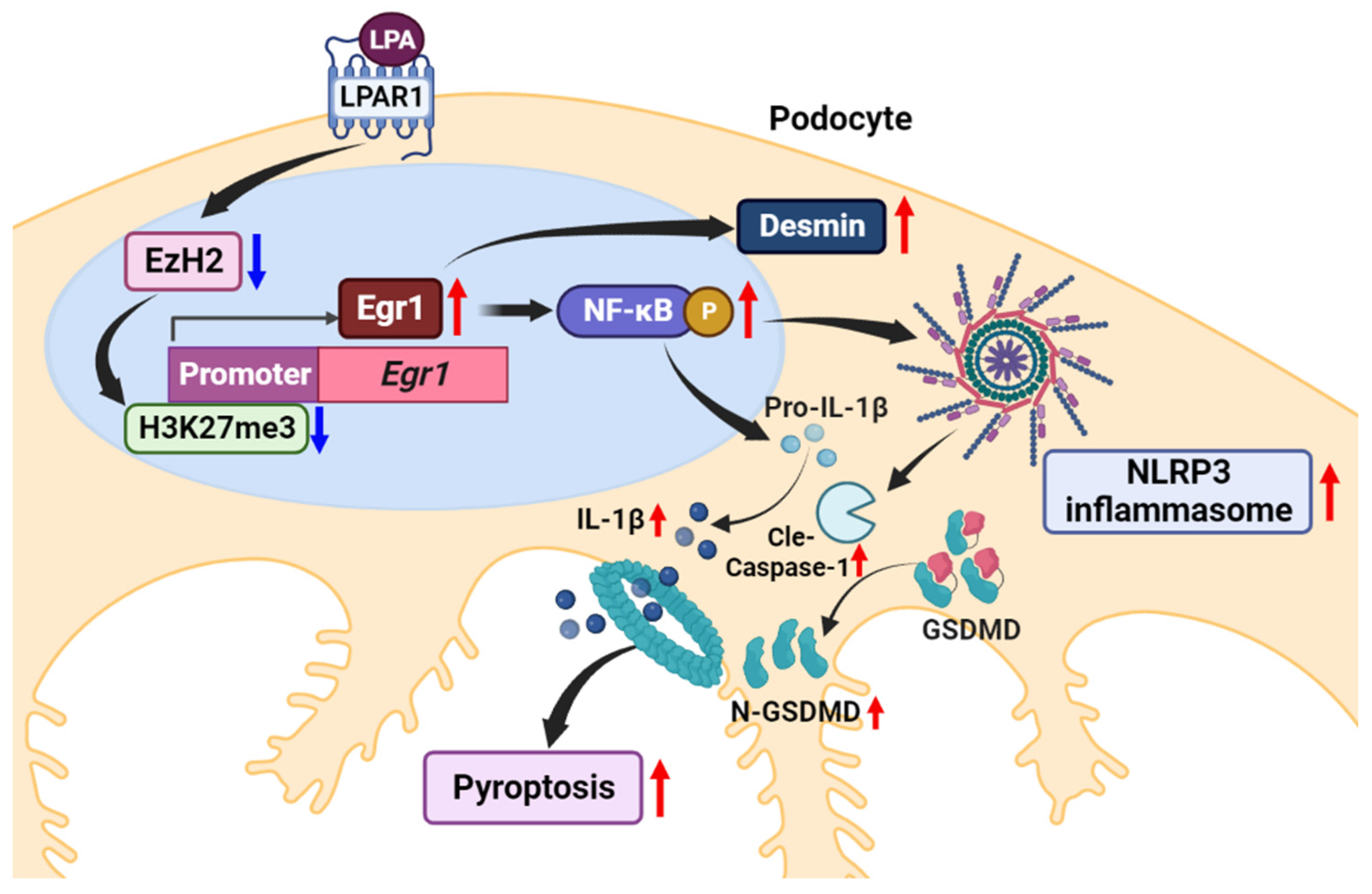
Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2
Abstract
1. Introduction
2. Results
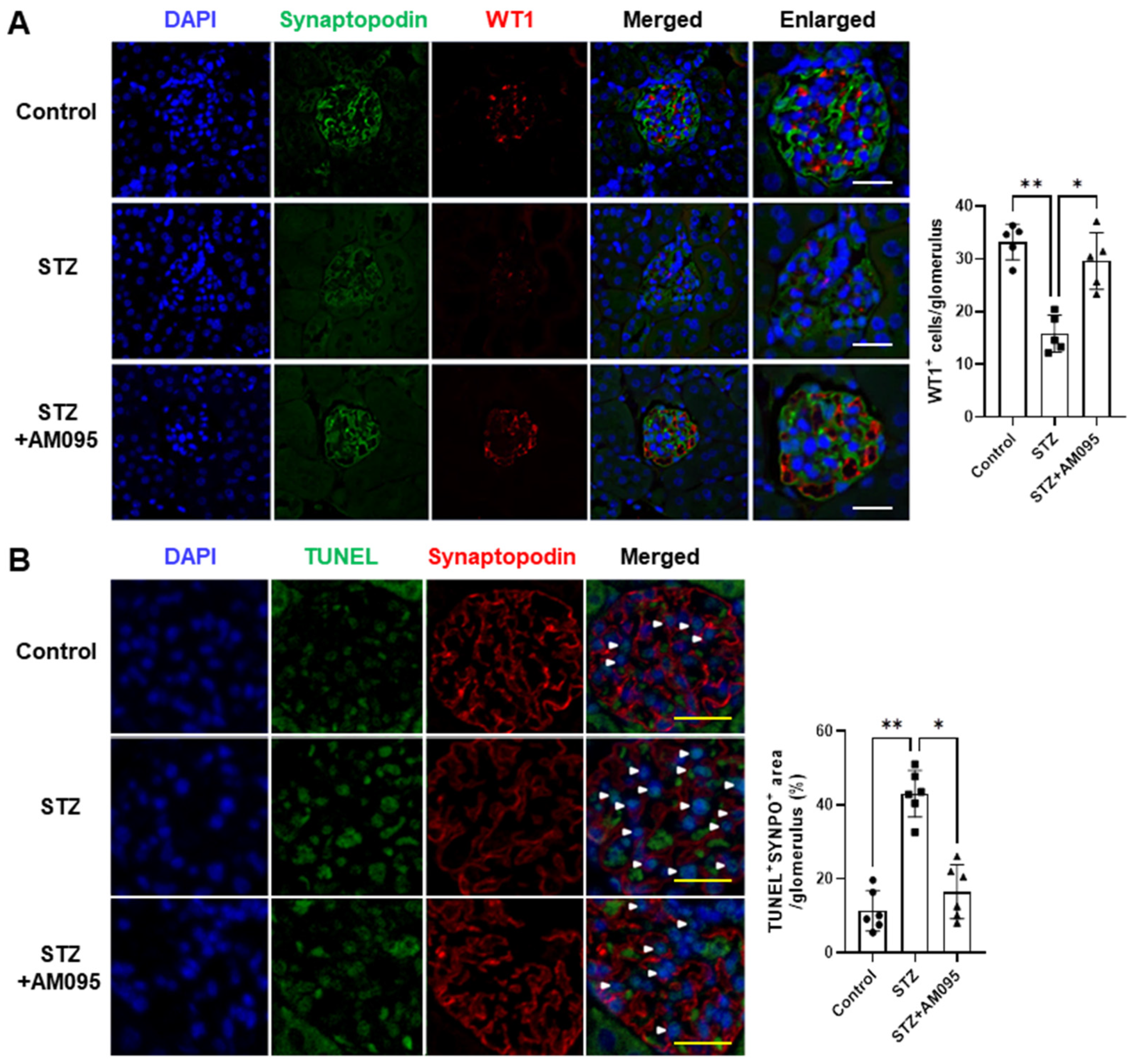
2.1. AM095 Inhibits Podocyte Loss and Death in the Kidney of STZ-Induced Diabetic Mice
2.2. AM095 Inhibits the Increased Expression of NLRP3 Inflammasome Factors in Podocytes of STZ-Induced Diabetic Mice
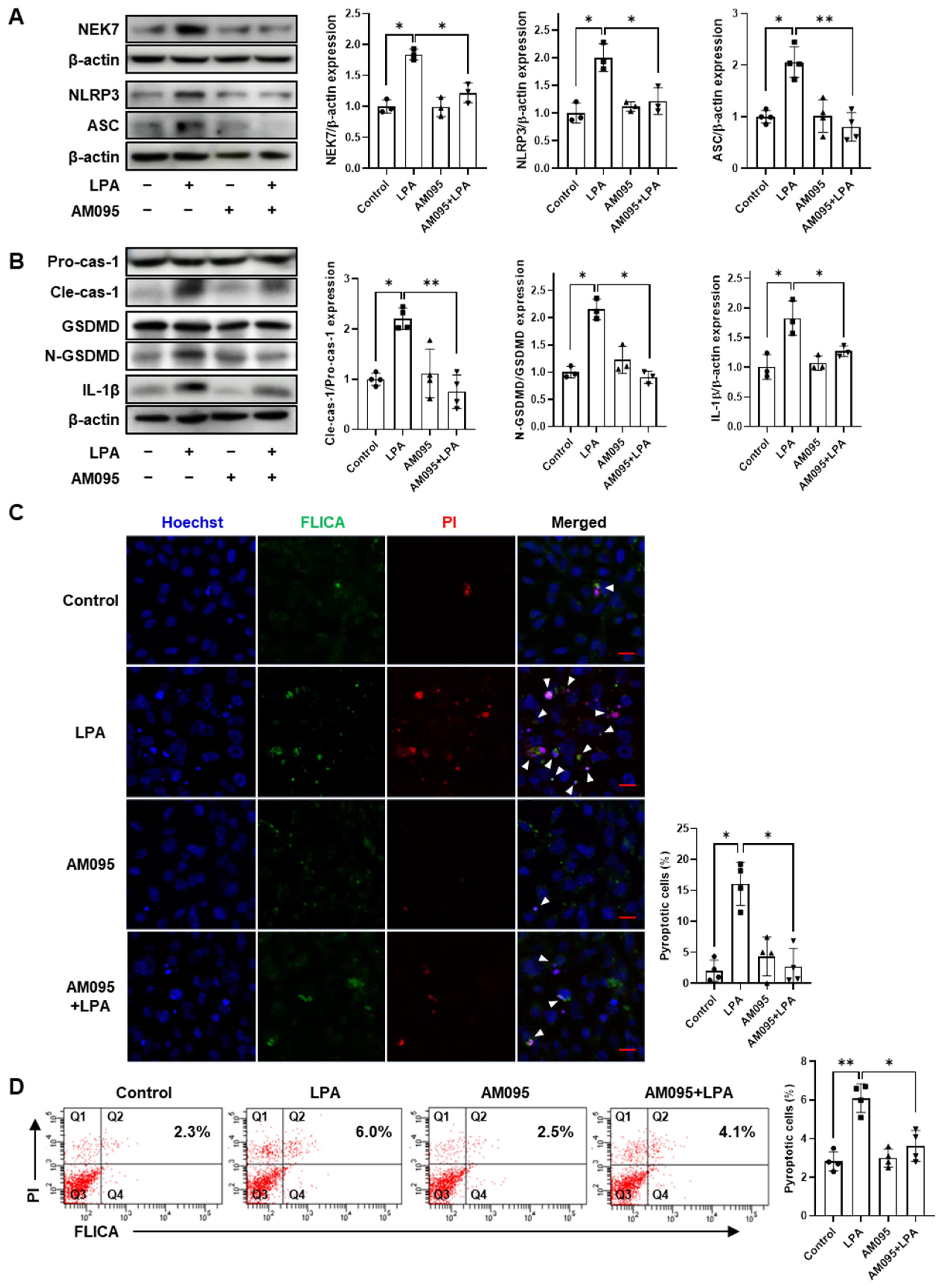
2.3. AM095 Inhibits LPA-Induced Pyroptosis in E11 Cells
2.4. Egr1 Is Required for LPA-Induced Pyroptosis in E11 Cells

2.5. Egr1 Knockdown Decreases LPA-Induced Nuclear Factor-κB (NF-κB) Activation and Desmin Expression in E11 Cells
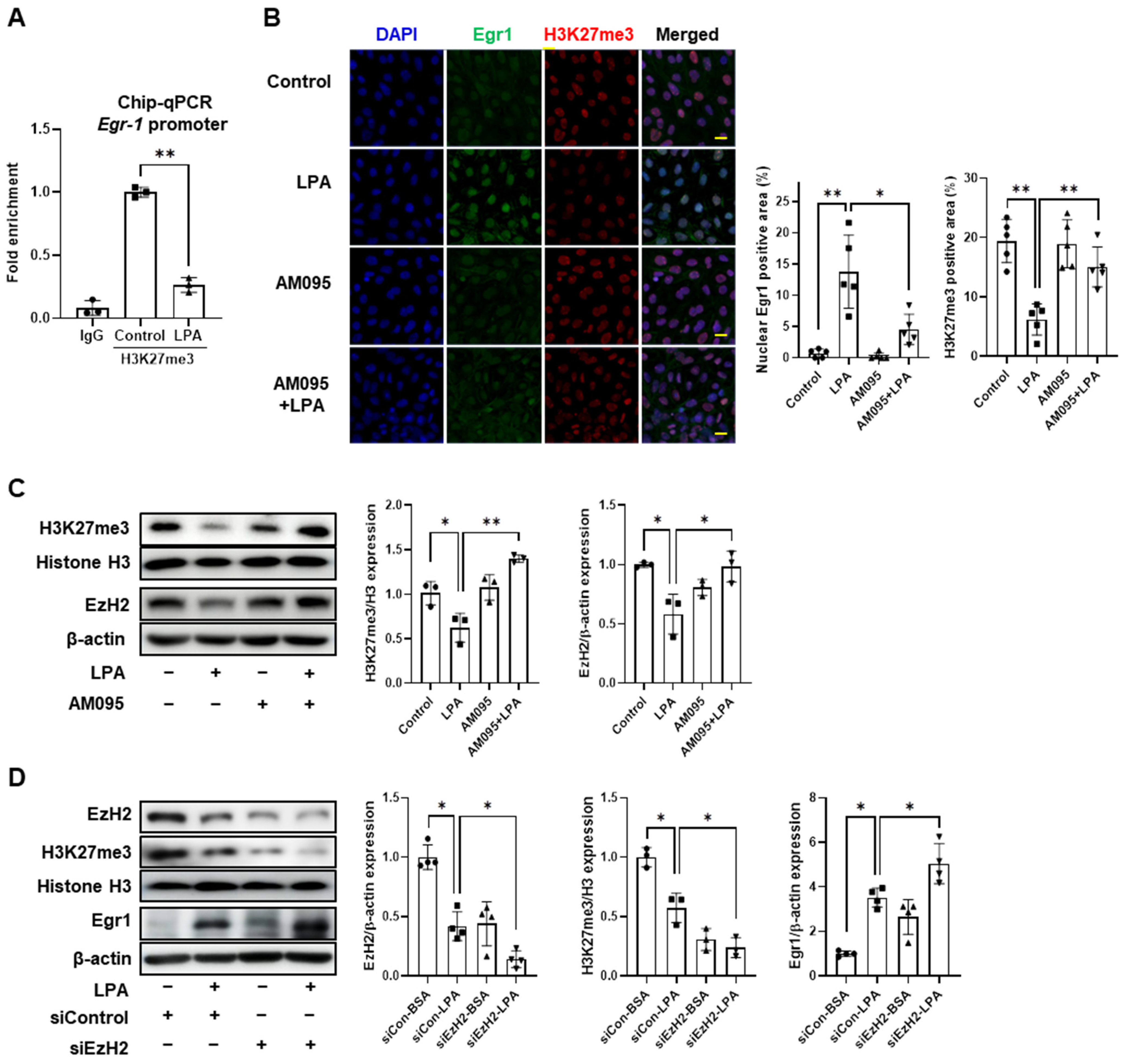
2.6. LPA Increased Egr1 Expression by Downregulation of EzH2 in E11 Cells
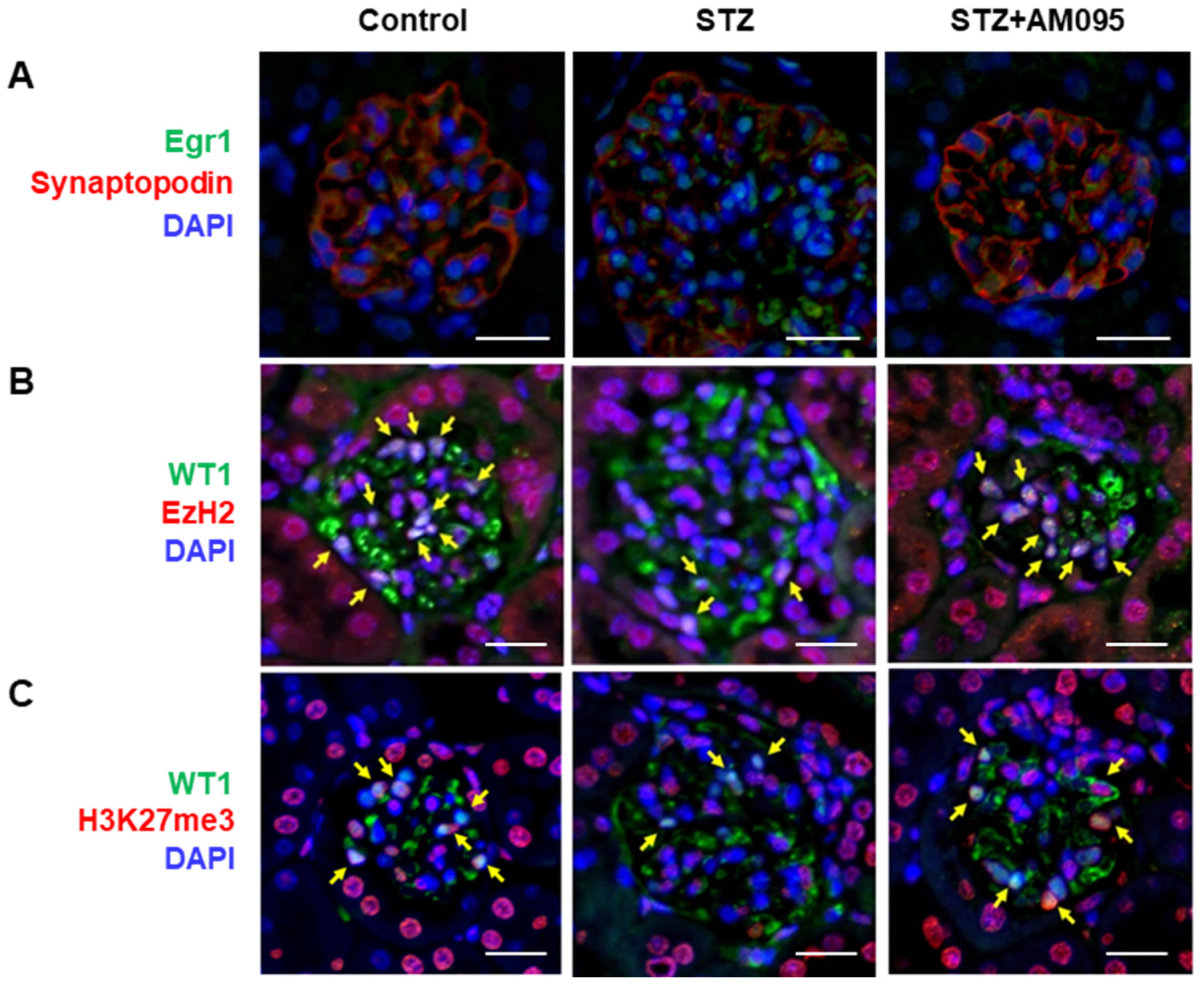
2.7. AM095 Decreases Egr1 Expression and Restores EzH2 and H3K27me3 Expression in Podocytes from STZ-Induced Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Immunohistochemistry (IHC) and Immunocytochemistry
4.3. TUNEL Assay
4.4. Cell Culture and Treatment
4.5. Transfection of E11 Podocytes
4.6. Western Blot Analysis
4.7. Determination of Pyroptosis
4.8. Reverse Transcription-Quantitative PCR (RT-qPCR) Analysis
4.9. Chromatin Immunoprecipitation (ChIP)-qPCR Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregg, E.W.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States. N. Engl. J. Med. 2014, 371, 286–287. [Google Scholar] [PubMed]
- Kainz, A.; Hronsky, M.; Stel, V.S.; Jager, K.J.; Geroldinger, A.; Dunkler, D.; Heinze, G.; Tripepi, G.; Oberbauer, R. Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol. Dial. Transplant. 2015, 30, iv113–iv118. [Google Scholar]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [PubMed]
- Liu, S.; Yuan, Y.; Xue, Y.; Xing, C.; Zhang, B. Podocyte Injury in Diabetic Kidney Disease: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2022, 10, 832887. [Google Scholar] [PubMed]
- Spurney, R.F.; Coffman, T.M. Stressed-out podocytes in diabetes? J. Am. Soc. Nephrol. 2008, 19, 2035–2037. [Google Scholar] [CrossRef]
