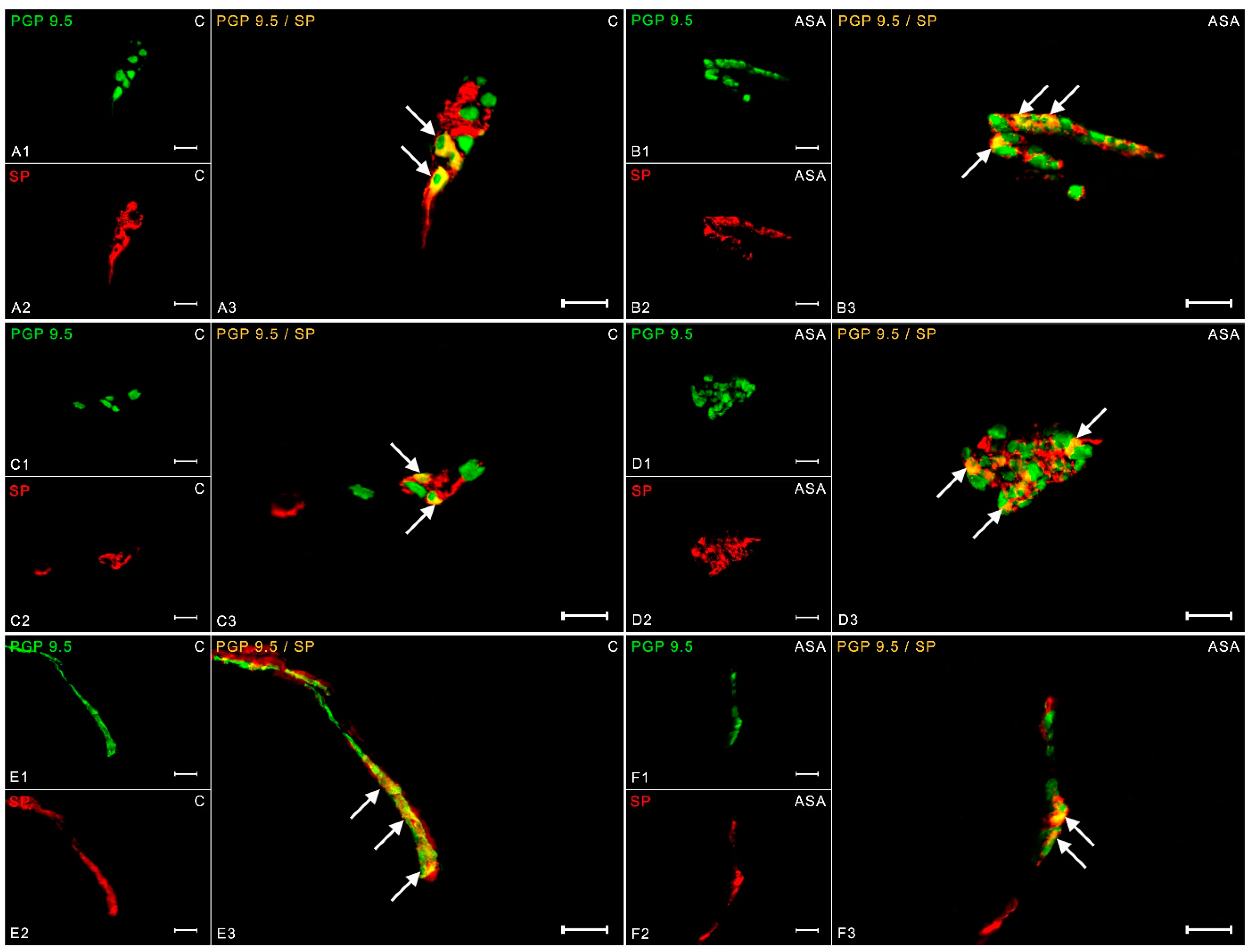
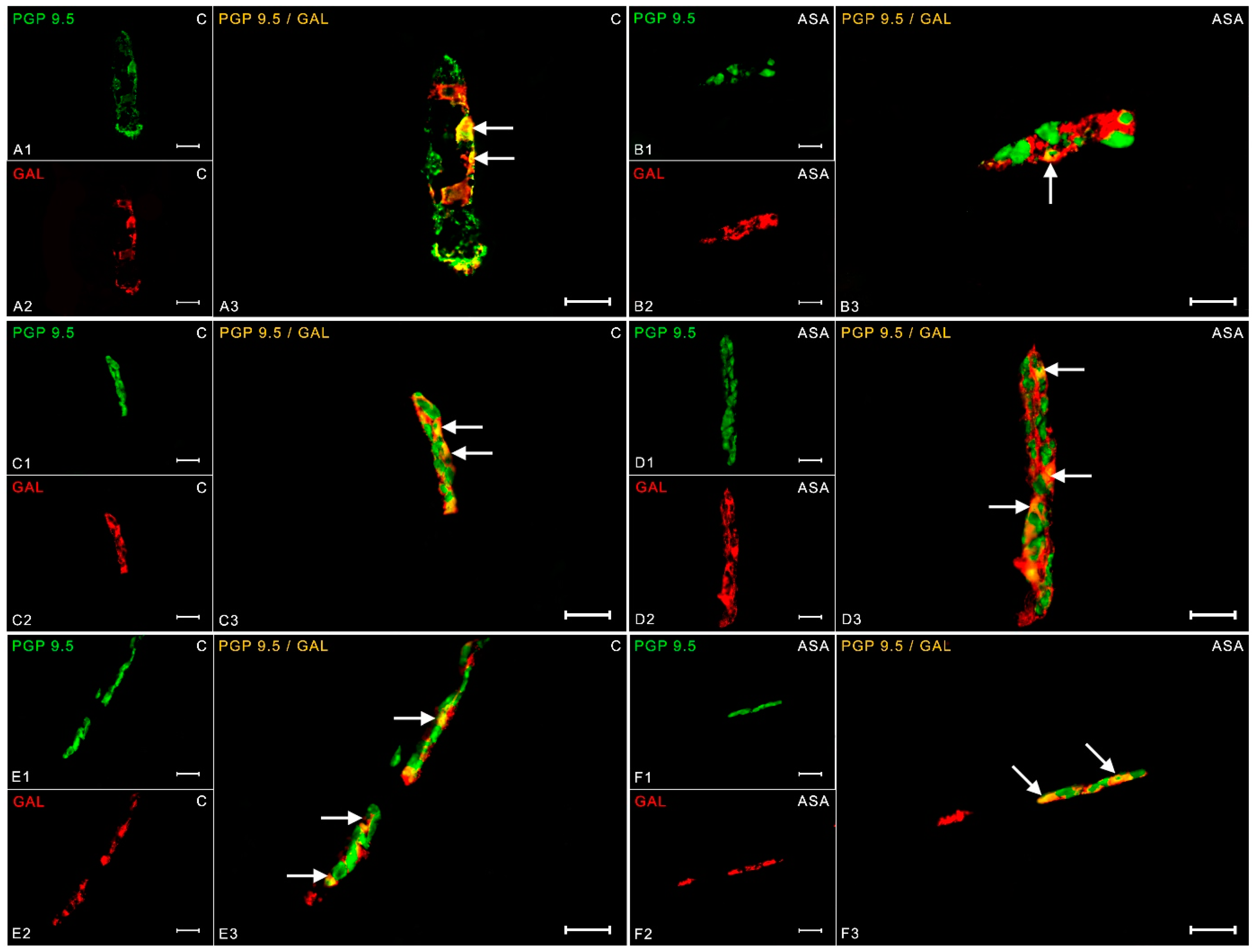
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, M.M.; Lichtenstein, D.R.; Singh, G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1999, 340, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001, 120, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Tacconelli, S.; Piazuelo, E.; Di Francesco, L.; Dovizio, M.; Sostres, C.; Marcantoni, E.; Guillem-Llobat, P.; Del Boccio, P.; Zucchelli, M.; et al. Reappraisal of the clinical pharmacology of low-dose aspirin by comparing novel direct and traditional indirect biomarkers of drug action. J. Thromb. Haemost. 2014, 12, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Shewmake, K.; Cryer, B. Time course inhibition of gastric and platelet COX activity by acetylsalicylic acid in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1113–G1120. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Rząp, D.; Czajkowska, M.; Całka, J. Neurochemical Plasticity of nNOS-, VIP- and CART-Immunoreactive Neurons Following Prolonged Acetylsalicylic Acid Supplementation in the Porcine Jejunum. Int. J. Mol. Sci. 2020, 21, 2157. [Google Scholar] [CrossRef]
- Bulc, M.; Całka, J.; Palus, K. Effect of Streptozotocin-Inducted Diabetes on the Pathophysiology of Enteric Neurons in the Small Intestine Based on the Porcine Diabetes Model. Int. J. Mol. Sci. 2020, 21, 2047. [Google Scholar] [CrossRef]
- Lomax, A.E.; Zhang, J.Y.; Furness, J.B. Origins of cholinergic inputs to the cell bodies of intestinofugal neurons in the guinea pig distal colon. J. Comp. Neurol. 2000, 416, 451–460. [Google Scholar] [CrossRef]
- Brzozowska, M.; Całka, J. Review: Occurrence and Distribution of Galanin in the Physiological and Inflammatory States in the Mammalian Gastrointestinal Tract. Front. Immunol. 2021, 11, 602070. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Barabasz, S.; Skobowiat, C.; Maksymowicz, W.; Majewski, M. Immunodetection of cocaine- and amphetamine-regulated transcript in the rumen, reticulum, omasum and abomasum of the sheep. Anat. Histol. Embryol. 2009, 38, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, M.; Jana, B.; Całka, J. Effect of NSAIDs Supplementation on the PACAP-, SP- and GAL-Immunoreactive Neurons in the Porcine Jejunum. Int. J. Mol. Sci. 2021, 22, 11689. [Google Scholar] [CrossRef] [PubMed]
