IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells
Abstract
:1. Introduction
2. Results
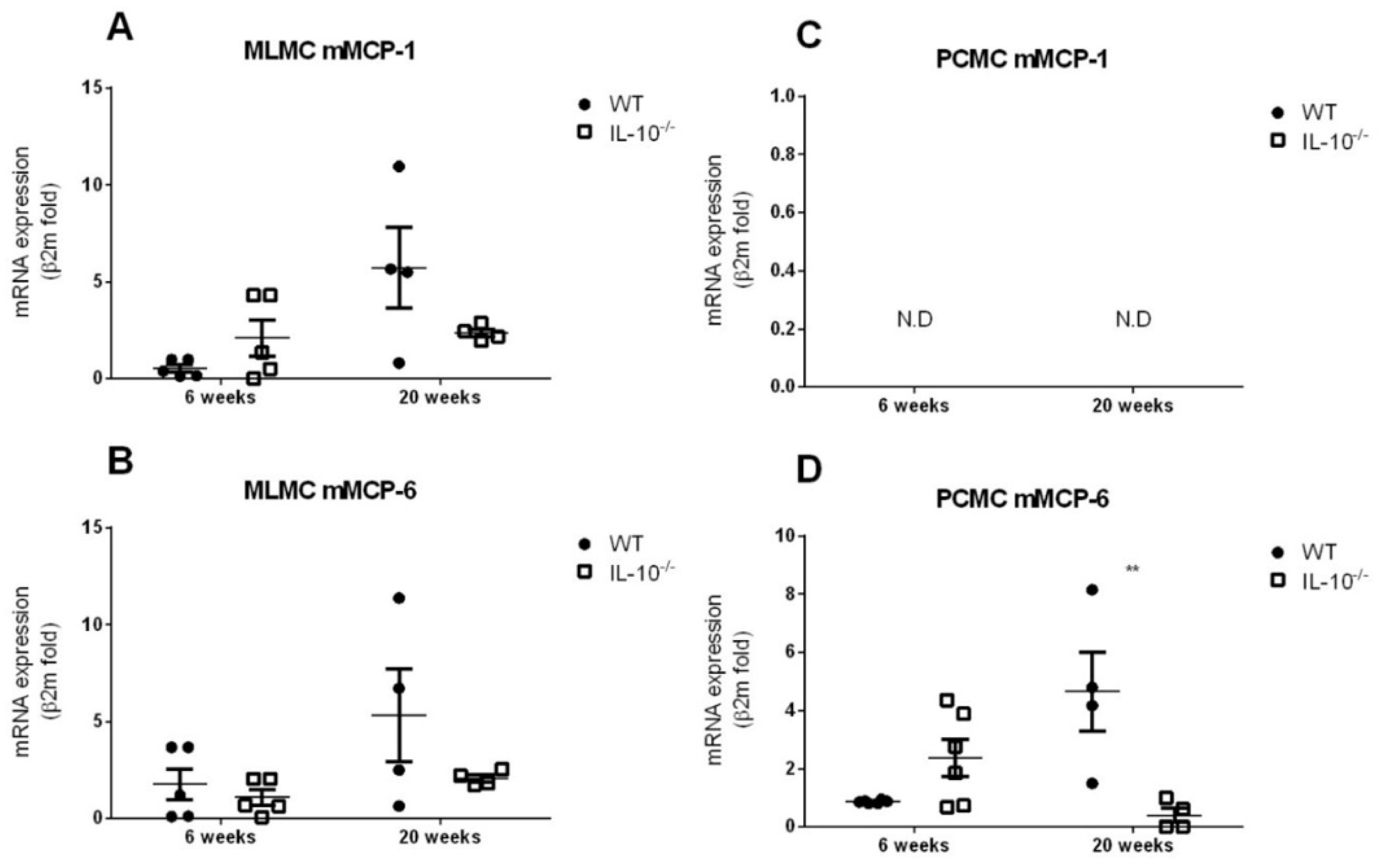
2.1. Morphological Analysis and mMCP-1 and mMCP-6 Characterization of MLMC and PCMC
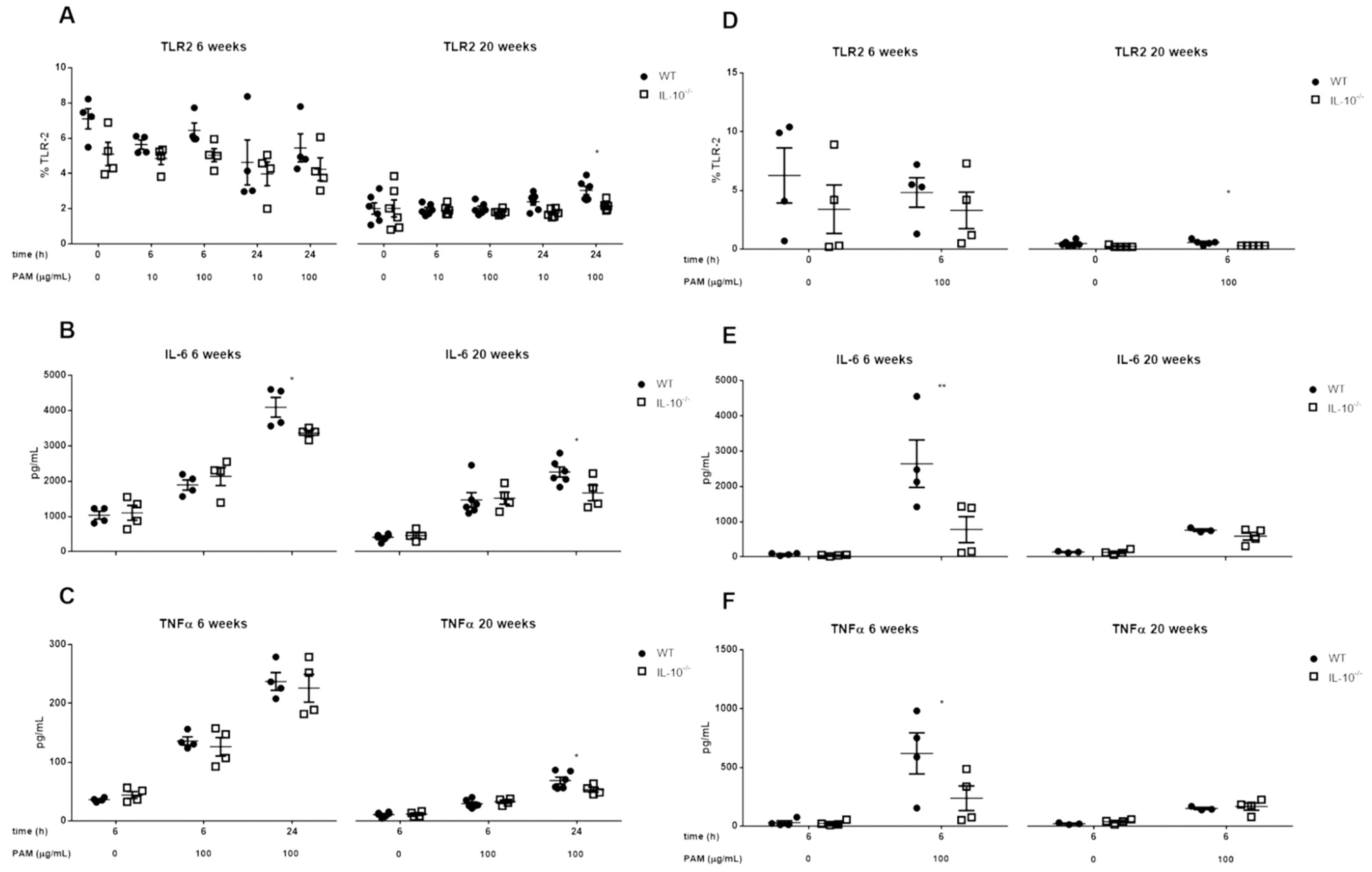
2.2. Effects of IL-10 Deletion on TLR2 Expression and Release of Inflammatory Mediators Related to Its Activation by Pam2CSK4 in MLMC and PCMC
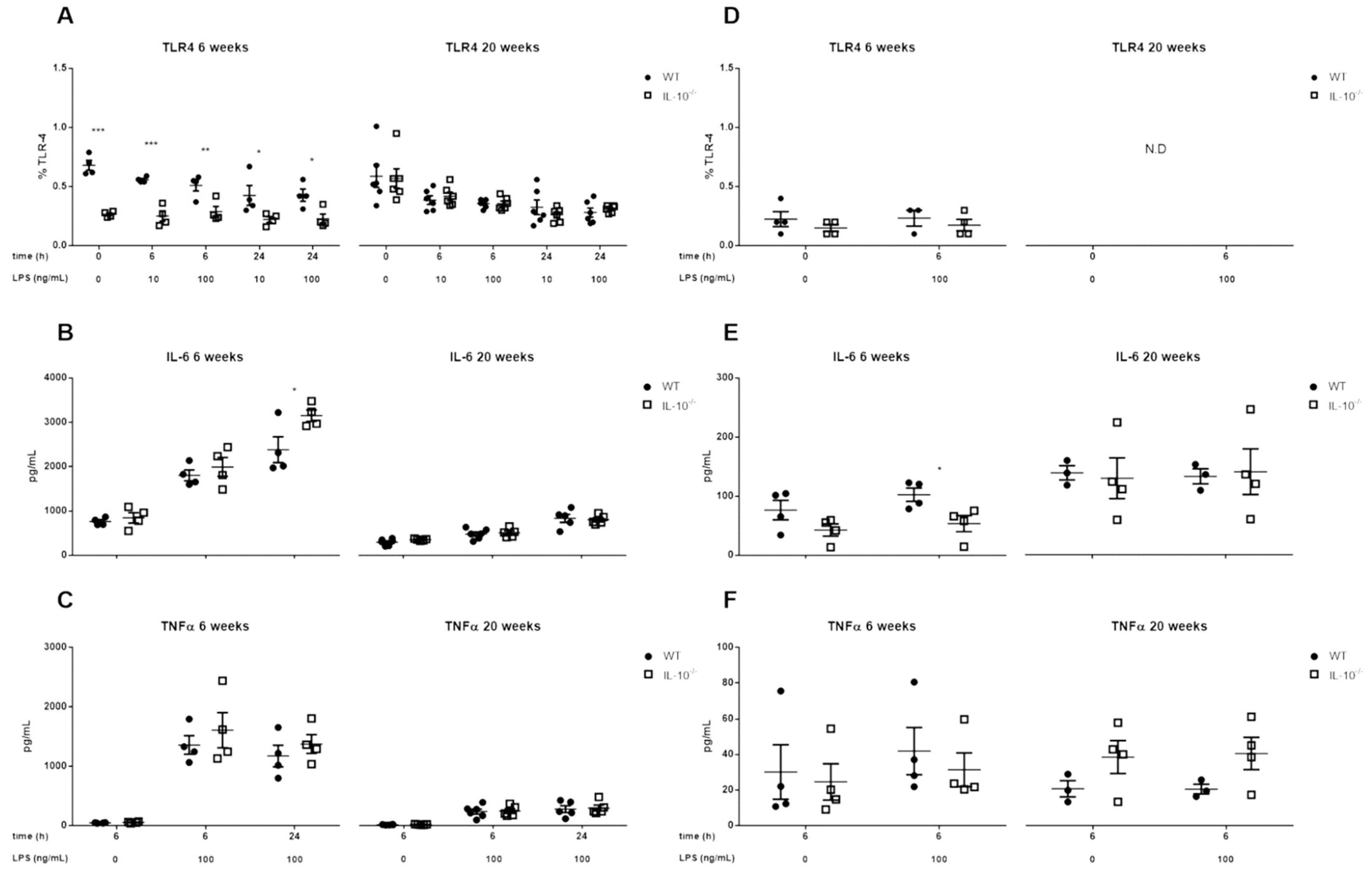
2.3. Effects of IL-10 Deletion on TLR4 Expression and Release of Inflammatory Mediators Related to Its Activation by LPS in MLMC and PCMC
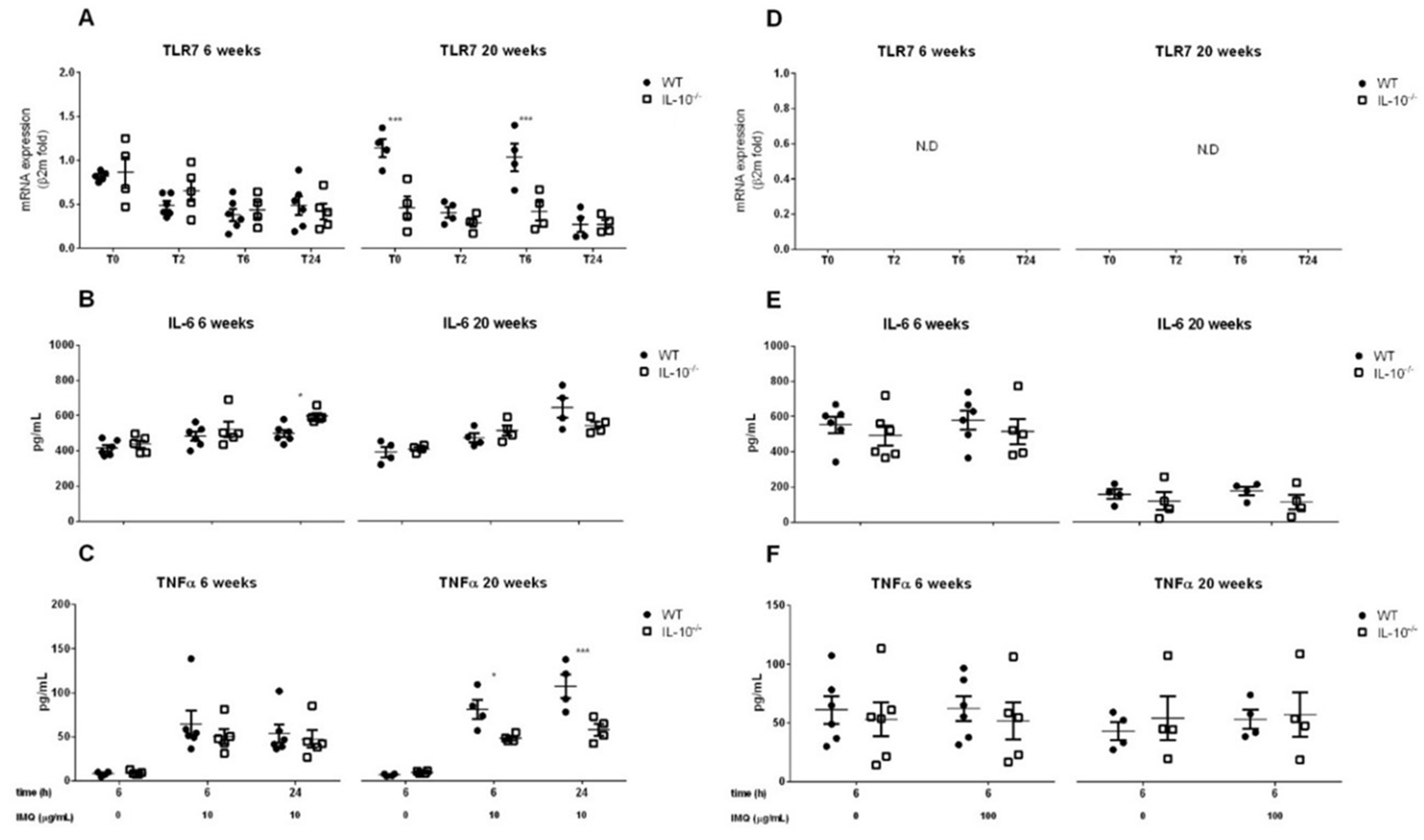
2.4. Effects of IL-10 Deletion on TLR7 Expression and Release of Inflammatory Mediators Related to Its Activation by Imiquimod in MLMC and PCMC
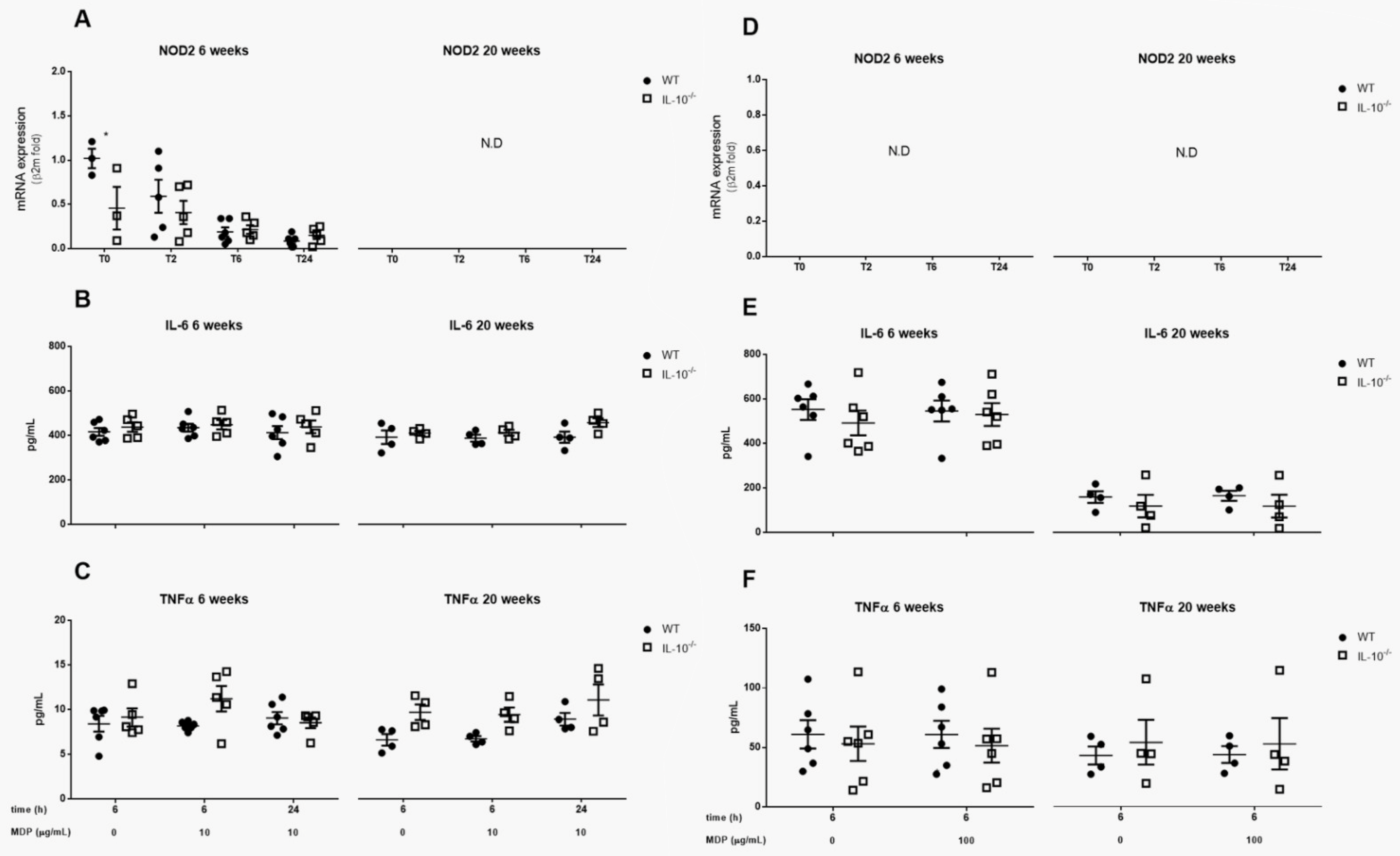
2.5. Effects of IL-10 Deletion on NOD2 Expression and Release of Inflammatory Mediators Related to Its Activation by MDP in MLMC and PCMC
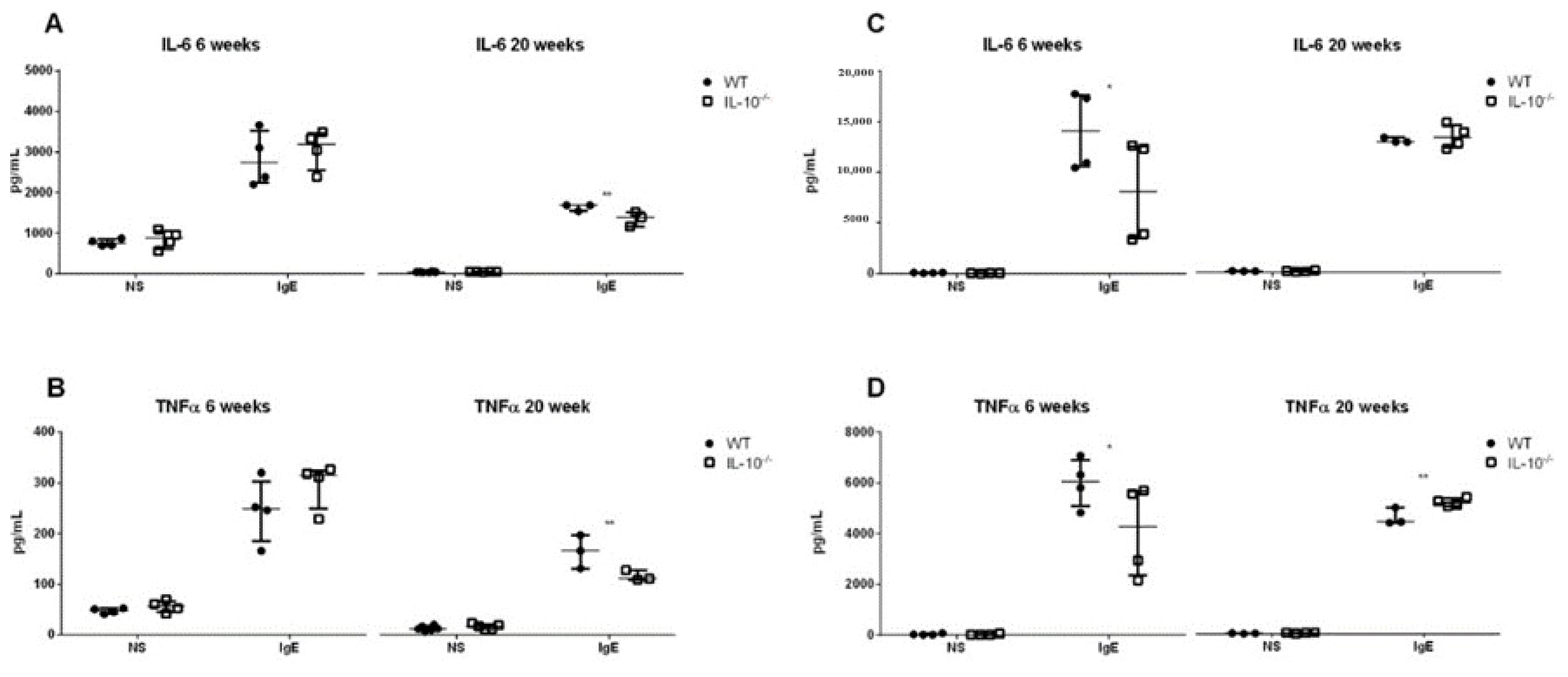
2.6. Effects of the Lack IL-10 on FcεRI-Mediated Responses in MLMC and PCMC
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Collection and Culture of Peritoneum-Derived Cultured Mast Cells (PCMC) and Mucosal-like Mast Cells (MLMC)
4.3. Cytospin and Staining of Mast Cell
4.4. In Vitro Cell Stimulation
4.5. RNA Extraction and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.6. Flow Cytometry
4.7. Quantification of Cytokines Concentration
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Gurish, M.F.; Tao, H.; Abonia, J.; Arya, A.; Friend, D.S.; Parker, C.M.; Austen, K.F. Intestinal Mast Cell Progenitors Require CD49dβ7 (α4β7 Integrin) for Tissue-specific Homing. J. Exp. Med. 2001, 194, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast Cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Galli, S.J. Mast Cells Can Amplify Airway Reactivity and Features of Chronic Inflammation in an Asthma Model in Mice. J. Exp. Med. 2000, 192, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, C.M.; Ullerås, E.; Nilsson, G. Differentiation of mast cell subpopulations from mouse embryonic stem cells. J. Immunol. Methods 2012, 382, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Boyce, J.A. Mast Cell Growth, Differentiation, and Death. Clin. Rev. Allergy Immunol. 2002, 22, 107–118. [Google Scholar] [CrossRef]