- Moolenaar, W.H.; van Meeteren, L.A.; Giepmans, B.N. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004, 26, 870–881. [Google Scholar]
- Pages, C.; Simon, M.F.; Valet, P.; Saulnier-Blache, J.S. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001, 64, 1–10. [Google Scholar]
- Geraldo, L.H.M.; Spohr, T.; Amaral, R.F.D.; Fonseca, A.; Garcia, C.; Mendes, F.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of lysophosphatidic acid and its receptors in health and disease: Novel therapeutic strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar]
- Grove, K.J.; Voziyan, P.A.; Spraggins, J.M.; Wang, S.; Paueksakon, P.; Harris, R.C.; Hudson, B.G.; Caprioli, R.M. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J. Lipid Res. 2014, 55, 1375–1385. [Google Scholar]
- Saulnier-Blache, J.S.; Feigerlova, E.; Halimi, J.M.; Gourdy, P.; Roussel, R.; Guerci, B.; Dupuy, A.; Bertrand-Michel, J.; Bascands, J.L.; Hadjadj, S.; et al. Urinary lysophopholipids are increased in diabetic patients with nephropathy. J. Diabetes Complicat. 2017, 31, 1103–1108. [Google Scholar]
- Lee, J.H.; Khin, P.P.; Lee, G.; Lim, O.K.; Jun, H.S. Effect of BBT-877, a novel inhibitor of ATX, on a mouse model of type 1 diabetic nephropathy. Aging 2022, 14, 6467–6480. [Google Scholar]
- Lee, J.H.; Sarker, M.K.; Choi, H.; Shin, D.; Kim, D.; Jun, H.S. Lysophosphatidic acid receptor 1 inhibitor, AM095, attenuates diabetic nephropathy in mice by downregulation of TLR4/NF-kappaB signaling and NADPH oxidase. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1332–1340. [Google Scholar] [PubMed]
- Li, H.Y.; Oh, Y.S.; Choi, J.W.; Jung, J.Y.; Jun, H.S. Blocking lysophosphatidic acid receptor 1 signaling inhibits diabetic nephropathy in db/db mice. Kidney Int. 2017, 91, 1362–1373. [Google Scholar]
- Zhang, M.Z.; Wang, X.; Yang, H.; Fogo, A.B.; Murphy, B.J.; Kaltenbach, R.; Cheng, P.; Zinker, B.; Harris, R.C. Lysophosphatidic Acid Receptor Antagonism Protects against Diabetic Nephropathy in a Type 2 Diabetic Model. J. Am. Soc. Nephrol. 2017, 28, 3300–3311. [Google Scholar] [PubMed]
