In Vitro Safety Assessment of In-House Synthesized Titanium Dioxide Nanoparticles: Impact of Washing and Temperature Conditions
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of TiO2 NPs
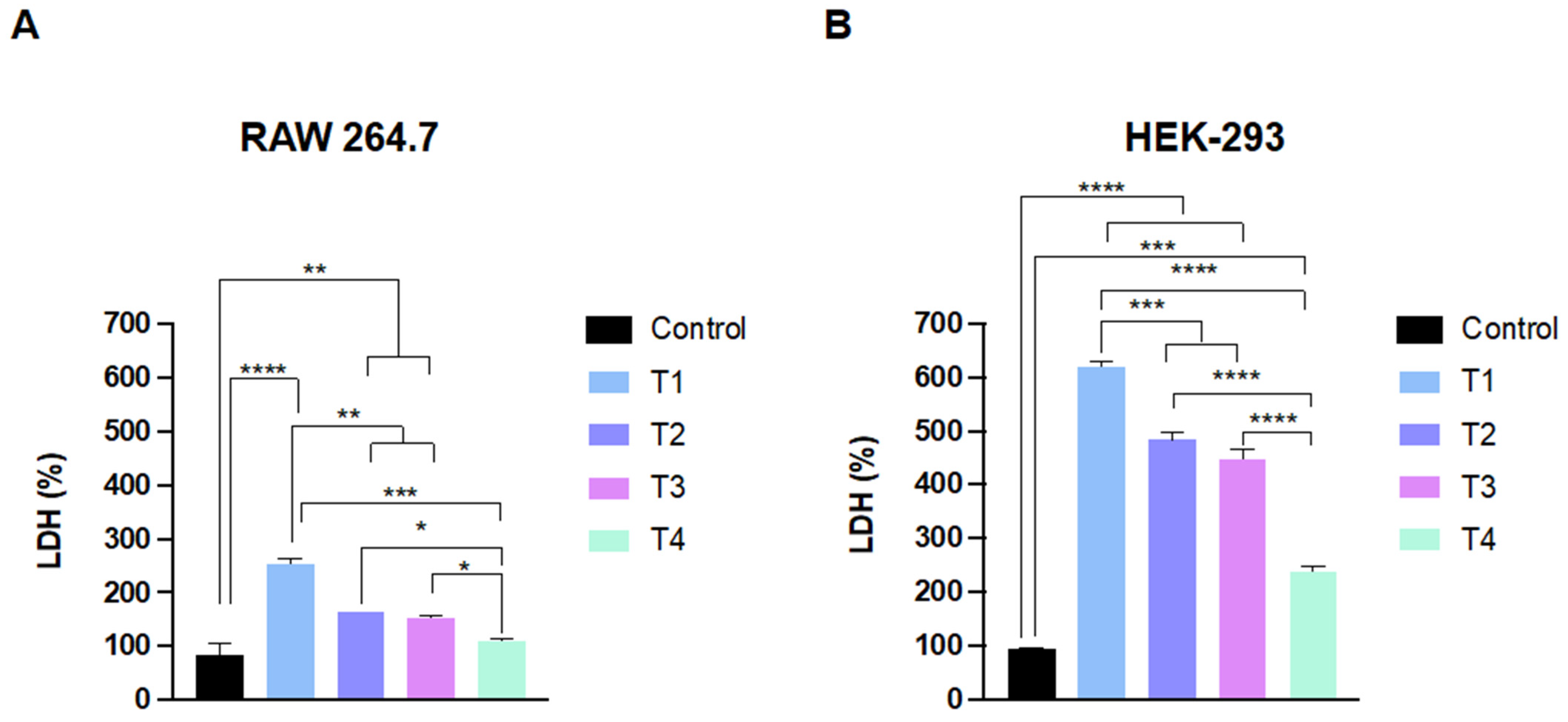
2.2. TiO2 NPs Affect the Cell Viability and Membrane Integrity of Phagocytic and Non-Phagocytic Cells
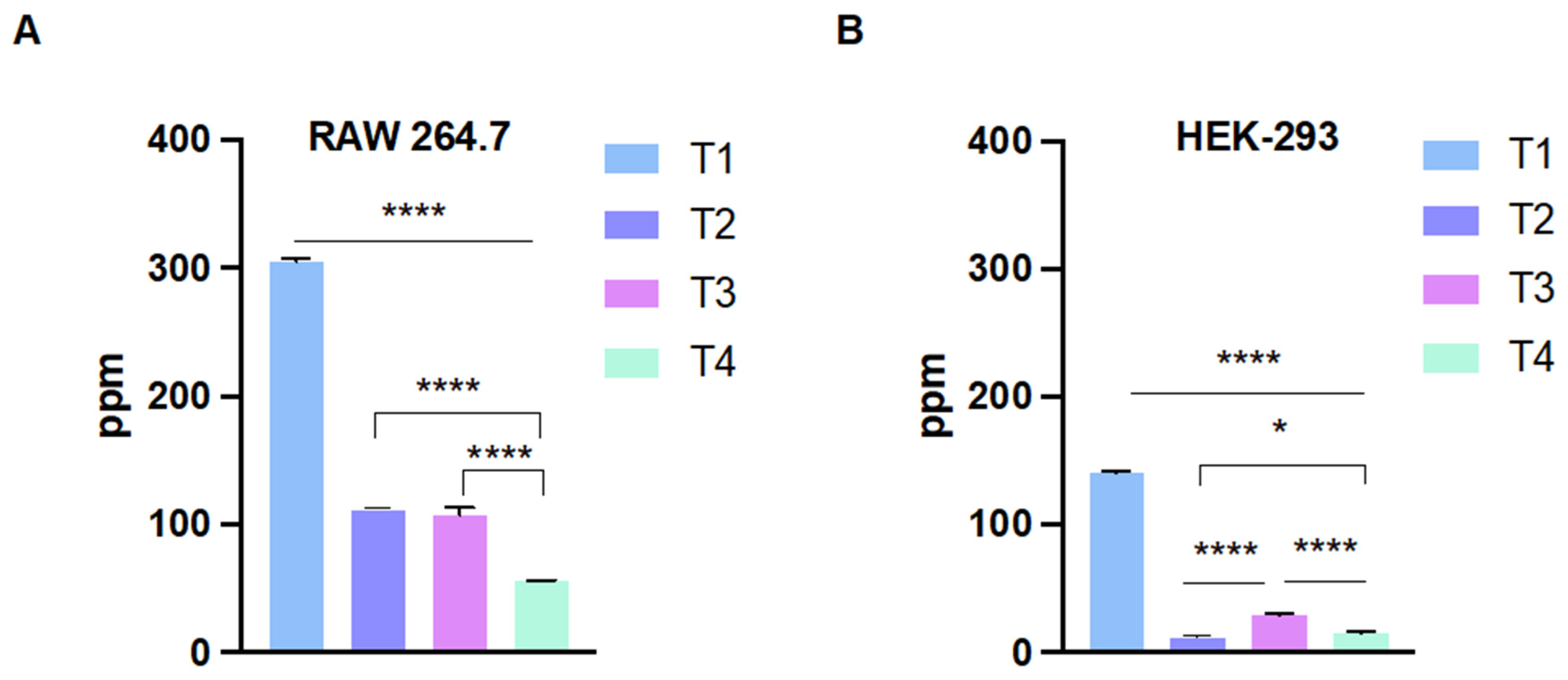
2.3. Cellular Internalization of the Nanoparticles
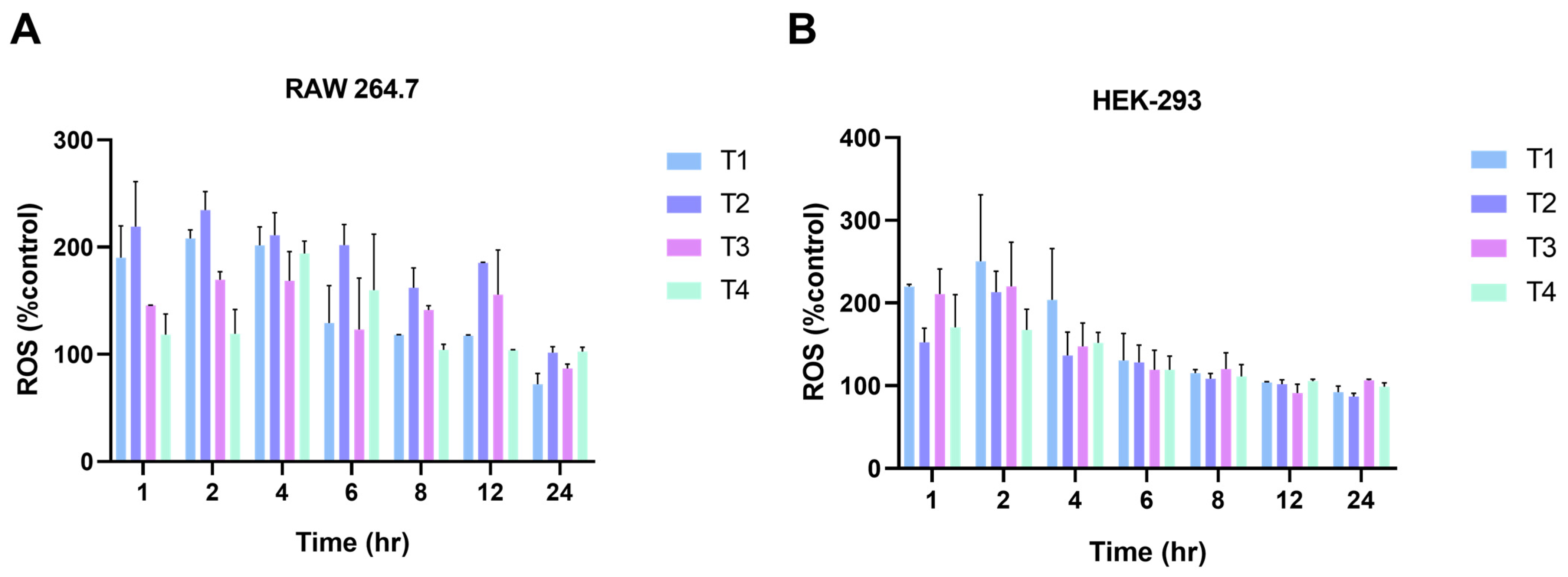
2.4. Generation of Intracellular Reactive Oxygen Species (ROS)
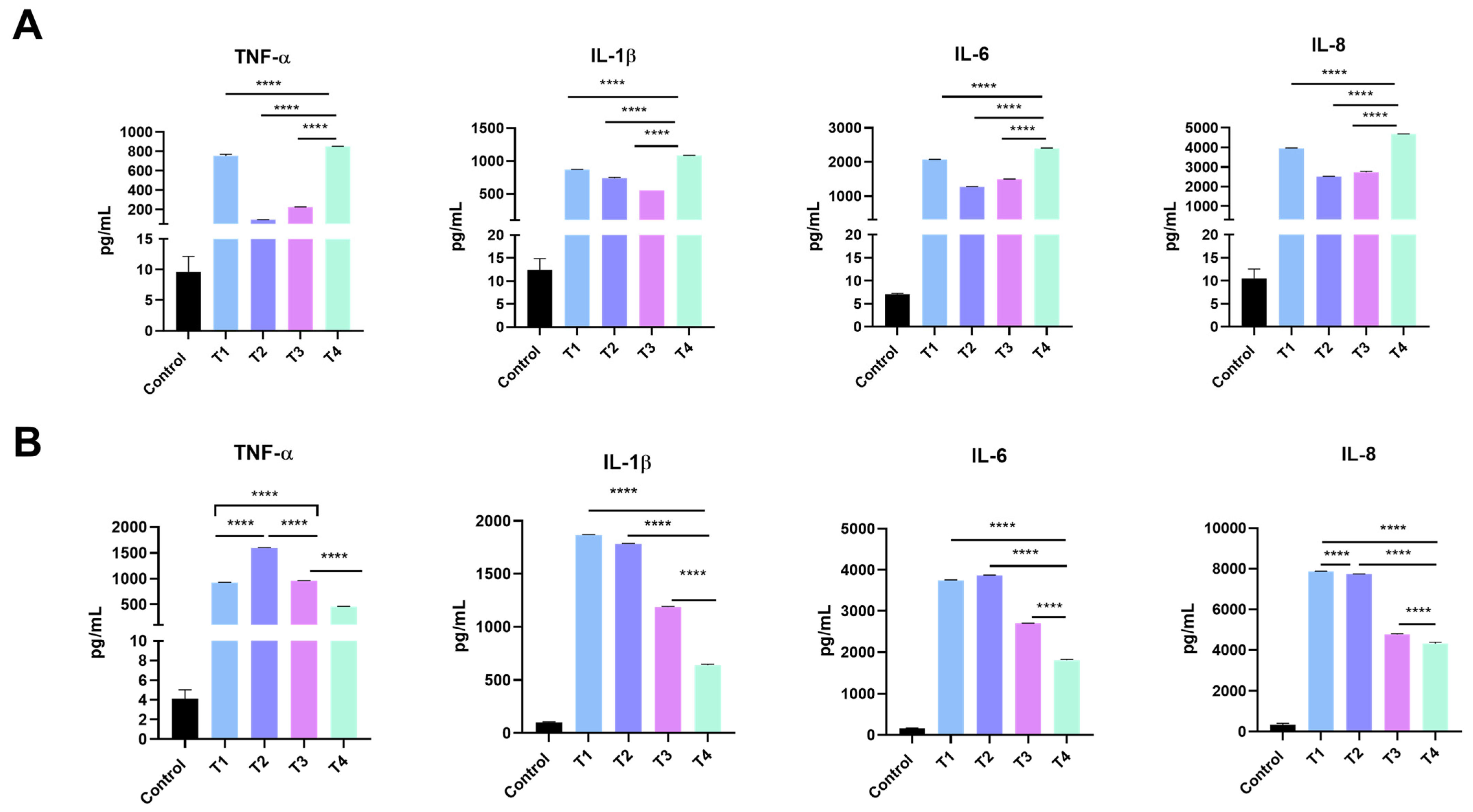
2.5. Release of Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Synthesis
4.2. Nanoparticle Characterization
4.3. Cell Culture
4.4. Cell Viability and Membrane Integrity
4.5. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.6. Cytokine Immunoassay
4.7. Cellular Uptake Measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, C.M. Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Baranowska-Wojcik, E.; Baranowska-Wojcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Xia, T. Continued Efforts on Nanomaterial-Environmental Health and Safety Is Critical to Maintain Sustainable Growth of Nanoindustry. Small 2020, 16, e2000603. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, N.B.; Brown, J.M. Immune responses to engineered nanomaterials: Current understanding and challenges. Curr. Opin. Toxicol. 2018, 10, 8–14. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug. Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Samadian, H.; Barabadi, H.; Azarnezhad, A.; Ahmadi, A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem. Biol. Interact. 2019, 312, 108814. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Alsaleh, N.B. Adverse cardiovascular responses of engineered nanomaterials: Current understanding of molecular mechanisms and future challenges. Nanomedicine 2021, 37, 102421. [Google Scholar] [CrossRef]
- Abukabda, A.B.; Stapleton, P.A.; McBride, C.R.; Yi, J.; Nurkiewicz, T.R. Heterogeneous Vascular Bed Responses to Pulmonary Titanium Dioxide Nanoparticle Exposure. Front. Cardiovasc. Med. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, C.E.; Shepherd, D.L.; Hathaway, Q.A.; Durr, A.J.; Thapa, D.; Abukabda, A.; Yi, J.; Nurkiewicz, T.R.; Hollander, J.M. Reactive oxygen species damage drives cardiac and mitochondrial dysfunction following acute nano-titanium dioxide inhalation exposure. Nanotoxicology 2018, 12, 32–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, I.L.; Huang, Y.J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci. Total. Env. 2011, 409, 1219–1228. [Google Scholar] [CrossRef]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Forster, I.; Schins, R.P. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef]
- Uboldi, C.; Urban, P.; Gilliland, D.; Bajak, E.; Valsami-Jones, E.; Ponti, J.; Rossi, F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol. Vitr. 2016, 31, 137–145. [Google Scholar] [CrossRef]