- Malbec, O.; Roget, K.; Schiffer, C.; Iannascoli, B.; Dumas, A.R.; Arock, M.; Daeron, M. Peritoneal Cell-Derived Mast Cells: An In Vitro Model of Mature Serosal-Type Mouse Mast Cells. J. Immunol. 2007, 178, 6465–6475. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Wershil, B.K. The two faces of the mast cell. Nature 1996, 381, 21–22. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental Origin and Functional Specialization of Mast Cell Subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Blank, U.; Falcone, F.; Nilsson, G. The history of mast cell and basophil research—Some lessons learnt from the last century. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 1093–1101. [Google Scholar] [CrossRef]
- Baran, J.; Sobiepanek, A.; Mazurkiewicz-Pisarek, A.; Rogalska, M.; Gryciuk, A.; Kuryk, L.; Abraham, S.N.; Staniszewska, M. Mast Cells as a Target—A Comprehensive Review of Recent Therapeutic Approaches. Cells 2023, 12, 1187. [Google Scholar] [CrossRef]
- Parente, R.; Giudice, V.; Cardamone, C.; Serio, B.; Selleri, C.; Triggiani, M. Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. Int. J. Mol. Sci. 2023, 24, 7071. [Google Scholar] [CrossRef]
- Chen, Y.; Griffiths, C.E.M.; Bulfone-Paus, S. Exploring Mast Cell–CD8 T Cell Interactions in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1564. [Google Scholar] [CrossRef]
- Woźniak, E.; Owczarczyk-Saczonek, A.; Lange, M.; Czarny, J.; Wygonowska, E.; Placek, W.; Nedoszytko, B. The Role of Mast Cells in the Induction and Maintenance of Inflammation in Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 7021. [Google Scholar] [CrossRef]
- Ozpinar, E.W.; Frey, M.A.L.; Cruse, G.; Freytes, D.O. Mast Cell–Biomaterial Interactions and Tissue Repair. Tissue Eng. Part B Rev. 2021, 27, 590–603. [Google Scholar] [CrossRef]