- Aimi, Y.; Kimura, H.; Kinoshita, T.; Minami, Y.; Fujimura, M.; Vincent, S.R. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience 1993, 53, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Martinez, C.; Juarranz, M.G.; Arranz, A.; Leceta, J.; Delgado, M.; Gomariz, R.P. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology 2003, 124, 961–971. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hanazono, Y.; Kunita, S. Swine used in the medical university: Overview of 20 years of experience. Exp. Anim. 2018, 67, 7–13. [Google Scholar] [CrossRef]
- Konturek, P.C.; Konturek, S.J.; Hahn, E.G. Duodenal alkaline secretion: Its mechanisms and role in mucosal protection against gastric acid. Dig. Liver Dis. 2004, 36, 505–512. [Google Scholar] [CrossRef]
- Takeuchi, K.; Kita, K.; Hayashi, S.; Aihara, E. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol. Ther. 2011, 130, 59–70. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Pawlik, T.; Sliwowski, Z.; Ochmański, W.; Hahn, E.G. Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J. Physiol. Pharmacol. 2004, 55, 5–17. [Google Scholar]
- Lanas, A.; Hirschowitz, B.I. Toxicity of NSAIDs in the stomach and duodenum. Eur. J. Gastroenterol. Hepatol. 1999, 11, 375–381. [Google Scholar] [CrossRef]
- Czajkowska, M.; Całka, J. Neurochemistry of Enteric Neurons Following Prolonged Indomethacin Administration in the Porcine Duodenum. Front. Pharmacol. 2020, 11, 564457. [Google Scholar] [CrossRef]
- Czajkowska, M.; Rychlik, A.; Całka, J. Long-term treatment with naproxen changes the chemical coding of the porcine intramural duodenum neurons. Ann. Anat. 2020, 227, 151425. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Cryer, B. Epidemiology and role of nonsteroidal antiinflammatory drugs in causing gastrointestinal bleeding. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, C.E.; Dubois, R.N. Eicosanoids and the gastrointestinal tract. Gastroenterology 1995, 109, 285–301. [Google Scholar] [CrossRef]
- McConalogue, K.; Furness, J.B. Gastrointestinal neurotransmitters. Bailliere’s Clin. Endocrinol. Metab. 1994, 8, 51–76. [Google Scholar] [CrossRef]