- Swaney, J.S.; Chapman, C.; Correa, L.D.; Stebbins, K.J.; Broadhead, A.R.; Bain, G.; Santini, A.M.; Darlington, J.; King, C.D.; Baccei, C.S.; et al. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J. Pharmacol. Exp. Ther. 2011, 336, 693–700. [Google Scholar] [PubMed]
- Castelino, F.V.; Seiders, J.; Bain, G.; Brooks, S.F.; King, C.D.; Swaney, J.S.; Lorrain, D.S.; Chun, J.; Luster, A.D.; Tager, A.M. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011, 63, 1405–1415. [Google Scholar] [PubMed]
- Naruse, T.; Otake, H.; Takahashi, T. Effects of a lysophosphatidic acid receptor 1 antagonist on hypertensive renal injury in Dahl-Iwai salt-sensitive rats. J. Pharmacol. Sci. 2022, 149, 179–188. [Google Scholar]
- Duran-Salgado, M.B.; Rubio-Guerra, A.F. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393–398. [Google Scholar]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar]
- Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Gadi, I.; Kohli, S.; Nazir, S.; Ghosh, S.; Ranjan, S.; Wang, H.; Madhusudhan, T.; et al. Caspase-1, but Not Caspase-3, Promotes Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2270–2275. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M.; et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; He, F.F.; Tang, H.; Lei, C.T.; Chen, S.; Meng, X.F.; Su, H.; Zhang, C. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015, 2015, 504761. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metab. Clin. Exp. 2021, 118, 154748. [Google Scholar] [CrossRef]
- Li, H.; Zhao, K.; Li, Y. Gasdermin D Protects Mouse Podocytes Against High-Glucose-Induced Inflammation and Apoptosis via the C-Jun N-Terminal Kinase (JNK) Pathway. Med. Sci. Monit. 2021, 27, e928411. [Google Scholar] [CrossRef]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Gruden, G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin. Sci. 2022, 136, 493–520. [Google Scholar] [CrossRef]
- Sborgi, L.; Ruhl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Muller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Hu, F.; Xue, M.; Li, Y.; Jia, Y.J.; Zheng, Z.J.; Yang, Y.L.; Guan, M.P.; Sun, L.; Xue, Y.M. Early Growth Response 1 (Egr1) Is a Transcriptional Activator of NOX4 in Oxidative Stress of Diabetic Kidney Disease. J. Diabetes Res. 2018, 2018, 3405695. [Google Scholar] [CrossRef]