- Bramhall, M.; Zaph, C. Mastering gut permeability: New roles for old friends. Eur. J. Immunol. 2017, 47, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- Hochdörfer, T.; Kuhny, M.; Zorn, C.N.; Hendriks, R.W.; Vanhaesebroeck, B.; Bohnacker, T.; Krystal, G.; Huber, M. Activation of the PI3K pathway increases TLR-induced TNF-α and IL-6 but reduces IL-1β production in mast cells. Cell Signal. 2011, 23, 866–875. [Google Scholar] [CrossRef]
- Rönnberg, E.; Calounova, G.; Guss, B.; Lundequist, A.; Pejler, G. Granzyme D Is a Novel Murine Mast Cell Protease That Is Highly Induced by Multiple Pathways of Mast Cell Activation. Infect. Immun. 2013, 81, 2085–2094. [Google Scholar] [CrossRef] [Green Version]
- Savage, A.; Risquez, C.; Gomi, K.; Schreiner, R.; Borczuk, A.C.; Worgall, S.; Silver, R.B. The mast cell exosome-fibroblast connection: A novel pro-fibrotic pathway. Front. Med. 2023, 10, 1139397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, G. Mast Cell and Autoimmune Diseases. Mediat. Inflamm. 2015, 2015, 246126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; Leal-Berumen, I.; Nielsen, L.; Glibetić, M.; Jordana, M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J. Clin. Investig. 1996, 97, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Chichlowski, M.; Westwood, G.S.; Abraham, S.N.; Hale, L.P. Role of Mast Cells in Inflammatory Bowel Disease and Inflammation-Associated Colorectal Neoplasia in IL-10-Deficient Mice. PLoS ONE 2010, 5, e12220. [Google Scholar] [CrossRef]
- Li, Z.; Dong, S.; Huang, S.; Sun, Y.; Sun, Y.; Zhao, B.; Qi, Q.; Xiong, L.; Hong, F.; Jiang, Y. Role of CD34 in inflammatory bowel disease. Front. Physiol. 2023, 14, 1144980. [Google Scholar] [CrossRef]
