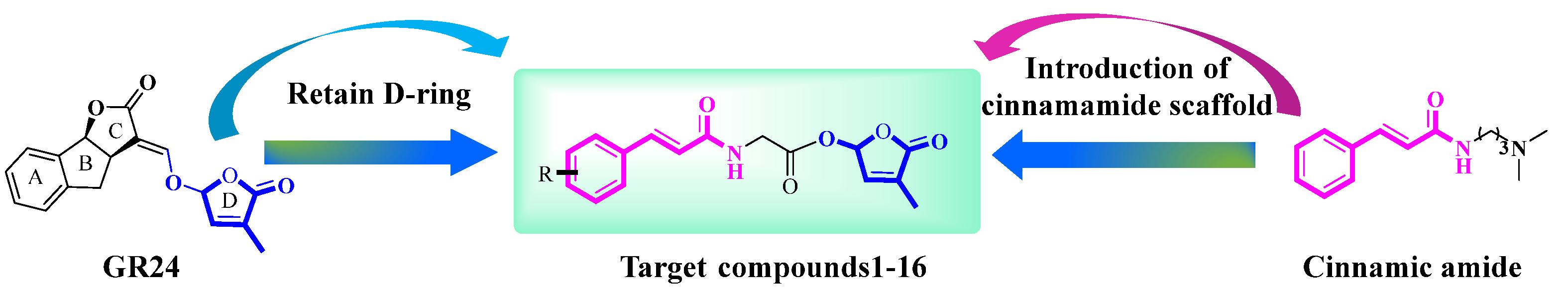
Discovery of Novel Hybrid-Type Strigolactone Mimics Derived from Cinnamic Amide
Abstract
:1. Introduction
2. Results and Discussion
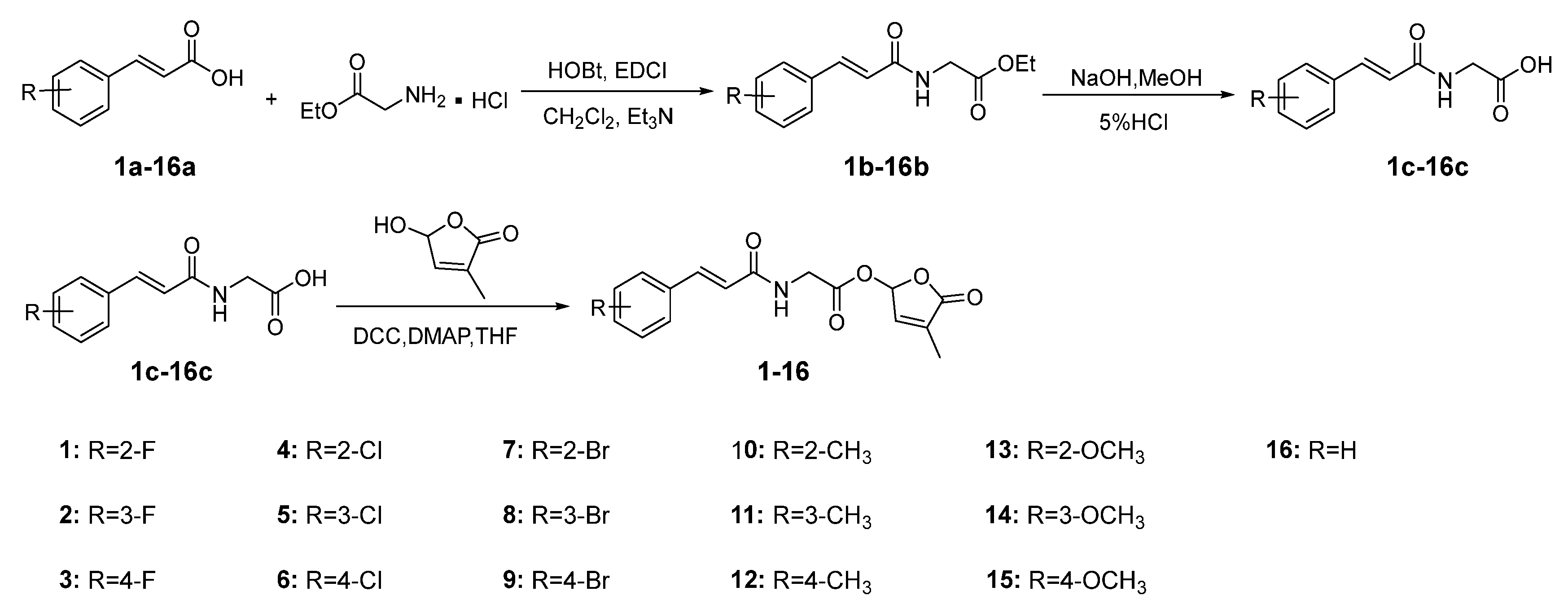
2.1. Synthesis of Target Compounds 1–16
2.2. The Biological Activity of Target Compounds
2.2.1. The Effect on Parasitic Weed Seeds
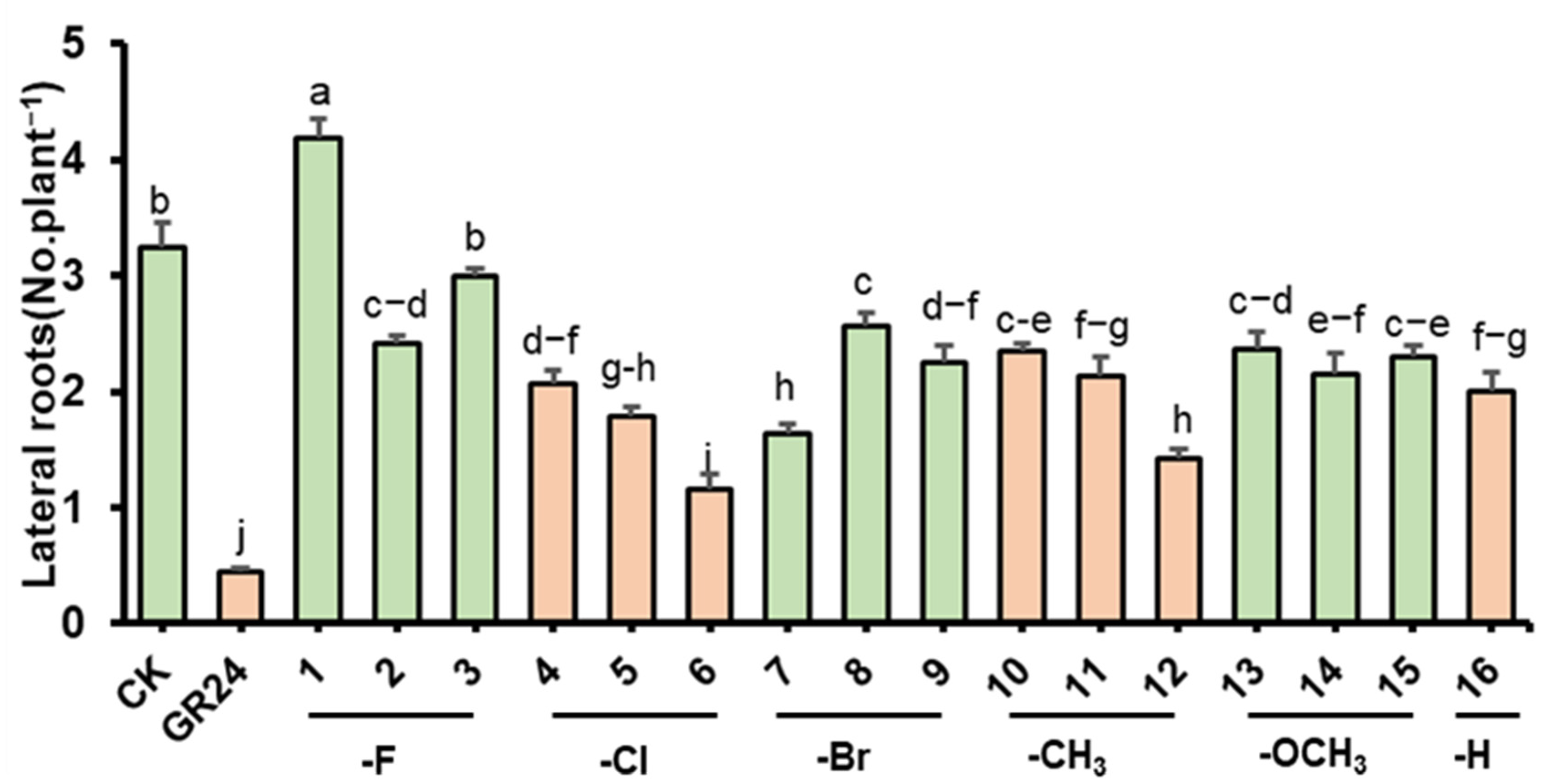
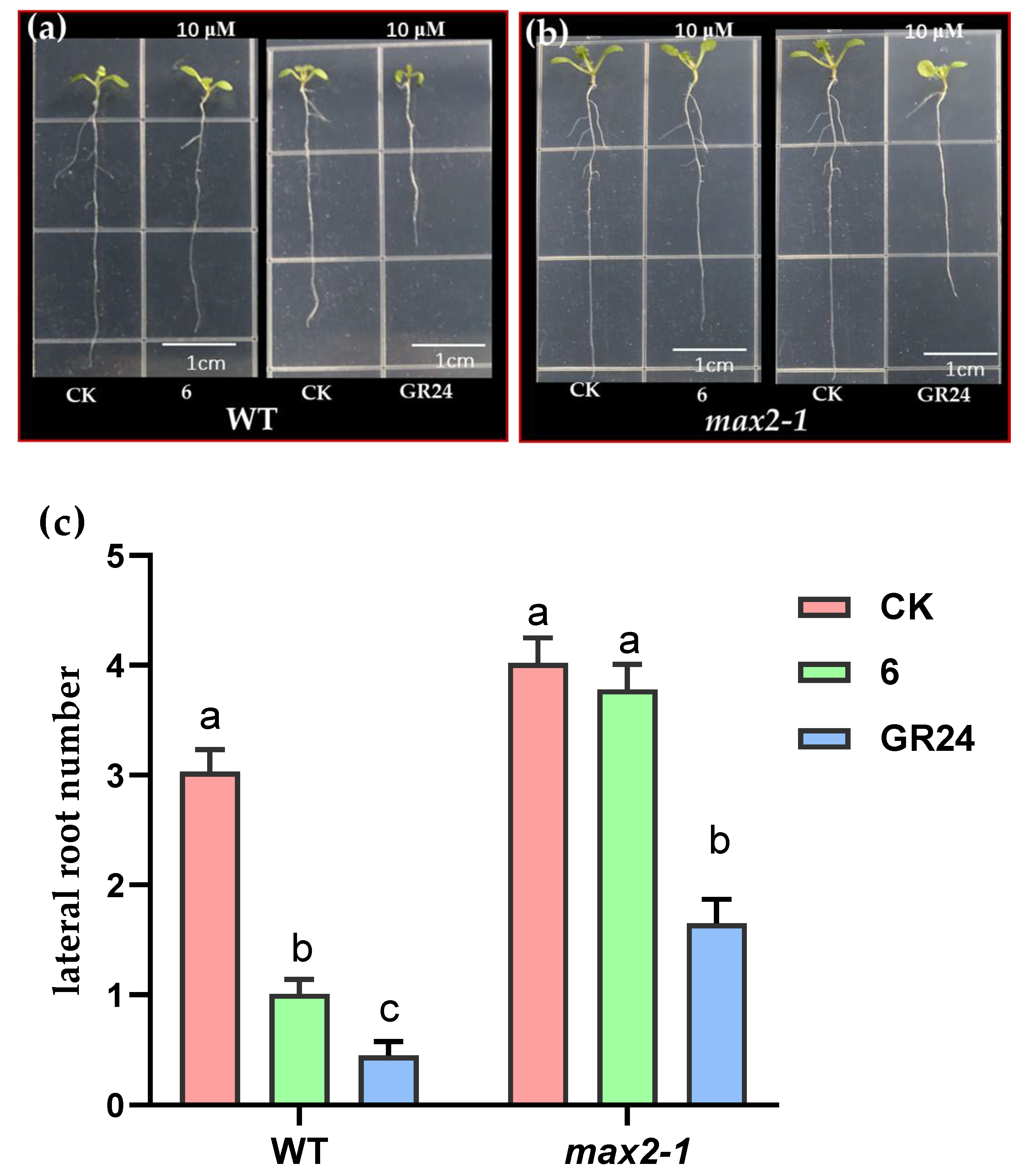
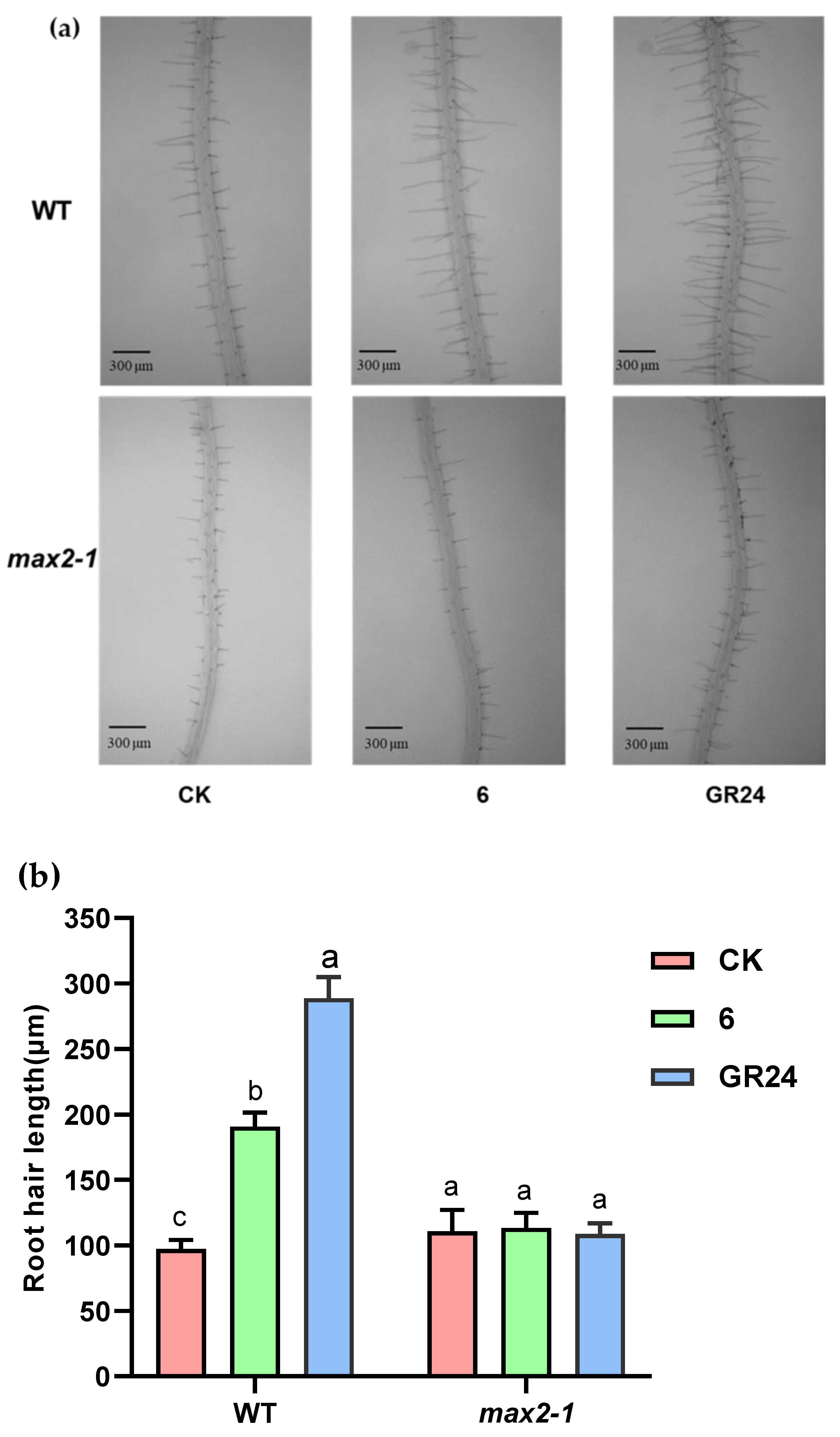
2.2.2. Evaluation of Effect on Arabidopsis Roots
2.2.3. Effect of Compound 6 on Arabidopsis Mutant Growth
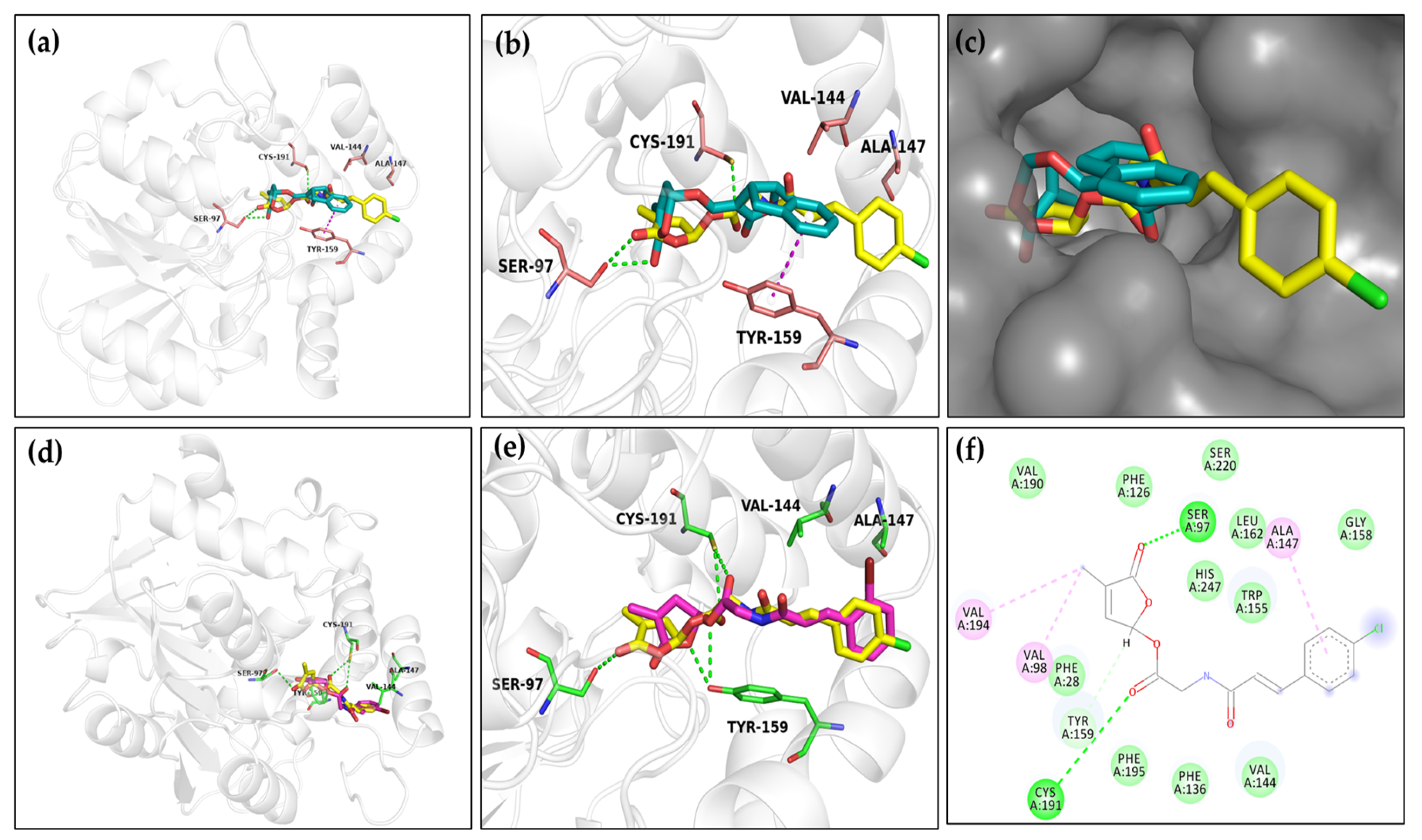
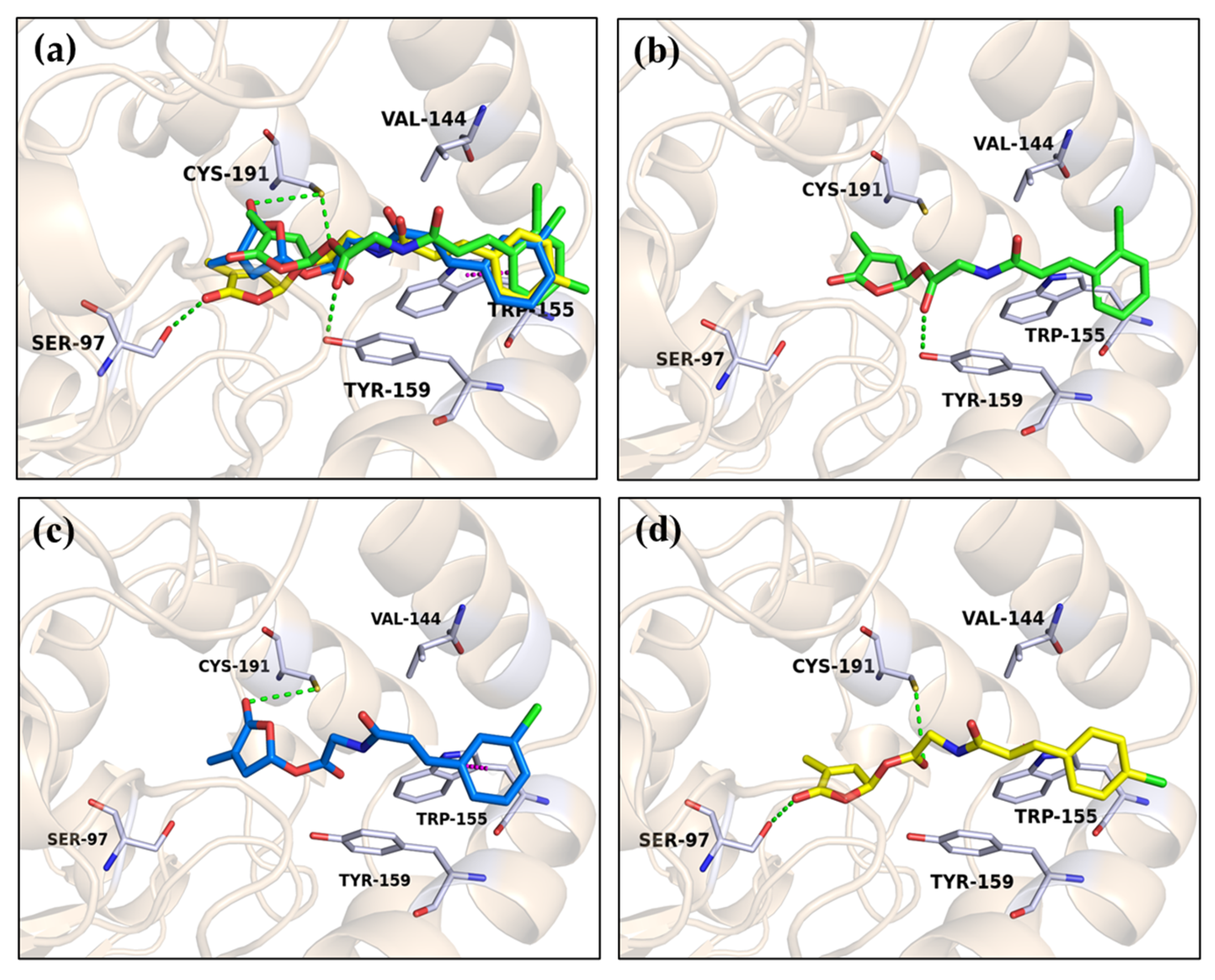
2.3. Molecular Docking of Ligands to OsD14
2.4. Hydrolytic Stability
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Target Compounds 1–16
3.3. Parasitic Weed Seed Germination Bioassays
3.4. Arabidopsis Primary Root Elongation, Lateral Root Formation, and Root Hair Elongation Assay
3.5. Molecular Docking
3.6. Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez-Roldon, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pil-lot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [Green Version]
- Reizelman, A.; Scheren, M.; Nefkens, G.; Zwanenburg, B. Synthesis of all eight stereoisomers of the germination stimulant strigol. Synthesis 2000, 13, 1944–1955. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R. The Role of Strigolactones in the Regulation of Root System Architecture in Grapevine (Vitis vinifera L.) in Response to Root-Restriction Cultivation. Int. J. Mol. Sci. 2021, 22, 8799–8818. [Google Scholar]
