New Insights into Immunopathology Associated to Bothrops lanceolatus Snake Envenomation: Focus on PLA2 Toxin
Abstract
:1. Introduction
2. Results
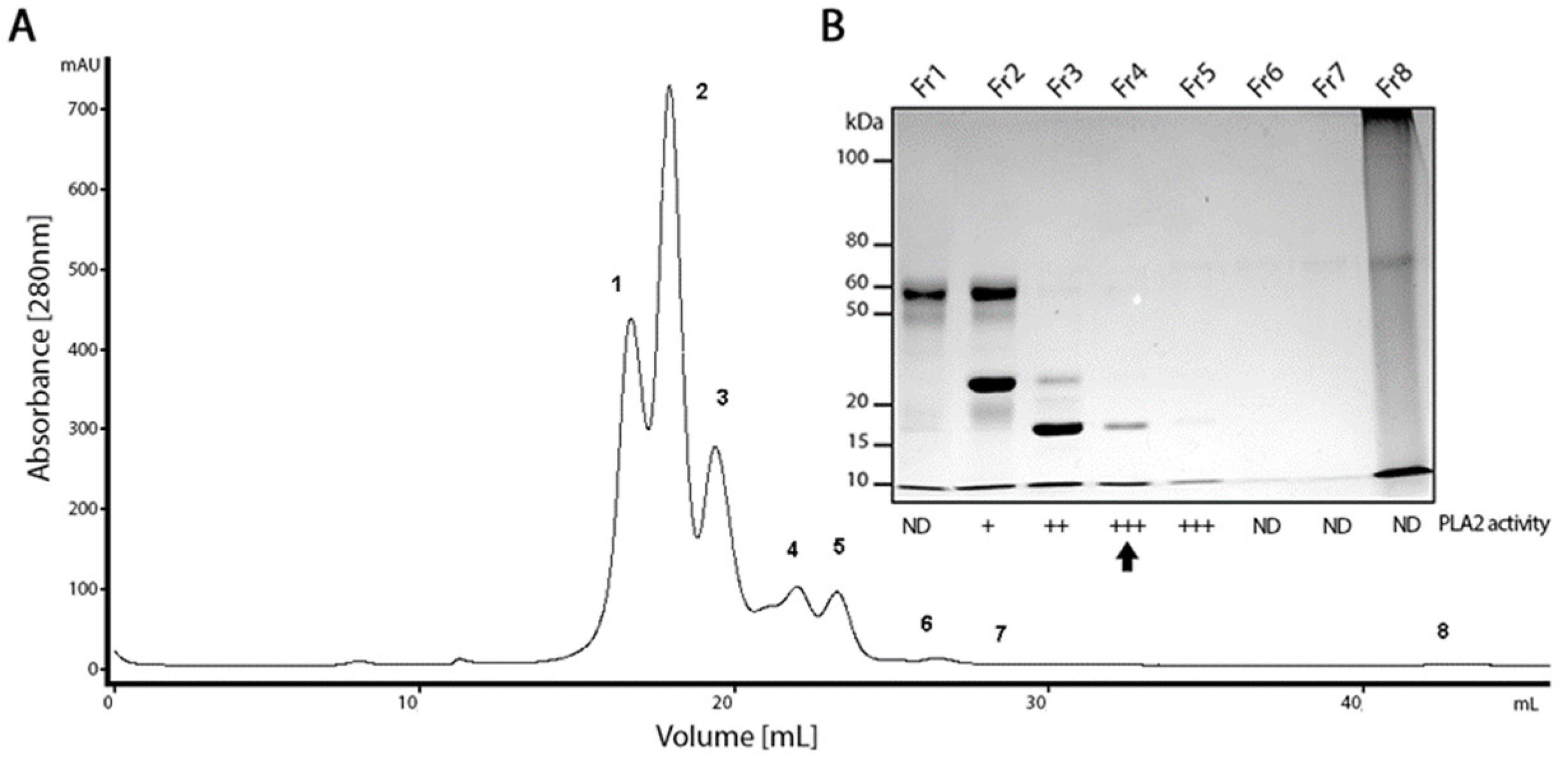
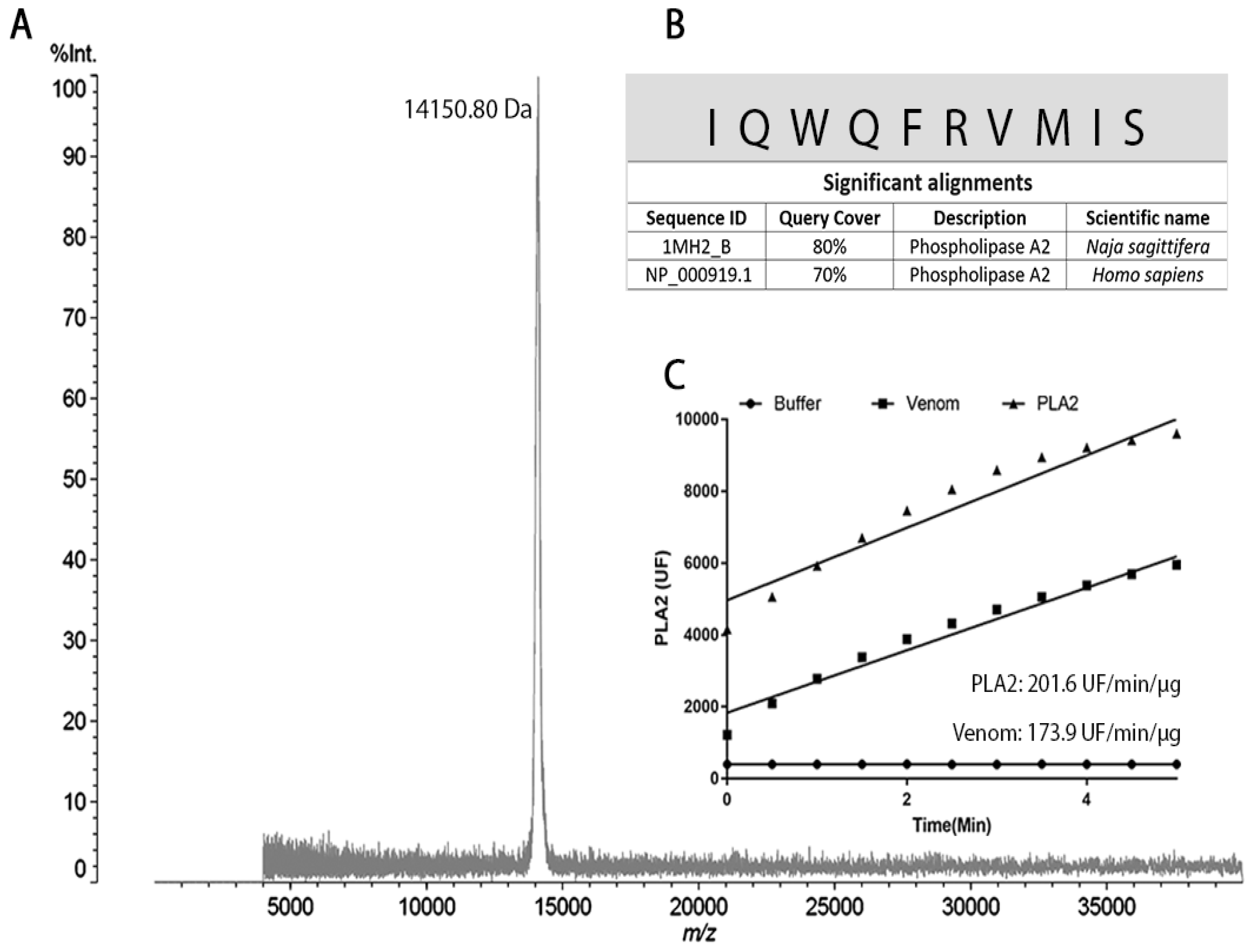
2.1. PLA2 Isolation from the B. lanceolatus Venom
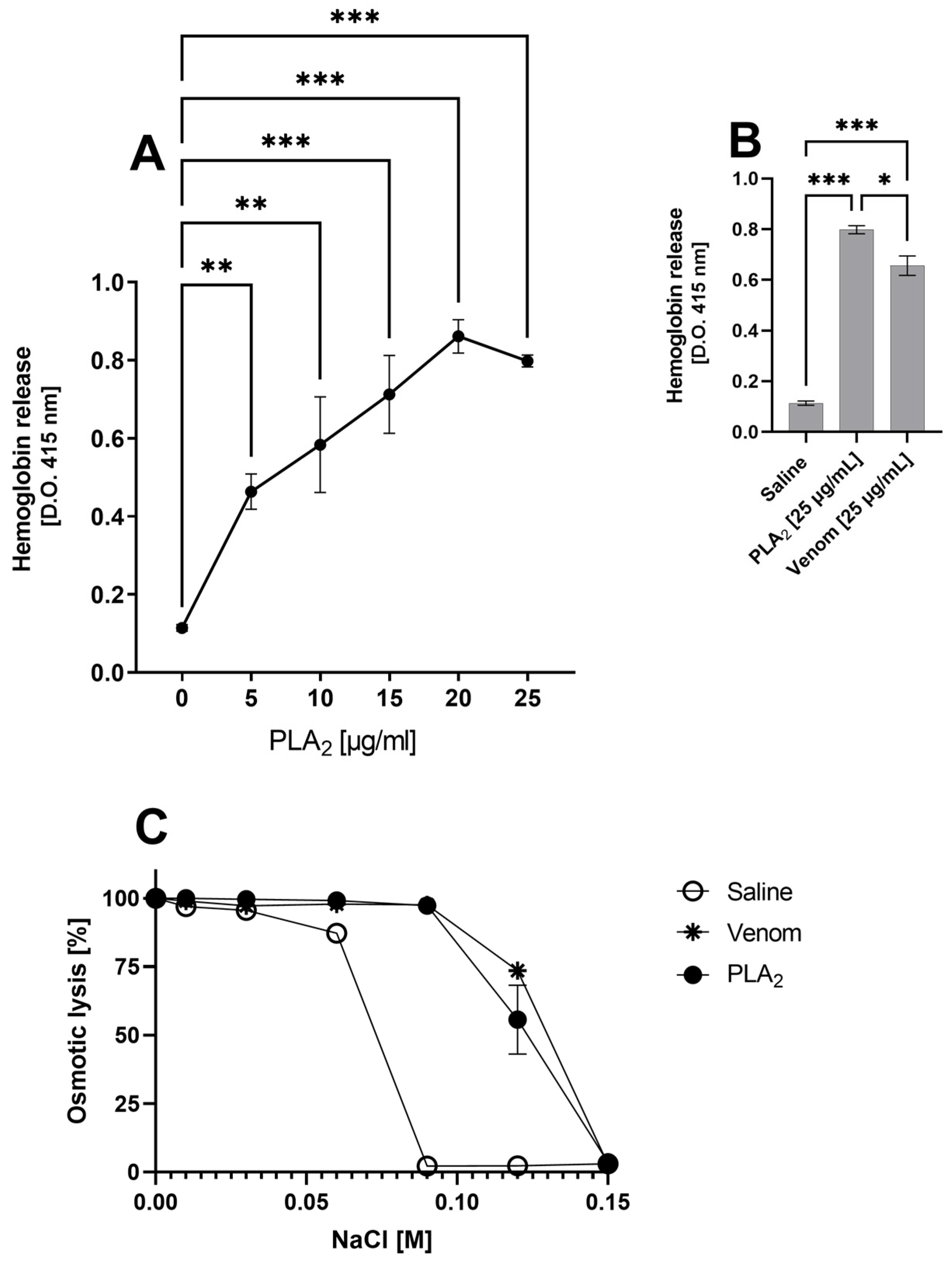
2.2. B. lanceolatus Venom PLA2 and Hemoglobin Release
2.3. B. lanceolatus Venom PLA2 Activates the Complement System in the Ex Vivo Human Whole Blood Model
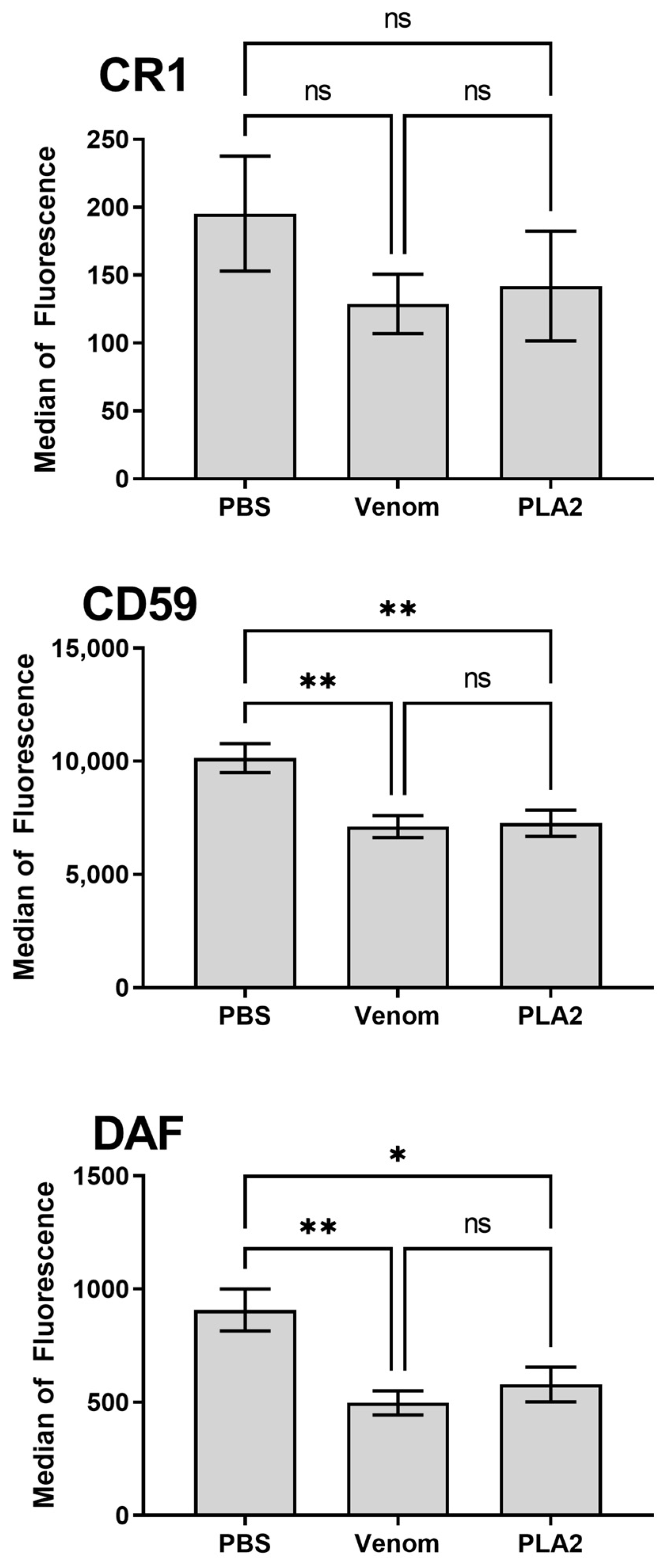
2.4. B. lanceolatus Venom PLA2 Induces Modulation of Erythrocyte Membrane Complement Regulators
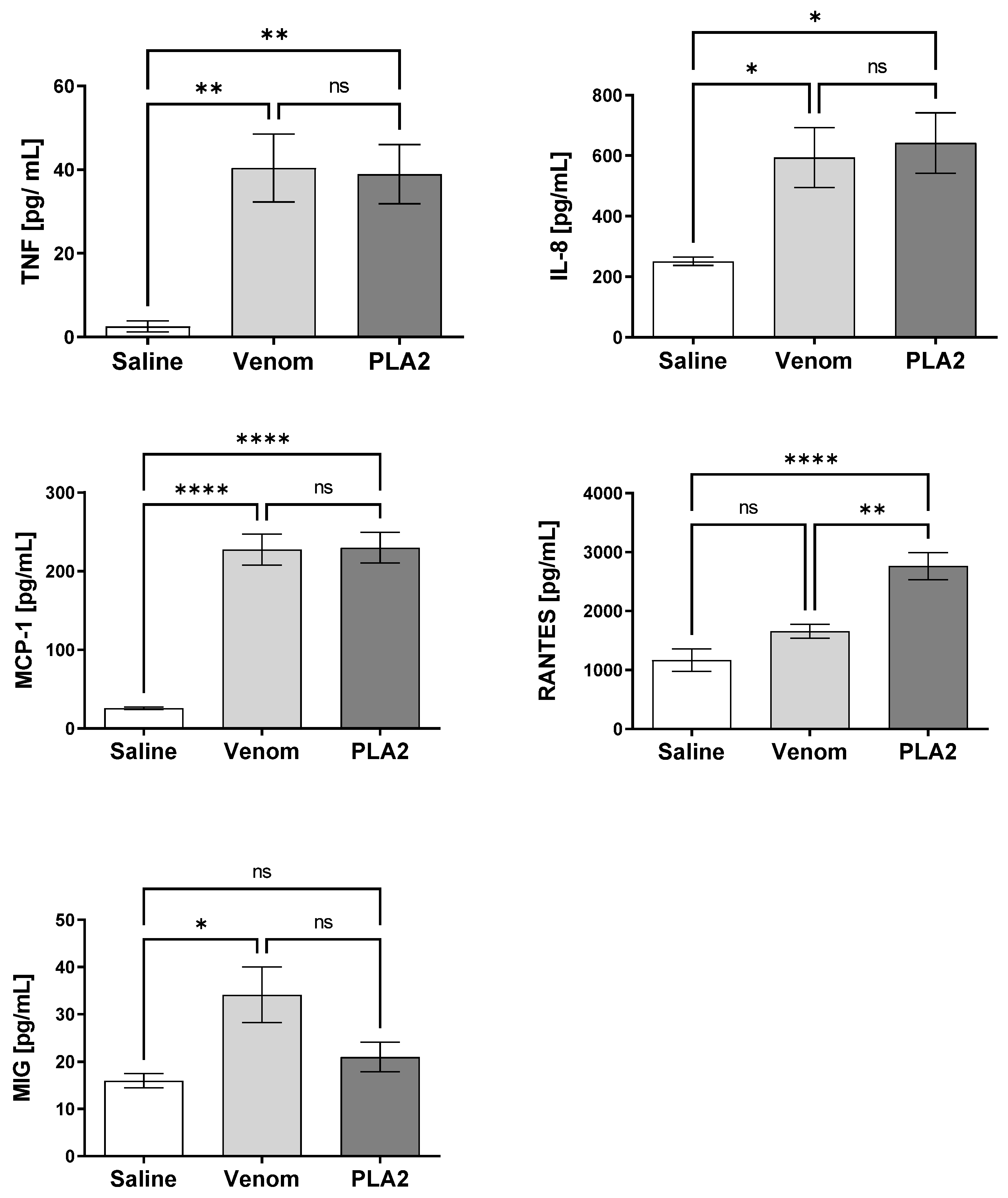
2.5. B. lanceolatus Venom PLA2 Induces Pro-Inflammatory Cytokines and Chemokines in Human Whole Blood
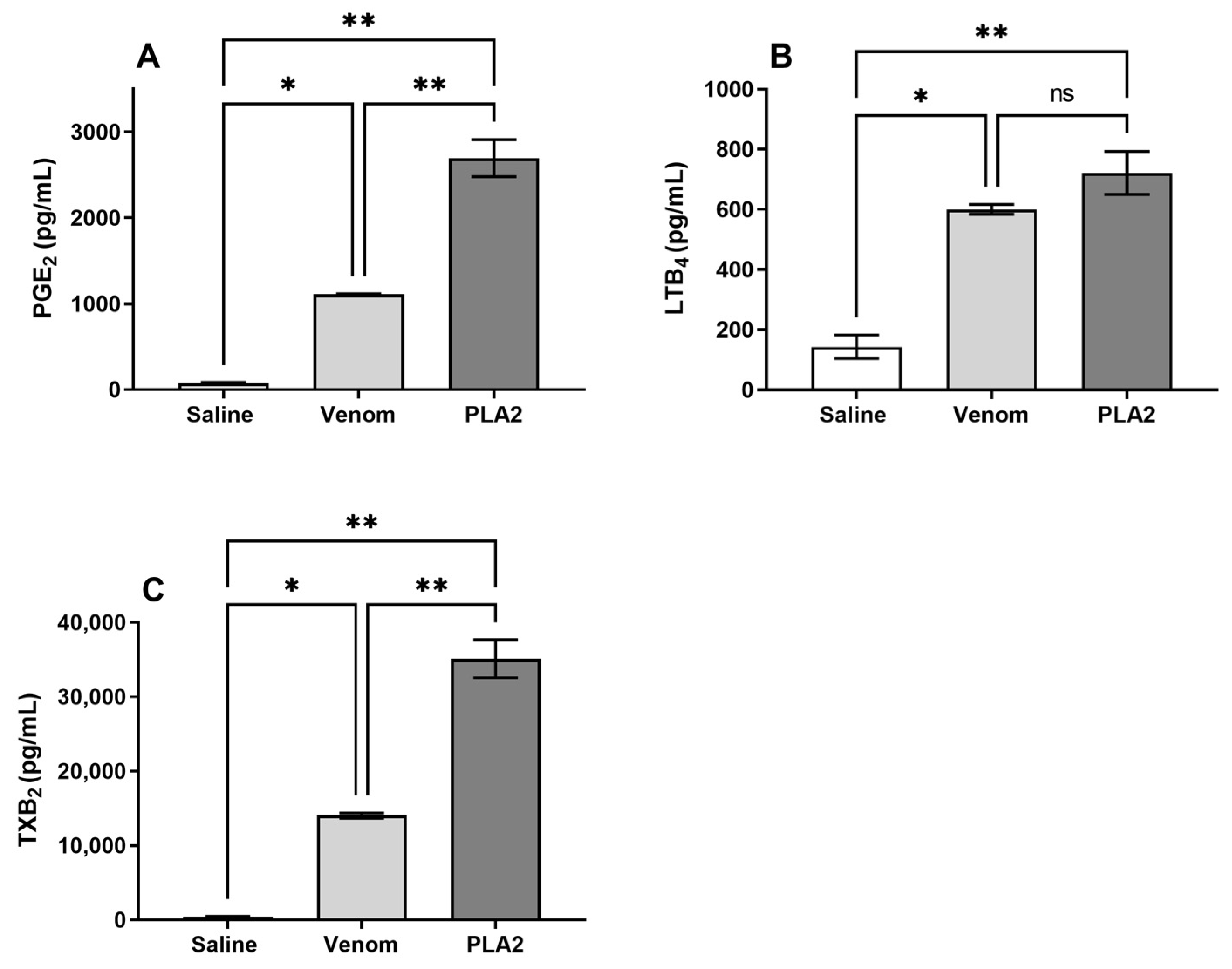
2.6. B. lanceolatus Venom Induces the Release of Lipid Mediators
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Venom
4.3. PLA2 Purification
4.4. MALDI-TOF MS
4.5. N-Terminal Sequencing
4.6. Phospholipase A2 Activity
4.7. Hemolysis Assay
4.8. Human Whole Blood Model
4.9. Osmotic Susceptibility
4.10. Detection of Anaphylatoxins and Soluble Terminal Complement Complex (sTCC) in Venom/Toxin Treated Samples
4.11. Analysis of Complement Regulators on Erythrocytes by Flow Cytometry
4.12. Dosage of Cytokines and Chemokines in Human Whole Blood
4.13. Quantification of Lipid Mediators
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resiere, D.; Megarbane, B.; Valentino, R.; Mehdaoui, H.; Thomas, L. Bothrops lanceolatus bites: Guidelines for severity assessment and emergent management. Toxins 2010, 2, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, J.L.C. Animais peçonhentos no Brasil: Biologia, clínica e terapêutica dos acidentes. In Animais Peçonhentos no Brasil: Biologia, Clínica e Terapêutica dos Acidentes; FAPESP: Savier, Brazil, 2009; p. 540. [Google Scholar]