- Nagalingam, N.A.; Robinson, C.J.; Bergin, I.L.; Eaton, K.A.; Huffnagle, G.B.; Young, V.B. The effects of intestinal microbial community structure on disease manifestation in IL-10-/- mice infected with Helicobacter hepaticus. Microbiome 2013, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; Vieira, P.; O’garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, 20190418. [Google Scholar] [CrossRef] [Green Version]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2008, 58, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glocker, E.-O.; Kotlarz, D.; Klein, C.; Shah, N.; Grimbacher, B. IL-10 and IL-10 receptor defects in humans. Ann. N. Y. Acad. Sci. 2011, 1246, 102–107. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schäffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory Bowel Disease and Mutations Affecting the Interleukin-10 Receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef] [Green Version]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A. A Multihit Model: Colitis Lessons from the Interleu-kin-10-Deficient Mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef]
- Ghildyal, N.; McNeil, H.P.; Stechschulte, S.; Austen, K.F.; Silberstein, D.; Gurish, M.F.; Somerville, L.L.; Stevens, R.L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J. Immunol. 1992, 149, 2123–2129. [Google Scholar] [CrossRef]
- Ghildyal, N.; McNeil, H.; Gurish, M.; Austen, K.; Stevens, R. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J. Biol. Chem. 1992, 267, 8473–8477. [Google Scholar] [CrossRef]
- Gillespie, S.R.; DeMartino, R.R.; Zhu, J.; Chong, H.J.; Ramirez, C.; Shelburne, C.P.; Bouton, L.A.; Bailey, D.P.; Gharse, A.; Mirmonsef, P.; et al. IL-10 Inhibits FcεRI Expression in Mouse Mast Cells. J. Immunol. 2004, 172, 3181–3188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xue, Y.; Wang, H.; Huang, Y.; Du, M.; Yang, Q.; Zhu, M.-J. Mast cell deficiency exacerbates inflammatory bowel symptoms in interleukin-10-deficient mice. World J. Gastroenterol. 2014, 20, 9106–9115. [Google Scholar]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef]
- Agier, J.; Różalska, S.; Wiktorska, M.; Żelechowska, P.; Pastwińska, J.; Brzezińska-Błaszczyk, E. The RLR/NLR expression and pro-inflammatory activity of tissue mast cells are regulated by cathelicidin LL-37 and defensin hBD-2. Sci. Rep. 2018, 8, 11750. [Google Scholar] [CrossRef] [Green Version]