- Wang, X.; Liu, D.; Lin, J.; Zhu, T.; Liu, N.; Yang, X.; Ma, J.; Sui, S. Carotenoid Cleavage Dioxygenase Genes of Chimonanthus praecox, CpCCD7 and CpCCD8, Regulate Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 8750. [Google Scholar] [CrossRef]
- Xie, X.N.; Yoneyama, K.; Kisugi, T.; Μchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, K. Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 2020, 45, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zealactones. Novel natural strigolactones from maize. Phytochemistry 2017, 137, 123–131. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.; Macias, F.A. Strategies for the synthesis of canonical, non-canonical and analogues of strigolactones, and evaluation of their parasitic weed germination activity. Phytochem. Rev. 2022, 21, 1627–1659. [Google Scholar] [CrossRef]
- Bromhead, L.J.; Mcerlean, C. Accessing Single Enantiomer Strigolactones: Progress and Opportunities. Eur. J. Org. Chem. 2017, 38, 5712–5723. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospisil, T.; Zeljkovic, S.C. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [Green Version]
- Hamiaux, C.; Drummond, R.S.M.; Janssen, B.J.; Ledger, S.E.; Cooney, J.M.; Newcomb, R.D.; Snowden, K.C. DAD2 is an alpha/beta hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012, 22, 2032–2036. [Google Scholar] [CrossRef] [Green Version]
- Fukui, K.; Ito, S.; Asami, T. Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol. Plant 2013, 6, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Li, Y.W.; Chen, L.H.; Zhang, C.; Wang, F.; Li, H.O.; Wang, M.; Wang, Y.P.; Nan, F.J.; Xie, D.X.; et al. Strigolactone mimic 2-nitrodebranone is highly active in Arabidopsis growth and development. Plant J. 2021, 107, 67–76. [Google Scholar] [CrossRef]
- Samejima, H.; Babiker, A.G.; Takikawa, H.; Sasaki, M.; Sugimoto, Y. Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag. Sci. 2016, 72, 2035–2042. [Google Scholar] [CrossRef]
- Hýlová, A.; Pospíšil, T.; Spíchal, L.; Mateman, J.J.; Blanco-Ania, D.; Zwanenburg, B. New hybrid type strigolactone mimics derived from plant growth regulator auxin. New Biotechnol. 2019, 48, 76–82. [Google Scholar] [CrossRef]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, K.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef]
- Yao, R.; Wang, F.; Ming, Z.; Du, X.; Chen, L.; Wang, Y.; Zhang, W.; Deng, H.; Xie, D. ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res. 2017, 27, 838–841. [Google Scholar] [CrossRef] [Green Version]