- Resiere, D.; Hossein, M.; Megarbane, B. Snake Bites by Bothrops lanceolatus in Martinique. Med. Sante Trop. 2018, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Tyburn, B.; Bucher, B.; Pecout, F.; Ketterle, J.; Rieux, D.; Smadja, D.; Garnier, D.; Plumelle, Y. Prevention of thromboses in human patients with Bothrops lanceolatus envenoming in Martinique: Failure of anticoagulants and efficacy of a monospecific antivenom. Research Group on Snake Bites in Martinique. Am. J. Trop. Med. Hyg. 1995, 52, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Severyns, M.; Neviere, R.; Resiere, D.; Andriamananaivo, T.; Decaestecker, L.; Mehdaoui, H.; Odri, G.A.; Rouvillain, J.L. Case Report: Bothrops lanceolatus Snakebite Surgical Management-Relevance of Fasciotomy. Am. J. Trop. Med. Hyg. 2018, 99, 1350–1353. [Google Scholar] [CrossRef] [Green Version]
- Malbranque, S.; Piercecchi-Marti, M.D.; Thomas, L.; Barbey, C.; Courcier, D.; Bucher, B.; Ridarch, A.; Smadja, D.; Warrell, D.A. Fatal diffuse thrombotic microangiopathy after a bite by the “Fer-de-Lance” pit viper (Bothrops lanceolatus) of Martinique. Am. J. Trop. Med. Hyg. 2008, 78, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Resiere, D.; Kallel, H.; Florentin, J.; Houcke, S.; Mehdaoui, H.; Gutierrez, J.M.; Neviere, R. Bothrops (Fer-de-lance) snakebites in the French departments of the Americas (Martinique and Guyana): Clinical and experimental studies and treatment by immunotherapy. PLoS Negl. Trop. Dis. 2023, 17, e0011083. [Google Scholar] [CrossRef]
- Estrade, G.; Garnier, D.; Bernasconi, F.; Donatien, Y. Embolie pulmonaire et coagulation intravasculaire dissémine après une morsure de serpent Bothrops lanceolatus. Arch. Mal. Cœur. Vaiss Pratique 1989, 82, 1903–1905. [Google Scholar]