- Sticlaru, L.; Stăniceanu, F.; Cioplea, M.; Nichita, L.; Bastian, A.; Micu, G.; Popp, C. Neuroimmune cross-talk in Helicobacter pylori infection: Analysis of substance P and vasoactive intestinal peptide expression in gastric enteric nervous system. J. Immunoass. Immunochem. 2018, 39, 660–671. [Google Scholar] [CrossRef]
- Voukali, E.; Shotton, H.R.; Lincoln, J. Selective responses of myenteric neurons to oxidative stress and diabetic stimuli. Neurogastroenterol. Motil. 2011, 23, 964-e411. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Dunay, I.R.; Schulze, S.; Fischer, A.; Grundmann, U.; Alutis, M.; Kühl, A.A.; Tamas, A.; Toth, G.; Dunay, M.P.; et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS ONE 2014, 9, e108389. [Google Scholar] [CrossRef]
- Satheeshkumar, P.S.; Mohan, M.P. Tachykinin peptide, substance P, and its receptor NK-1R play an important role in alimentary tract mucosal inflammation during cytotoxic therapy. Dig. Dis. Sci. 2014, 59, 2864–2873. [Google Scholar] [CrossRef]
- Goode, T.; O’Connell, J.; Anton, P.; Wong, H.; Reeve, J.; O’Sullivan, G.C.; Collins, J.K.; Shanahan, F. Neurokinin-1 receptor expression in inflammatory bowel disease: Molecular quantitation and localisation. Gut 2000, 47, 387–396. [Google Scholar] [CrossRef]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 2019, 31, e13580. [Google Scholar] [CrossRef]
- Renzi, D.; Evangelista, S.; Mantellini, P.; Surrenti, C. Decrease of duodenal calcitonin gene-related peptide- and substance P-like immunoreactivity in rat duodenal ulcers. Adv. Exp. Med. Biol. 1991, 298, 129–135. [Google Scholar]
- Schoen, R.T.; Vender, R.J. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Am. J. Med. 1989, 86, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Cryer, B. The role of cyclooxygenase selective inhibitors in the gastrointestinal tract. Curr. Gastroenterol. Rep. 2003, 5, 453–458. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Zhou, Y.; Dial, E.J.; Raphael, R.M. NSAID injury to the gastrointestinal tract: Evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J. Pharm. Pharmacol. 2008, 58, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Sánchez-Fidalgo, S.; Calvo, J.R.; Motilva, V. Chronic administration of galanin attenuates the TNBS-induced colitis in rats. Regul. Pept. 2007, 141, 96–104. [Google Scholar] [CrossRef]