- Germain, A.D.S.; Clavé, G.; Badet-Denisot, M.-A.; Pillot, J.-P.; Cornu, D.; Le Caer, J.-P.; Burger, M.; Pelissier, F.; Retailleau, P.; Turnbull, C.; et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016, 12, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Soudek, P.; Vanek, T. Triazolide strigolactone mimics influence root development in Arabidopsis. J. Nat. Prod. 2017, 80, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.G.; Cala, A.; Fernandez-Aparicio, M.; Molinillo, J.M.G.; Boaventura, M.A.D.; Macias, F.A. Gibberellic and kaurenoic hybrid strigolactone mimics for seed germination of parasitic weeds. Pest Manag. Sci. 2017, 73, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ania, D.; Mateman, J.J.; Hýlová, A.; Spíchal, L.; Debie, L.M.; Zwanenburg, B. Hybrid-type strigolactone analogues derived from auxins. Pest Manag. Sci. 2019, 75, 3113–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure-activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.S.; Nayak, V.L.; Rao, A.V.S.; Hussaini, S.M.A.; Sunkari, S.; Alarifi, A.; Kamal, A. Arylcinnamido-propionone conjugates as tubulin polymerization inhibitors and apoptotic inducers. Arab. J. Chem. 2019, 12, 4740–4755. [Google Scholar]
- Seelolla, G.; Cheera, P.; Ponneri, V. Synthesis, antimicrobial and antioxidant activities of novel series of cinnamamide derivatives having morpholine moiety. Med. Chem. Res. 2014, 4, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Gu, Q.; Morris-Natschke, S.L.; Chen, C.H.; Lee, K.H. Incorporation of