- Bucher, B.; Canonge, D.; Thomas, L.; Tyburn, B.; Robbe-Vincent, A.; Choumet, V.; Bon, C.; Ketterle, J.; Lang, J. Clinical indicators of envenoming and serum levels of venom antigens in patients bitten by Bothrops lanceolatus in Martinique. Research Group on Snake Bites in Martinique. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 186–190. [Google Scholar] [CrossRef]
- Thomas, L.; Chausson, N.; Uzan, J.; Kaidomar, S.; Vignes, R.; Plumelle, Y.; Bucher, B.; Smadja, D. Thrombotic stroke following snake bites by the “Fer-de-Lance” Bothrops lanceolatus in Martinique despite antivenom treatment: A report of three recent cases. Toxicon 2006, 48, 23–28. [Google Scholar] [CrossRef]
- Larreche, S.; Chippaux, J.P.; Chevillard, L.; Mathe, S.; Resiere, D.; Siguret, V.; Megarbane, B. Bleeding and Thrombosis: Insights into Pathophysiology of Bothrops Venom-Related Hemostasis Disorders. Int. J. Mol. Sci. 2021, 22, 9643. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Sanz, L.; Escolano, J.; Fernandez, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake venomics of the Lesser Antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus: Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef] [PubMed]
- Delafontaine, M.; Villas-Boas, I.M.; Pidde, G.; van den Berg, C.W.; Mathieu, L.; Blomet, J.; Tambourgi, D.V. Venom from Bothrops lanceolatus, a Snake Species Native to Martinique, Potently Activates the Complement System. J. Immunol. Res. 2018, 2018, 3462136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva de Franca, F.; Gabrili, J.J.M.; Mathieu, L.; Burgher, F.; Blomet, J.; Tambourgi, D.V. Bothrops lanceolatus snake (Fer-de-lance) venom triggers inflammatory mediators’ storm in human blood. Arch. Toxicol. 