- Kose, O.; Stalet, M.; Leclerc, L.; Forest, V. Influence of the physicochemical features of TiO2 nanoparticles on the formation of a protein corona and impact on cytotoxicity. RSC Adv. 2020, 10, 43950–43959. [Google Scholar] [CrossRef]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.B.; Hochepied, J.F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the Physicochemical Features of TiO2 Nanoparticles on Their In Vitro Toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; Van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H.; et al. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef]
- Kheder, W.; Soumya, S.; Samsudin, A.R. Impact of titanium dioxide particle size on macrophage production of intracellular reactive oxygen species. Arch. Oral. Biol. 2021, 127, 105133. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, Y.; Guan, C.; Wang, L.; Xu, L.; Li, D.; Zhang, S.; Zhang, L.; Yang, D.; Xu, Y. Crystal Phase-Related Toxicity of One-Dimensional Titanium Dioxide Nanomaterials on Kidney Cells. ACS Appl. Bio Mater. 2021, 4, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, H.; Peng, Q.; Li, Y.; Liu, Z.; Li, M. Different toxicity of anatase and rutile TiO2 nanoparticles on macrophages: Involvement of difference in affinity to proteins and phospholipids. J. Hazard. Mater. 2017, 335, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2 Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef] [PubMed]
- Mortein, S.; Søgaard, E.G. Sol–gel reactions of titanium alkoxides and water: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar]
- Falk, G.; Borlaf, M.; López-Muñoz, M.; Fariñas, J.; Rodrigues Neto, J.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanoparticle Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Keerthana, B.G.T.; Solaiyammal, T.; Muniyappan, S.; Murugakoothan, P. Hydrothermal synthesis and characterization of TiO2 nanostructures prepared using different solvents. Mater. Lett. 2018, 220, 20–23. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Cui, X.; Wang, J.; Zhang, X.; Wang, Q.; Song, M.; Chai, J. Preparation of Nano-TiO2 by a Surfactant-Free Microemulsion–Hydrothermal Method and Its Photocatalytic Activity. Langmuir 2019, 35, 9255–9263. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium dioxide: From engineering to applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.; Garcia Serrano, J.; Santiago, P.; Pal, U. Size-controlled synthesis of spherical TiO2 nanoparticles: Morphology, crystallization, and phase transition. J. Phys. Chem. C. 2007, 111, 96–102. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core–shell structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Tichagwa, L.; Greyling, C. The influence of carbon doping on TiO2 nanoparticle size, surface area, anatase to rutile phase transformation and photocatalytic activity. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012. [Google Scholar]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B. 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2008, 11, 77–89. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Karakus, C.O.; Bilgi, E.; Winkler, D.A. Biomedical nanomaterials: Applications, toxicological concerns, and regulatory needs. Nanotoxicology 2021, 15, 331–351. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef] [PubMed]
- Cesmeli, S.; Avci, C.B. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug. Target. 2019, 27, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Mao, L.; Johnson, O.; Li, K.; Phan, J.; Yin, Q.; Li, L.; Zhang, J.; Chen, W.; Zhang, Y. Exploration of TiO2 nanoparticle mediated microdynamic therapy on cancer treatment. Nanomedicine 2019, 18, 272–281. [Google Scholar] [CrossRef]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef] [Green Version]
- Allegri, M.; Bianchi, M.G.; Chiu, M.; Varet, J.; Costa, A.L.; Ortelli, S.; Blosi, M.; Bussolati, O.; Poland, C.A.; Bergamaschi, E. Shape-Related Toxicity of Titanium Dioxide Nanofibres. PLoS ONE 2016, 11, e0151365. [Google Scholar] [CrossRef] [Green Version]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.D.; Armato, C.; Rossi, A.M.; Schiliro, T. Shape-engineered titanium dioxide nanoparticles (TiO2-NPs): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. Bionanoscience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Liu, H.; Zhou, M.; Duan, Y.; Li, N.; Gong, X.; Hu, R.; Hong, M.; Hong, F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. A 2011, 96, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kongseng, S.; Yoovathaworn, K.; Wongprasert, K.; Chunhabundit, R.; Sukwong, P.; Pissuwan, D. Cytotoxic and inflammatory responses of TiO2 nanoparticles on human peripheral blood mononuclear cells. J. Appl. Toxicol. 2016, 36, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, M.K.; Jang, A.S. Effect of TiO2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness. Allergy Asthma Immunol. Res. 2017, 9, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugita, M.; Morimoto, N.; Nakayama, M. SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 2017, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Ji, J.; Ze, X.; Zhou, Y.; Ze, Y. Liver Inflammation and Fibrosis Induced by Long-Term Exposure to Nano Titanium Dioxide (TiO2) Nanoparticles in Mice and Its Molecular Mechanism. J. Biomed. Nanotechnol. 2020, 16, 616–625. [Google Scholar] [CrossRef]
- Song, Y.J.; Kim, A.; Kim, G.T.; Yu, H.Y.; Lee, E.S.; Park, M.J.; Kim, Y.J.; Shim, S.M.; Park, T.S. Inhibition of lactate dehydrogenase A suppresses inflammatory response in RAW 264.7 macrophages. Mol. Med. Rep. 2019, 19, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Fialek, B.; Pruc, M.; Smereka, J.; Jas, R.; Rahnama-Hezavah, M.; Denegri, A.; Szarpak, A.; Jaguszewski, M.J.; Peacock, F.W.; Szarpak, L. Diagnostic value of lactate dehydrogenase in COVID-19: A systematic review and meta-analysis. Cardiol. J. 2022, 29, 751–758. [Google Scholar] [CrossRef]
- Coreas, R.; Cao, X.; DeLoid, G.M.; Demokritou, P.; Zhong, W. Lipid and protein corona of food-grade TiO2 nanoparticles in simulated gastrointestinal digestion. NanoImpact 2020, 20, 100272. [Google Scholar] [CrossRef] [PubMed]
- Korabkova, E.; Kasparkova, V.; Jasenska, D.; Moricova, D.; Dadova, E.; Truong, T.H.; Capakova, Z.; Vicha, J.; Pelkova, J.; Humpolicek, P. Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. Int. J. Mol. Sci. 2021, 22, 10614. [Google Scholar] [CrossRef] [PubMed]
- Deciga-Alcaraz, A.; Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Rodriguez-Ibarra, C.; Ganem-Rondero, A.; Vazquez-Zapien, G.J.; Mata-Miranda, M.M.; Limon-Pacheco, J.H.; Garcia-Cuellar, C.M.; Sanchez-Perez, Y.; et al. Toxicity of engineered nanomaterials with different physicochemical properties and the role of protein corona on cellular uptake and intrinsic ROS production. Toxicology 2020, 442, 152545. [Google Scholar] [CrossRef] [PubMed]
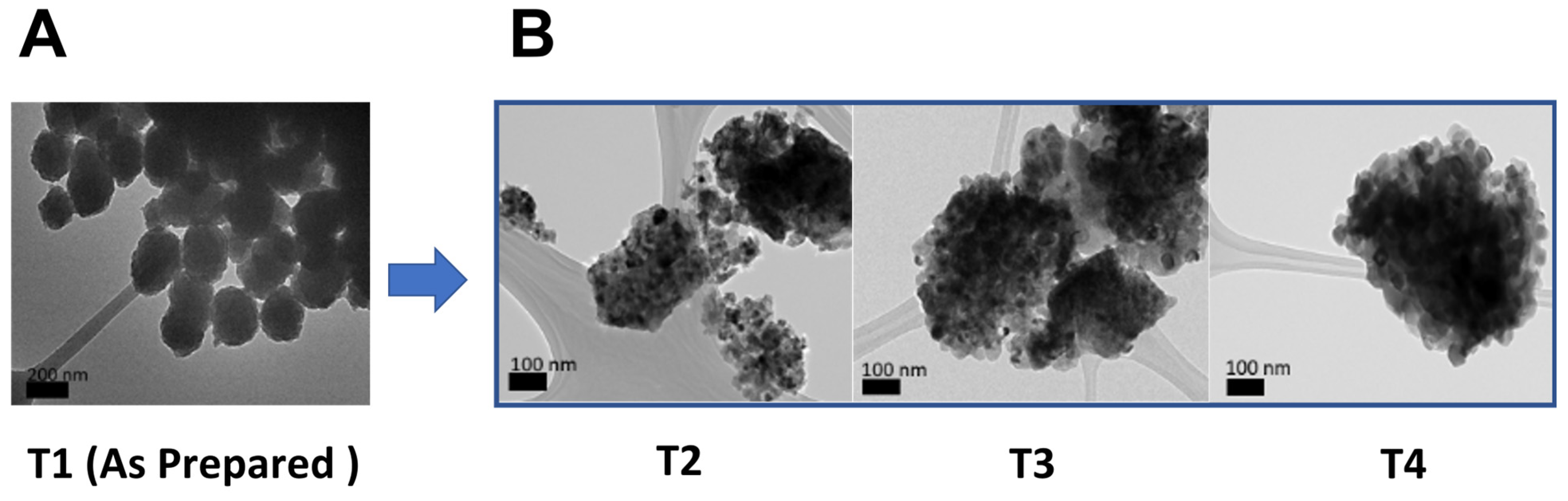
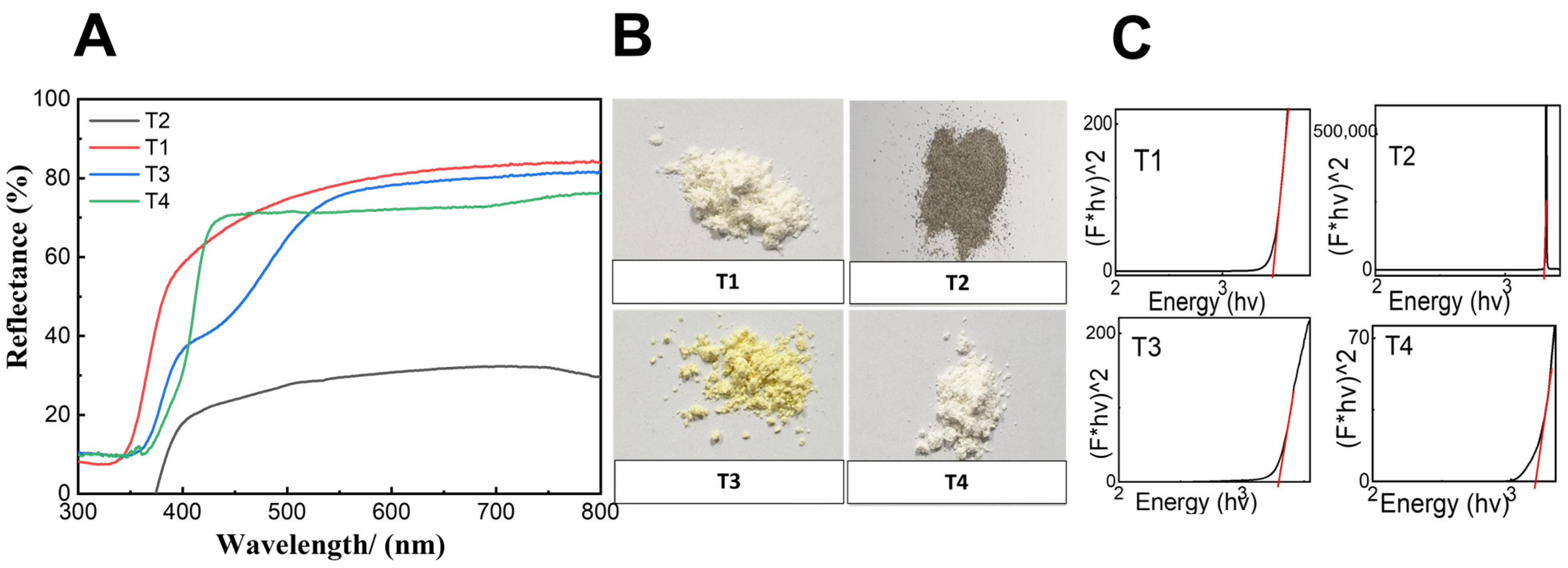

| Sample | Size * (nm) | Surface Area (m2/g) | Zeta-Potential (mV) | Band Gap (eV) | Crystalline Form |
|---|---|---|---|---|---|