Privileged Structures into Bevirimat Can Improve Activity against Wild-Type and Bevirimat-Resistant HIV-1. J. Med. Chem. 2016, 59, 9262–9268. [Google Scholar] [CrossRef] [Green Version]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Di Marino, C.; Golino, A.; Previtera, L.; Zarrelli, A. Cinnamic acid amides from Chenopodium album: Effects on seeds germination and plant growth. Phytochemistry 2003, 64, 1381–1387. [Google Scholar] [CrossRef]
- Chen, W.; Xu, L. Growth-regulating activity of cinnamamide and betaine cinnamamide on wheat. Adv. J. Food Sci. Technol. 2014, 7, 584–588. [Google Scholar]
- Ridwan, A.Y.; Matoba, R.; Jing, W.; Choi, J.; Hirai, H.; Kawagishi, H. A novel plant growth regulator from Pholiota lubrica. Tetrahedron Lett. 2018, 59, 2559–2561. [Google Scholar] [CrossRef]
- Kang, S.; Song, B.; Wu, J.; He, M.; Hu, D.; Jin, L.; Zeng, S.; Xue, W.; Yang, S. Design, synthesis and insecticidal activities of novel acetamido derivatives containing N-pyridylpyrazole carboxamides. Eur. J. Med. Chem. 2013, 67, 14–18. [Google Scholar] [CrossRef]
- Kang, Y.; Pang, Z.; Xu, N.; Chen, F.; Jin, Z.; Xu, X. Strigolactone Analogues Derived from Dihydroflavonoids as Potent Seed Germinators for the Broomrapes. J. Agric. Food Chem. 2020, 68, 11077–11087. [Google Scholar] [CrossRef]