2021, 95, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.Q.; Cruz-Hofling, M.A.; Ferreira de Araujo, P.M.; Bon, C.; Lobo de Araujo, A. Pharmacological and histopathological characterization of Bothrops lanceolatus (Fer de lance) venom-induced edema. Inflamm. Res. 2004, 53, 284–291. [Google Scholar] [CrossRef]
- Shina, R.; Yates, S.L.; Ghassemi, A.; Rosenberg, P.; Condrea, E. Inhibitory effect of EDTA.Ca2+ on the hydrolysis of synaptosomal phospholipids by phospholipase A2 toxins and enzymes. Biochem. Pharmacol. 1990, 40, 2233–2239. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [Green Version]
- Pirolla, R.A.; Baldasso, P.A.; Marangoni, S.; Moran, P.J.; Rodrigues, J.A.R. Evaluation of snake venom phospholipase A2: Hydrolysis of non-natural esters. J. Braz. Chem. Soc. 2011, 22, 300–307. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A(2) catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 766–771. [Google Scholar]
- Braud, S.; Bon, C.; Wisner, A. Snake venom proteins acting on hemostasis. Biochimie 2000, 82, 851–859. [Google Scholar] [CrossRef]
- Chacur, M.; Milligan, E.D.; Sloan, E.M.; Wieseler-Frank, J.; Barrientos, R.M.; Martin, D.; Poole, S.; Lomonte, B.; Gutierrez, J.M.; Maier, S.F.; et al. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: Involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain 2004, 108, 180–191. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Diaz, C.; Angulo, Y.; Gutierrez, J.M.; Lomonte, B. Role of enzymatic activity in muscle damage and cytotoxicity induced by Bothrops asper Asp49 phospholipase A2 myotoxins: Are there additional effector mechanisms involved? PeerJ 2014, 