- Talero, E.; Sánchez-Fidalgo, S.; Ramón Calvo, J.; Motilva, V. Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int. Immunopharmacol. 2006, 6, 1404–1412. [Google Scholar] [CrossRef]
- McDonald, A.C.; Schuijers, J.A.; Gundlach, A.L.; Grills, B.L. Galanin treatment offsets the inhibition of bone formation and downregulates the increase in mouse calvarial expression of TNFalpha and GalR2 mRNA induced by chronic daily injections of an injurious vehicle. Bone 2007, 40, 895–903. [Google Scholar] [CrossRef]
- Ekblad, E.; Kuhar, M.; Wierup, N.; Sundler, F. Cocaine- and amphetamine-regulated transcript: Distribution and function in rat gastrointestinal tract. Neurogastroenterol. Motil. 2003, 15, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.; Sigthorsson, G.; Simpson, R.J.; Watts, J.; Jacob, M.; Tavares, I.A.; Rafi, S.; Roseth, A.; Foster, R.; Price, A.B.; et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharmacol. Ther. 2000, 14, 639–650. [Google Scholar] [CrossRef]
- Magazine, H.I.; Liu, Y.; Bilfinger, T.V.; Fricchione, G.L.; Stefano, G.B. Morphine-induced conformational changes in human monocytes, granulocytes, and endothelial cells and in invertebrate immunocytes and microglia are mediated by nitric oxide. J. Immunol. 1996, 156, 4845–4850. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Sadowska-Krowicka, H.; Chotinaruemol, S.; Kakkis, J.L.; Clark, D.A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J. Pharmacol. Exp. Ther. 1993, 264, 11–16. [Google Scholar] [PubMed]
- Makowska, K.; Rytel, L.; Lech, P.; Osowski, A.; Kruminis-Kaszkiel, E.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in the enteric nervous system of the porcine esophagus. Comptes Rendus Biol. 2018, 341, 325–333. [Google Scholar] [CrossRef]
- Cornet, A.; Savidge, T.C.; Cabarrocas, J.; Deng, W.L.; Colombel, J.F.; Lassmann, H.; Desreumaux, P.; Liblau, R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. USA 2001, 98, 13306–13311. [Google Scholar] [CrossRef]