- Agier, J.; Brzezińska-Błaszczyk, E.; Żelechowska, P.; Wiktorska, M.; Pietrzak, J.; Różalska, S.; Zelechowska, P.; Wiktorska, M.; Pietrzak, J.; Rózalska, S. Cathelicidin LL-37 Affects Surface and Intracellular Toll-like Receptor Expression in Tissue Mast Cells. J. Immunol. Res. 2018, 2018, 7357162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host Recognition of Bacterial Muramyl Dipeptide Mediated through NOD2. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.S.; McCurdy, J.D.; Olynych, T. Toll-Like Receptor-Mediated Activation of Mast Cells: Implications for Allergic Disease? Int. Arch. Allergy Immunol. 2003, 132, 87–97. [Google Scholar] [CrossRef]
- Mrabet-Dahbi, S.; Metz, M.; Dudeck, A.; Zuberbier, T.; Maurer, M. Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp. Dermatol. 2009, 18, 437–444. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Gong, F.; Xia, C.; Chen, J.; Song, Y.; Shen, A.; Song, J. Intracellular Staphylococcus aureus-induced NF-κB activation and proinflammatory responses of P815 cells are mediated by NOD2. J. Huazhong Univ. Sci. Technol. 2012, 32, 317–323. [Google Scholar] [CrossRef]
- Ikeda, T.; Funaba, M. Altered function of murine mast cells in response to lipopolysaccharide and peptidoglycan. Immunol. Lett. 2003, 88, 21–26. [Google Scholar] [CrossRef]
- Varadaradjalou, S.; Féger, F.; Thieblemont, N.; Ben Hamouda, N.; Pleau, J.-M.; Dy, M.; Arock, M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur. J. Immunol. 2003, 33, 899–906. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [Green Version]
- Supajatura, V.; Ushio, H.; Nakao, A.; Okumura, K.; Ra, C.; Ogawa, H. Protective Roles of Mast Cells Against Enterobacterial Infection Are Mediated by Toll-like Receptor 4. J. Immunol. 2001, 167, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; King, C.A.; McCurdy, J. Mast Cell Cytokine and Chemokine Responses to Bacterial and Viral Infection. Curr. Pharm. Des. 2003, 9, 11–24. [Google Scholar] [CrossRef]