2, e569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoni, M.; Schiavo, G.; Weston, A.E.; Caccin, P.; Allegrini, F.; Pennuto, M.; Valtorta, F.; Montecucco, C.; Rossetto, O. Snake presynaptic neurotoxins with phospholipase A2 activity induce punctate swellings of neurites and exocytosis of synaptic vesicles. J. Cell Sci. 2004, 117, 3561–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.K.; Gowda, T.V. Isolation and characterization of a lethal phospholipase A2 (NN-IVb1-PLA2) from the Indian cobra (Naja naja naja) venom. Biochem. Int. 1991, 25, 1023–1034. [Google Scholar] [PubMed]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Pidde-Queiroz, G.; Pessoa, L.A.; Portaro, F.C.; Furtado Mde, F.; Tambourgi, D.V. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon 2008, 52, 842–851. [Google Scholar] [CrossRef]
- Delafontaine, M.; Villas-Boas, I.M.; Mathieu, L.; Josset, P.; Blomet, J.; Tambourgi, D.V. Enzymatic and Pro-Inflammatory Activities of Bothrops lanceolatus Venom: Relevance for Envenomation. Toxins 2017, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory Effects of Bothrops Phospholipases A(2): Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins 2021, 13, 868. [Google Scholar] [CrossRef]
- Giannotti, K.C.; Weinert, S.; Viana, M.N.; Leiguez, E.; Araujo, T.L.S.; Laurindo, F.R.M.; Lomonte, B.; Braun-Dullaeus, R.; Teixeira, C. A Secreted Phospholipase A(2) Induces Formation of Smooth Muscle Foam Cells Which Transdifferentiate to Macrophage-Like State. Molecules 2019, 24, 3244. [Google Scholar] [CrossRef] [Green Version]
- Leiguez, E.; Motta, P.; Maia Marques, R.; Lomonte, B.; Sampaio, S.V.; Teixeira, C. A Representative GIIA Phospholipase A(2) Activates Preadipocytes to Produce Inflammatory Mediators Implicated in Obesity Development. Biomolecules 2020, 10, 1593. [Google Scholar] [CrossRef]