| T1 | 220.5 ± 18.2 | 200.483 | −44.9 ± 1.15 | 3.480 | Amorphous |
| T2 | 150.9 ± 20.4 | 4.766 | −7.39 ± 1.18 | 3.303 | Anatase |
| T3 | 169.8 ± 26.3 | 10.458 | −25.8 ± 0.70 | 3.299 | Anatase (high intensity) |
| T4 | 232.4 ± 19.7 | 7.053 | −27.1 ± 0.40 | 3.178 | Anatase and rutile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomen, A.; Alsaleh, N.B.; El-Toni, A.M.; EL-Mahrouky, M.A.; Alhowyan, A.A.; Alkholief, M.; Alshamsan, A.; Khurana, N.; Ghandehari, H. In Vitro Safety Assessment of In-House Synthesized Titanium Dioxide Nanoparticles: Impact of Washing and Temperature Conditions. Int. J. Mol. Sci. 2023, 24, 9966. https://doi.org/10.3390/ijms24129966
Almomen A, Alsaleh NB, El-Toni AM, EL-Mahrouky MA, Alhowyan AA, Alkholief M, Alshamsan A, Khurana N, Ghandehari H. In Vitro Safety Assessment of In-House Synthesized Titanium Dioxide Nanoparticles: Impact of Washing and Temperature Conditions. International Journal of Molecular Sciences. 2023; 24(12):9966. https://doi.org/10.3390/ijms24129966
Chicago/Turabian StyleAlmomen, Aliyah, Nasser B. Alsaleh, Ahmed Mohamed El-Toni, Mohamed A. EL-Mahrouky, Adel Ali Alhowyan, Musaed Alkholief, Aws Alshamsan, Nitish Khurana, and Hamidreza Ghandehari. 2023. "In Vitro Safety Assessment of In-House Synthesized Titanium Dioxide Nanoparticles: Impact of Washing and Temperature Conditions" International Journal of Molecular Sciences 24, no. 12: 9966. https://doi.org/10.3390/ijms24129966