- Okumura, S.; Yuki, K.; Kobayashi, R.; Okamura, S.; Ohmori, K.; Saito, H.; Ra, C.; Okayama, Y. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: Preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin. Immunol. 2009, 130, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Mekori, Y.A.; Metcalfe, D.D. Mast cell–T cell interactions. J. Allergy Clin. Immunol. 1999, 104, 517–523. [Google Scholar] [CrossRef]
- Kalesnikoff, J.; Galli, S.J. New developments in mast cell biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Frossi, B.; Gri, G.; Tripodo, C.; Pucillo, C. Exploring a regulatory role for mast cells: ‘MCregs’? Trends Immunol. 2010, 31, 97–102. [Google Scholar] [CrossRef]
- Yu, C.; Cantor, A.B.; Yang, H.; Browne, C.; Wells, R.A.; Fujiwara, Y.; Orkin, S.H. Targeted Deletion of a High-Affinity GATA-binding Site in the GATA-1 Promoter Leads to Selective Loss of the Eosinophil Lineage In Vivo. J. Exp. Med. 2002, 195, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Meurer, S.K.; Neß, M.; Weiskirchen, S.; Kim, P.; Tag, C.G.; Kauffmann, M.; Huber, M.; Weiskirchen, R. Isolation of Mature (Peritoneum-Derived) Mast Cells and Immature (Bone Marrow-Derived) Mast Cell Precursors from Mice. PLoS ONE 2016, 11, e0158104. [Google Scholar] [CrossRef] [Green Version]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Kasakura, K.; Nagata, K.; Miura, R.; Iida, M.; Nakaya, H.; Okada, H.; Arai, T.; Kawakami, Y.; Kawakami, T.; Yashiro, T.; et al. Cooperative Regulation of the Mucosal Mast Cell–Specific Protease Genes Mcpt1 and Mcpt2 by GATA and Smad Transcription Factors. J. Immunol. 2020, 204, 1641–1649. [Google Scholar] [CrossRef]
- Wright, S.H.; Brown, J.; Knight, P.A.; Thornton, E.M.; Kilshaw, P.J.; Miller, H.R.P. Transforming growth factor-bβ1 mediates coexpression of the integrin subunit aαE and the chymase mouse mast cell protease-1 during the early differentiation of bone marrow-derived mucosal mast cell homologues. Clin. Exp. Allergy 2002, 32, 315–324. [Google Scholar] [CrossRef]