- Martins, L.J.; de Araújo, P.M.F.; Bon, C.; Hyslop, S.; de Araújo, A.L. In vitro hemolytic activity of Bothrops lanceolatus (fer-de-lance) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 498–508. [Google Scholar] [CrossRef]
- Warrell, D. Snakebites in Central and South America: Epidemiology, clinical features, and clinical management. In The Venomous Reptiles of the Western Hemisphere; Campbell, J., Lamar, W., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; Volume 2, pp. 709–761. [Google Scholar]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pao, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Silva de Franca, F.; Villas-Boas, I.M.; Cogliati, B.; Woodruff, T.M.; Reis, E.D.S.; Lambris, J.D.; Tambourgi, D.V. C5a-C5aR1 Axis Activation Drives Envenomation Immunopathology by the Snake Naja annulifera. Front. Immunol. 2021, 12, 652242. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.; Yan, J. The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy. Front. Immunol. 2019, 10, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimat, M.; Tabarin, F.; Dimitrov, J.D.; Poitou, C.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 2013, 122, 282–292. [Google Scholar] [CrossRef]
- Yamamoto, C.; Tsuru, D.; Oda-Ueda, N.; Ohno, M.; Hattori, S.; Kim, S.T. Trimeresurus flavoviridis (habu snake) venom induces human erythrocyte lysis through enzymatic lipolysis, complement activation and decreased membrane expression of CD55 and CD59. Pharmacol. Toxicol. 2001, 89, 188–194. [Google Scholar] [CrossRef]