- Matthys, C.; Walton, A.; Struk, S.; Stes, E.; Boyer, F.D.; Gevaert, K.; Goormachtig, S. The whats, the wheres and the hows of strigolactone action in the roots. Planta 2016, 243, 1327–1337. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones. Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Dvorakova, M.; Hylova, A.; Soudek, P.; Retzer, K.; Spichal, L.; Vanek, T. Resorcinol-Type Strigolactone Mimics as Potent Germinators of the Parasitic Plants Striga hermonthica and Phelipanche ramose. J. Nat. Prod. 2018, 81, 2321–2328. [Google Scholar] [CrossRef]
- Jamil, M.; Kountche, B.A.; Haider, I.; Guo, X.; Ntui, V.O.; Jia, K.P.; Ali, S.; Hameed, U.S.; Nakamura, H.; Lyu, Y.; et al. Methyl phenlactonoates are efficient strigolactone analogs with simple structure. J. Exp. Bot. 2018, 69, 2319–2331. [Google Scholar] [CrossRef]
- Yang, Z.K.; Wang, J.E.; Tian, H.; He, Y.; Duan, H.X.; Duan, L.S.; Tan, W.M. Design, synthesis, biological activities, and dynamic simulation study of novel thiourea derivatives with gibberellin activity towards Arabidopsis thaliana. Bioorg. Med. Chem. 2019, 27, 114969. [Google Scholar] [CrossRef]

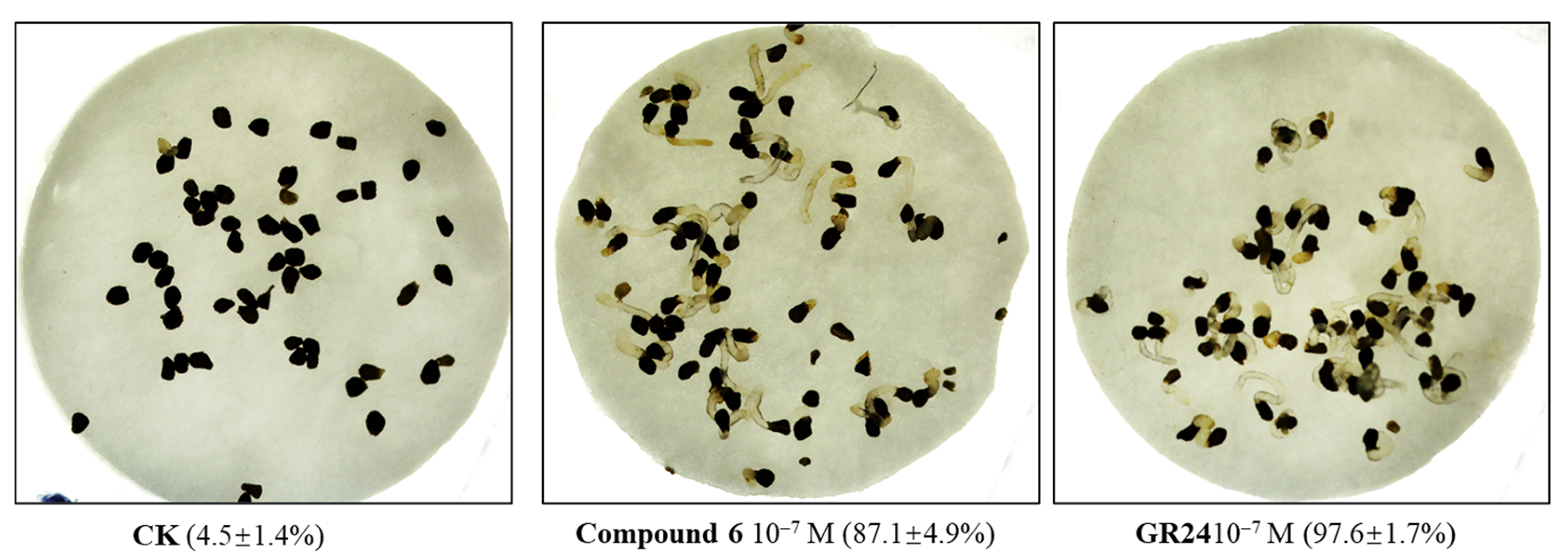
| Compds | R | Germination Rate (%) a | EC50 (M) | ||
|---|---|---|---|---|---|
| 10−5 (M) | 10−6 (M) | 10−7 (M) | |||
| 1 | 2-F | 51.7 ± 8.8 | 35.6 ± 0.8 | 26.0 ± 2.2 | n.d |
| 2 | 3-F | 56.0 ± 6.2 | 44.1 ± 9.2 | 30.8 ± 8.0 | n.d |
| 3 | 4-F | 52.7 ± 5.7 | 47.9 ± 3.1 | 40.1 ± 6.4 | 1.15 × 10−6 |
| 4 | 2-Cl | 57.3 ± 5.7 | 42.5 ± 7.6 | 27.8 ± 5.2 | 1.72 × 10−6 |