- Shahrestanifar, M.; Fan, X.; Manning, D.R. Lysophosphatidic acid activates NF-kappaB in fibroblasts. A requirement for multiple inputs. J. Biol. Chem. 1999, 274, 3828–3833. [Google Scholar] [CrossRef]
- Cummings, R.; Zhao, Y.; Jacoby, D.; Spannhake, E.W.; Ohba, M.; Garcia, J.G.; Watkins, T.; He, D.; Saatian, B.; Natarajan, V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J. Biol. Chem. 2004, 279, 41085–41094. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Govind, D.; Becker, J.U.; Miecznikowski, J.; Rosenberg, A.Z.; Dang, J.; Tharaux, P.L.; Yacoub, R.; Thaiss, F.; Hoyer, P.F.; Manthey, D.; et al. PodoSighter: A Cloud-Based Tool for Label-Free Podocyte Detection in Kidney Whole-Slide Images. J. Am. Soc. Nephrol. 2021, 32, 2795–2813. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.J.; Mishra, R.; Schneider, K.S.; Medard, G.; Wettmarshausen, J.; Dittlein, D.C.; Shi, H.; Gorka, O.; Koenig, P.A.; Fromm, S.; et al. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 2016, 45, 761–773. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Wang, Q.; Yuan, L. NEK7: A potential therapy target for NLRP3-related diseases. Biosci. Trends 2020, 14, 74–82. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Kim, D.; Li, H.Y.; Lee, J.H.; Oh, Y.S.; Jun, H.S. Lysophosphatidic acid increases mesangial cell proliferation in models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Guan, M.P.; Zheng, Z.J.; Li, W.Q.; Lyv, F.P.; Pang, R.Y.; Xue, Y.M. Transcription Factor Egr1 is Involved in High Glucose-Induced Proliferation and Fibrosis in Rat Glomerular Mesangial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C.; Sung, J.M.; Shen, Y.T.; Jheng, H.F.; Chen, S.H.; Tsai, P.J.; Tsai, Y.S. Egr-1 deficiency protects from renal inflammation and fibrosis. J. Mol. Med. 2016, 94, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; McCormick, C.; Cheng, Z. Early Growth Response 1 Deficiency Protects the Host against Pseudomonas aeruginosa Lung Infection. Infect. Immun. 2019, 88, e00678-19. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Y.S.; Dai, C.; Kiss, L.P.; Wen, X.; Liu, Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 2008, 172, 299–308. [Google Scholar] [CrossRef]