- Yamamoto, C.; Tsuru, D.; Oda-Ueda, N.; Ohno, M.; Hattori, S.; Kim, S.T. Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726-Ser727 of human C3 and acts as a novel, heterologous C3 convertase. Immunology 2002, 107, 111–117. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; Morgan, B.P.; de Andrade, R.M.; Magnoli, F.C.; van Den Berg, C.W. Loxosceles intermedia spider envenomation induces activation of an endogenous metalloproteinase, resulting in cleavage of glycophorins from the erythrocyte surface and facilitating complement-mediated lysis. Blood 2000, 95, 683–691. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Fang, C.J.; Atkinson, J.P. Inhibiting complement activation on cells at the step of C3 cleavage. Vaccine 2008, 26, I22–I27. [Google Scholar] [CrossRef] [Green Version]
- Liszewski, M.K.; Kolev, M.; Le Friec, G.; Leung, M.; Bertram, P.G.; Fara, A.F.; Subias, M.; Pickering, M.C.; Drouet, C.; Meri, S.; et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 2013, 39, 1143–1157. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, A.; Fauquert, J.L.; Thomas, C.; Kemper, C.; Drouet, C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol. Immunol. 2014, 8, 98–107. [Google Scholar] [CrossRef]
- Arbore, G.; West, E.E.; Rahman, J.; Le Friec, G.; Niyonzima, N.; Pirooznia, M.; Tunc, I.; Pavlidis, P.; Powell, N.; Li, Y.; et al. Complement receptor CD46 co-stimulates optimal human CD8+ T cell effector function via fatty acid metabolism. Nat. Commun. 2018, 9, 4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Acosta, A.; Uzcategui, W.; Azuaje, R.; Giron, M.E.; Aguilar, I. ELISA assays for the detection of Bothrops lanceolatus venom in envenomed patient plasmas. Roum. Arch. Microbiol. Immunol. 1998, 57, 271–278. [Google Scholar] [PubMed]