- Fernández-Blanco, J.A.; Estévez, J.; Shea-Donohue, T.; Martínez, V.; Vergara, P. Changes in Epithelial Barrier Function in Response to Parasitic Infection: Implications for IBD Pathogenesis. J. Crohn’s Colitis 2015, 9, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Austen, K.F.; Gurish, M.F.; Jones, T.G. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 14210–14215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Kambe, N.; Saito, M.; Nishikomori, R.; Kim, Y.-G.; Murakami, M.; Núñez, G.; Matsue, H. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J. Exp. Med. 2009, 206, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandig, H.; Bulfone-Paus, S. TLR signaling in mast cells: Common and unique features. Front. Immunol. 2012, 3, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidl, I.D.; McAlpine, S.M.; Marshall, J.S. Enhancement of Mast Cell IL-6 Production by Combined Toll-like and Nucleotide-Binding Oligomerization Domain-Like Receptor Activation. Int. Arch. Allergy Immunol. 2010, 154, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, M.; Rockwell, J.; Engleman, C.; Gewirtz, A.; Katz, J.; Sambhara, S. Cutting Edge: Impaired Toll-like Receptor Expression and Function in Aging. J. Immunol. 2002, 169, 4697–4701. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function: A New Vision of an Old Cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Polukort, S.H.; Rovatti, J.; Carlson, L.; Thompson, C.; Ser-Dolansky, J.; Kinney, S.R.M.; Schneider, S.S.; Mathias, C.B. IL-10 Enhances IgE-Mediated Mast Cell Responses and Is Essential for the Development of Experimental Food Allergy in IL-10–Deficient Mice. J. Immunol. 2016, 196, 4865–4876. [Google Scholar] [CrossRef] [Green Version]
- McCurdy, J.D.; Lin, T.-J.; Marshall, J.S. Toll-like receptor 4-mediated activation of murine mast cells. J. Leukoc. Biol. 2001, 70, 977–984. [Google Scholar] [CrossRef]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef] [Green Version]
- Bijjiga, E.; Martino, A.T. Interleukin 10 (IL-10) Regulatory Cytokine and its Clinical Consequences. J. Clin. Cell Immunol. 2011, 1–6. [Google Scholar] [CrossRef]