- Song, Z.; Xiao, C.; Jia, X.; Luo, C.; Shi, L.; Xia, R.; Zhu, J.; Zhang, S. Vitamin D/VDR Protects Against Diabetic Kidney Disease by Restoring Podocytes Autophagy. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.; Shao, N.; Gao, P.; Tang, H.; Su, H.; Zhang, C.; Meng, X.F. The Expression and Significance of NLRP3 Inflammasome in Patients with Primary Glomerular Diseases. Kidney Blood Press. Res. 2015, 40, 344–354. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, H.; Zhang, Z.; Shao, S.; Liu, D.; Li, S.; Yan, Y. Transcription factor EGR1 promotes differentiation of bovine skeletal muscle satellite cells by regulating MyoG gene expression. J. Cell. Physiol. 2018, 233, 350–362. [Google Scholar] [CrossRef]
- Bottinger, E.P. TGF-beta in renal injury and disease. Semin. Nephrol. 2007, 27, 309–320. [Google Scholar] [CrossRef]
- Nemeth, A.; Mozes, M.M.; Calvier, L.; Hansmann, G.; Kokeny, G. The PPARgamma agonist pioglitazone prevents TGF-beta induced renal fibrosis by repressing EGR-1 and STAT3. BMC Nephrol. 2019, 20, 245. [Google Scholar] [CrossRef]
- Xu, P.; Zhan, H.; Zhang, R.; Xu, X.J.; Zhang, Y.; Le, Y.; Bi, J.G. Early growth response factor 1 upregulates pro-fibrotic genes through activation of TGF-beta1/Smad pathway via transcriptional regulation of PAR1 in high-glucose treated HK-2 cells. Mol. Cell. Endocrinol. 2023, 572, 111953. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Hao, J.; Zhou, Y.; Liu, W. High glucose increases Cdk5 activity in podocytes via transforming growth factor-beta1 signaling pathway. Exp. Cell Res. 2014, 326, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Huang, S.; Shen, L.; Tang, Y.; Li, H.; Shi, Y. Long noncoding RNA NONHSAG053901 promotes diabetic nephropathy via stimulating Egr-1/TGF-beta-mediated renal inflammation. J. Cell. Physiol. 2019, 234, 18492–18503. [Google Scholar] [CrossRef] [PubMed]