- Pawluczkowycz, A.W.; Lindorfer, M.A.; Waitumbi, J.N.; Taylor, R.P. Hematin promotes complement alternative pathway-mediated deposition of C3 activation fragments on human erythrocytes: Potential implications for the pathogenesis of anemia in malaria. J. Immunol. 2007, 179, 5543–5552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Morrissey, J.H. Silver stain for proteins in polyacrylamide gels: A modified procedure with enhanced uniform sensitivity. Anal. Biochem. 1981, 117, 307–310. [Google Scholar] [CrossRef]
- Mollnes, T.E.; Brekke, O.L.; Fung, M.; Fure, H.; Christiansen, D.; Bergseth, G.; Videm, V.; Lappegard, K.T.; Kohl, J.; Lambris, J.D. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002, 100, 1869–1877. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrili, J.J.M.; Pidde, G.; Magnoli, F.C.; Marques-Porto, R.; Villas-Boas, I.M.; Squaiella-Baptistão, C.C.; Silva-de-França, F.; Burgher, F.; Blomet, J.; Tambourgi, D.V. New Insights into Immunopathology Associated to Bothrops lanceolatus Snake Envenomation: Focus on PLA2 Toxin. Int. J. Mol. Sci. 2023, 24, 9931. https://doi.org/10.3390/ijms24129931
Gabrili JJM, Pidde G, Magnoli FC, Marques-Porto R, Villas-Boas IM, Squaiella-Baptistão CC, Silva-de-França F, Burgher F, Blomet J, Tambourgi DV. New Insights into Immunopathology Associated to Bothrops lanceolatus Snake Envenomation: Focus on PLA2 Toxin. International Journal of Molecular Sciences. 2023; 24(12):9931. https://doi.org/10.3390/ijms24129931
Chicago/Turabian StyleGabrili, Joel J. M., Giselle Pidde, Fabio Carlos Magnoli, Rafael Marques-Porto, Isadora Maria Villas-Boas, Carla Cristina Squaiella-Baptistão, Felipe Silva-de-França, François Burgher, Joël Blomet, and Denise V. Tambourgi. 2023. "New Insights into Immunopathology Associated to Bothrops lanceolatus Snake Envenomation: Focus on PLA2 Toxin" International Journal of Molecular Sciences 24, no. 12: 9931. https://doi.org/10.3390/ijms24129931