- Rühl, A.; Franzke, S.; Stremmel, W. IL-1beta and IL-10 have dual effects on enteric glial cell proliferation. Neurogastroenterol. Motil. 2001, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Aubé, A.C.; Cabarrocas, J.; Bauer, J.; Philippe, D.; Aubert, P.; Doulay, F.; Liblau, R.; Galmiche, J.P.; Neunlist, M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 2006, 55, 630–637. [Google Scholar] [CrossRef]




| Primary Antibodies | ||||
| Antigen | Species | Dilution | Code | Supplier |
| PGP 9.5 | Mouse | 1:1000 | 7863-2004 | BioRad, Hercules, CA, USA |
| VIP | Rabbit | 1:2000 | ab22736 | Abcam, UK |
| SP | Rabbit | 1:1000 | 8450-0004 | BioRad, Hercules, CA, USA |
| nNOS | Rabbit | 1:3000 | AB5380 | Chemicon, USA |
| GAL | Rabbit | 1:2000 | 4600-5004 | Biogenesis, UK |
| PACAP | Guinea Pig | 1:3000 | T-5039 | Peninsula, San Carlos, CA, USA |
| CART | Rabbit | 1:16,000 | H-003-61 | Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA |
| Secondary Antibodies | ||||
| Reagents | Dilution | Code | Supplier | |
| Alexa Fluor 488 donkey anti-mouse IgG | 1:1000 | A21202 | ThermoFisher Scientific, Waltham, MA, USA | |
| Alexa Fluor 546 donkey anti-rabbit IgG | 1:1000 | A11010 | ThermoFisher Scientific, Waltham, MA, USA | |
| Alexa Fluor 546 donkey anti-guinea pig IgG | 1:1000 | A11074 | ThermoFisher Scientific, Waltham, MA, USA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzozowska, M.; Całka, J. Acetylsalicylic Acid Supplementation Affects the Neurochemical Phenotyping of Porcine Duodenal Neurons. Int. J. Mol. Sci. 2023, 24, 9871. https://doi.org/10.3390/ijms24129871
Brzozowska M, Całka J. Acetylsalicylic Acid Supplementation Affects the Neurochemical Phenotyping of Porcine Duodenal Neurons. International Journal of Molecular Sciences. 2023; 24(12):9871. https://doi.org/10.3390/ijms24129871
Chicago/Turabian StyleBrzozowska, Marta, and Jarosław Całka. 2023. "Acetylsalicylic Acid Supplementation Affects the Neurochemical Phenotyping of Porcine Duodenal Neurons" International Journal of Molecular Sciences 24, no. 12: 9871. https://doi.org/10.3390/ijms24129871
APA StyleBrzozowska, M., & Całka, J. (2023). Acetylsalicylic Acid Supplementation Affects the Neurochemical Phenotyping of Porcine Duodenal Neurons. International Journal of Molecular Sciences, 24(12), 9871. https://doi.org/10.3390/ijms24129871