- Raneros, A.B.; Bernet, C.R.; Florez, A.B.; Suarez-Alvarez, B. An Epigenetic Insight into NLRP3 Inflammasome Activation in Inflammation-Related Processes. Biomedicines 2021, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kaang, B.K. Epigenetic regulation and chromatin remodeling in learning and memory. Exp. Mol. Med. 2017, 49, e281. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Ross, P.J. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian development. Epigenetics 2012, 7, 976–981. [Google Scholar] [CrossRef]
- Han, D.; Huang, M.; Wang, T.; Li, Z.; Chen, Y.; Liu, C.; Lei, Z.; Chu, X. Lysine methylation of transcription factors in cancer. Cell Death Dis. 2019, 10, 290. [Google Scholar] [CrossRef]
- Liebisch, M.; Wolf, G. AGE-Induced Suppression of EZH2 Mediates Injury of Podocytes by Reducing H3K27me3. Am. J. Nephrol. 2020, 51, 676–692. [Google Scholar] [CrossRef]
- Siddiqi, F.S.; Majumder, S.; Thai, K.; Abdalla, M.; Hu, P.; Advani, S.L.; White, K.E.; Bowskill, B.B.; Guarna, G.; Dos Santos, C.C.; et al. The Histone Methyltransferase Enzyme Enhancer of Zeste Homolog 2 Protects against Podocyte Oxidative Stress and Renal Injury in Diabetes. J. Am. Soc. Nephrol. 2016, 27, 2021–2034. [Google Scholar] [CrossRef]
- Komers, R.; Mar, D.; Denisenko, O.; Xu, B.; Oyama, T.T.; Bomsztyk, K. Epigenetic changes in renal genes dysregulated in mouse and rat models of type 1 diabetes. Lab. Investig. A J. Tech. Methods Pathol. 2013, 93, 543–552. [Google Scholar] [CrossRef]
- Majumder, S.; Thieme, K.; Batchu, S.N.; Alghamdi, T.A.; Bowskill, B.B.; Kabir, M.G.; Liu, Y.; Advani, S.L.; White, K.E.; Geldenhuys, L.; et al. Shifts in podocyte histone H3K27me3 regulate mouse and human glomerular disease. J. Clin. Investig. 2018, 128, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hung, M.C. Regulation and Role of EZH2 in Cancer. Cancer Res. Treat. 2014, 46, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Lin, C.Y.; Liao, T.W.; Peng, C.M.; Lee, S.C.; Liu, Y.J.; Chan, W.P.; Chou, R.H. EZH2 in Cancer Progression and Potential Application in Cancer Therapy: A Friend or Foe? Int. J. Mol. Sci. 2017, 18, 1172. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kaneko, S.; Yanai, K.; Aomatsu, A.; Hirai, K.; Ookawara, S.; Ishibashi, K.; Morishita, Y. MicroRNAs in Podocyte Injury in Diabetic Nephropathy. Front. Genet. 2020, 11, 993. [Google Scholar] [CrossRef]
- Sahay, D.; Leblanc, R.; Grunewald, T.G.; Ambatipudi, S.; Ribeiro, J.; Clezardin, P.; Peyruchaud, O. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget 2015, 6, 20604–20620. [Google Scholar] [CrossRef]
- Kim, D.; Nam, G.Y.; Seo, E.; Jun, H.S. Inhibition of ChREBP ubiquitination via the ROS/Akt-dependent downregulation of Smurf2 contributes to lysophosphatidic acid-induced fibrosis in renal mesangial cells. J. Biomed. Sci. 2022, 29, 31. [Google Scholar] [CrossRef]
- Gao, J.; Peng, S.; Shan, X.; Deng, G.; Shen, L.; Sun, J.; Jiang, C.; Yang, X.; Chang, Z.; Sun, X.; et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019, 10, 957. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.J.; Baek, J.O.; Roh, J.Y.; Jun, H.S. Lysophosphatidic Acid Mediates Imiquimod-Induced Psoriasis-like Symptoms by Promoting Keratinocyte Proliferation through LPAR1/ROCK2/PI3K/AKT Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 10777. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Ma, R.; Jiang, Y.; Liu, J.; Lin, Y.; Chen, S.; Xia, M.; Zou, F.; Zhang, J.; et al. UHRF1 Controls Thymocyte Fate Decisions through the Epigenetic Regulation of EGR1 Expression. J. Immunol. 2020, 204, 3248–3261. [Google Scholar] [CrossRef]


| Antibody | Company | Cat. No. | Dilution Ratio |
|---|---|---|---|
| β-actin (C4) | Santa Cruz Biotechnology (Dallas, TX, USA) | SC-47778 | WB 1:3000 |
| Egr-1 (C-19) (rabbit) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-189 | WB 1:1000; IFA 1:200 |
| Egr-1 (B-6) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-515830 | IFA 1:200 |
| Human ZIF268/EGR1 Antibody | LSBio (Lynnwood, WA, USA) | LS-C104940-100 | IFA 1:400 |
| Desmin (RD301) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-23879 | WB 1:1000; IFA 1:200 |