| 5 | 3-Cl | 80.5 ± 7.9 | 81.6 ± 8.0 | 53.3 ± 4.2 | 8.02 × 10−8 |
| 6 | 4-Cl | 93.7 ± 7.1 | 90.7 ± 4.3 | 87.1 ± 4.9 | 2.36 × 10−8 |
| 7 | 2-Br | 92.4 ± 3.4 | 68.3 ± 4.9 | 63.5 ± 7.8 | 4.47 × 10−8 |
| 8 | 3-Br | 81.1 ± 8.3 | 53.8 ± 0.2 | 56.3 ± 2.4 | 6.05 × 10−8 |
| 9 | 4-Br | 53.4 ± 6.5 | 23.9 ± 4.8 | 30.1 ± 7.2 | n.d |
| 10 | 2-CH3 | 50.2 ± 6.9 | 25.9 ± 6.5 | 27.6 ± 7.2 | n.d |
| 11 | 3-CH3 | 78.0 ± 5.0 | 25.0 ± 6.1 | 26.3 ± 9.0 | 3.42 × 10−6 |
| 12 | 4-CH3 | 21.6 ± 8.1 | 12.1 ± 3.0 | 22.2 ± 8.9 | n.d |
| 13 | 2-OCH3 | 68.6 ± 7.0 | 39.0 ± 5.4 | 40.0 ± 6.3 | n.d |
| 14 | 3-OCH3 | 23.1 ± 6.6 | 14.6 ± 5.0 | 16.6 ± 5.5 | n.d |
| 15 | 4-OCH3 | 31.9 ± 6.0 | 29.7 ± 6.3 | 27.2 ± 6.7 | n.d |
| 16 | H | 44.7 ± 2.0 | 39.4 ± 2.9 | 25.5 ± 7.7 | n.d |
| GR24 | - | 95.3 ± 1.7 | 96.5 ± 3.2 | 97.6 ± 1.7 | 3.09 × 10−9 |
| Compds | R | Inhibition Rate (%) | IC50 (μM) | ||
|---|---|---|---|---|---|
| 1 (μM) | 50 (μM) | 100 (μM) | |||
| 1 | 2-F | −17.4 ± 2.1 | 37.0 ± 1.9 | 74.5 ± 1.7 | 62.96 |
| 2 | 3-F | −10.7 ± 1.8 | 63.0 ± 1.9 | 74.8 ± 2.8 | 42.91 |
| 3 | 4-F | −0.4 ± 1.3 | 65.8 ± 3.2 | 76.0 ± 3.2 | 38.80 |
| 4 | 2-Cl | −3.5 ± 7.6 | 57.9 ± 4.0 | 72.6 ± 2.4 | 47.02 |
| 5 | 3-Cl | −5.3 ± 0.7 | 30.7 ± 2.1 | 64.9 ± 2.8 | 74.13 |
| 6 | 4-Cl | 2.7 ± 4.3 | 83.2 ± 0.6 | 91.3 ± 2.1 | 25.21 |
| 7 | 2-Br | 8.0 ± 4.4 | 52.8 ± 5.5 | 68.6 ± 2.4 | 47.67 |
| 8 | 3-Br | 3.3 ± 5.0 | 48.6 ± 6.1 | 76.5 ± 7.0 | 49.41 |