- Cyktor, J.C.; Turner, J. Interleukin-10 and Immunity against Prokaryotic and Eukaryotic Intracellular Pathogens. Infect. Immun. 2011, 79, 2964–2973. [Google Scholar] [CrossRef] [Green Version]
- Paul, G.; Khare, V.; Gasche, C. Inflamed gut mucosa: Downstream of interleukin-10. Eur. J. Clin. Investig. 2011, 42, 95–109. [Google Scholar] [CrossRef]
- Lang, R.; Patel, D.; Morris, J.J.; Rutschman, R.L.; Murray, P.J. Shaping Gene Expression in Activated and Resting Primary Macrophages by IL-10. J. Immunol. 2002, 169, 2253–2263. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, A.P.; Takahashi, Y.; Miranda-Saavedra, D. Genomic analysis of LPS-stimulated myeloid cells identifies a common pro-inflammatory response but divergent IL-10 anti-inflammatory responses. Sci. Rep. 2015, 5, srep09100. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, A.P.; Diez, D.; Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief. Funct. Genom. 2013, 12, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riquelme-Neira, R.; Walker-Vergara, R.; Fernández-Blanco, J.A.; Vergara, P. IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells. Int. J. Mol. Sci. 2023, 24, 9875. https://doi.org/10.3390/ijms24129875
Riquelme-Neira R, Walker-Vergara R, Fernández-Blanco JA, Vergara P. IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells. International Journal of Molecular Sciences. 2023; 24(12):9875. https://doi.org/10.3390/ijms24129875
Chicago/Turabian StyleRiquelme-Neira, Roberto, Romina Walker-Vergara, Joan Antoni Fernández-Blanco, and Patrocinio Vergara. 2023. "IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells" International Journal of Molecular Sciences 24, no. 12: 9875. https://doi.org/10.3390/ijms24129875
APA StyleRiquelme-Neira, R., Walker-Vergara, R., Fernández-Blanco, J. A., & Vergara, P. (2023). IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells. International Journal of Molecular Sciences, 24(12), 9875. https://doi.org/10.3390/ijms24129875