| Caspase-1 | Enzo Life Sciences (Farmingdale, NY, USA) | ALX-210-804-C100 | WB 1:1300 |
| IL-1β | Santa Cruz Biotechnology (Dallas, TX, USA) | SC-52012 | WB 1:1000 |
| Ezh2 (D2C9) | CST (Danvers, MA, USA) | 5246 | WB 1:2000; IFA 1:200 |
| Tri-Methyl-Histone H3 (Lys27) (C36B11) (rabbit) | CST (Danvers, MA, USA) | 9733S | WB 1:2000; IFA 1:200 |
| Histone H3 (D1H2) XP® (rabbit) | CST (Danvers, MA, USA) | 4499S | WB 1:2000 |
| SYNPO Polyclonal Antibody | Invitrogen (Waltham, MA, USA) | PA5-56997 | WB 1:1000; IFA 1:400 |
| WT1(C-19) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-192 | WB 1:500; IFA 1:100 |
| NEK7 (A-12) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-398439 | WB 1:1000; IFA 1:100 |
| NLRP3/NALP3 Antibody—BSA Free (rabbit) | Novus Biologicals (Littleton, CO, USA) | NBP2-12446 | WB 1:2000 |
| Human/Mouse NLRP3/NALP3 Antibody (rat) | R&D system (Minnesota, MN, USA) | MAB7578 | IFA 1:200 |
| ASC (B-3) | Santa Cruz Biotechnology (Dallas, TX, USA) | sc-514414 | WB 1:1000; IFA 1:100 |
| GSDMDC1 | Novus Biologicals (Littleton, CO, USA) | NBP2-33422 | WB 1:1000 |
| Phospho-NF-κB p65 (Ser536) (93H1) (rabbit) | CST (Danvers, MA, USA) | 3033 | WB 1:2000 |
| NF-κB p65 (C22B4) (rabbit) | CST (Danvers, MA, USA) | 4764 | WB 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Ban, K.-Y.; Lee, G.-H.; Jun, H.-S. Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2. Int. J. Mol. Sci. 2023, 24, 9968. https://doi.org/10.3390/ijms24129968
Kim D, Ban K-Y, Lee G-H, Jun H-S. Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2. International Journal of Molecular Sciences. 2023; 24(12):9968. https://doi.org/10.3390/ijms24129968
Chicago/Turabian StyleKim, Donghee, Ka-Yun Ban, Geon-Ho Lee, and Hee-Sook Jun. 2023. "Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2" International Journal of Molecular Sciences 24, no. 12: 9968. https://doi.org/10.3390/ijms24129968
APA StyleKim, D., Ban, K.-Y., Lee, G.-H., & Jun, H.-S. (2023). Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2. International Journal of Molecular Sciences, 24(12), 9968. https://doi.org/10.3390/ijms24129968