| 9 | 4-Br | −2.6 ± 7.7 | 47.3 ± 5.8 | 84.1 ± 2.5 | 52.66 |
| 10 | 2-CH3 | −6.9 ± 6.2 | 67.3 ± 2.0 | 72.8 ± 3.1 | 40.08 |
| 11 | 3-CH3 | 0.3 ± 4.4 | 53.5 ± 4.9 | 69.1 ± 2.2 | 49.40 |
| 12 | 4-CH3 | 1.3 ± 0.8 | 71.2 ± 2.8 | 76.2 ± 2.3 | 37.37 |
| 13 | 2-OCH3 | 8.4 ± 1.6 | 25.3 ± 1.3 | 72.4 ± 0.8 | 71.91 |
| 14 | 3-OCH3 | −1.3 ± 2.5 | 64.1 ± 0.4 | 67.0 ± 2.3 | 44.84 |
| 15 | 4-OCH3 | 2.3 ± 0.5 | 66.5 ± 5.6 | 70.7 ± 2.8 | 41.24 |
| 16 | H | 2.3 ± 1.8 | 49.9 ± 4.1 | 66.5 ± 1.2 | 54.08 |
| GR24 | - | 3.0 ± 4.3 | 94.4 ± 1.2 | 96.6 ± 0.9 | 9.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, B.; Yang, Z.; Du, L.; Yu, C.; Zhou, Y.; Zhao, H.; Wang, Y.; Duan, L. Discovery of Novel Hybrid-Type Strigolactone Mimics Derived from Cinnamic Amide. Int. J. Mol. Sci. 2023, 24, 9967. https://doi.org/10.3390/ijms24129967
Wang C, Guo B, Yang Z, Du L, Yu C, Zhou Y, Zhao H, Wang Y, Duan L. Discovery of Novel Hybrid-Type Strigolactone Mimics Derived from Cinnamic Amide. International Journal of Molecular Sciences. 2023; 24(12):9967. https://doi.org/10.3390/ijms24129967
Chicago/Turabian StyleWang, Chunying, Bingbo Guo, Zhaokai Yang, Lin Du, Chunxin Yu, Yuyi Zhou, Hanqing Zhao, Ye Wang, and Liusheng Duan. 2023. "Discovery of Novel Hybrid-Type Strigolactone Mimics Derived from Cinnamic Amide" International Journal of Molecular Sciences 24, no. 12: 9967. https://doi.org/10.3390/ijms24129967






