Activation of Gq-Coupled Receptors in Astrocytes Restores Cognitive Function in Alzheimer’s Disease Mice Model
Abstract
:1. Introduction
2. Results
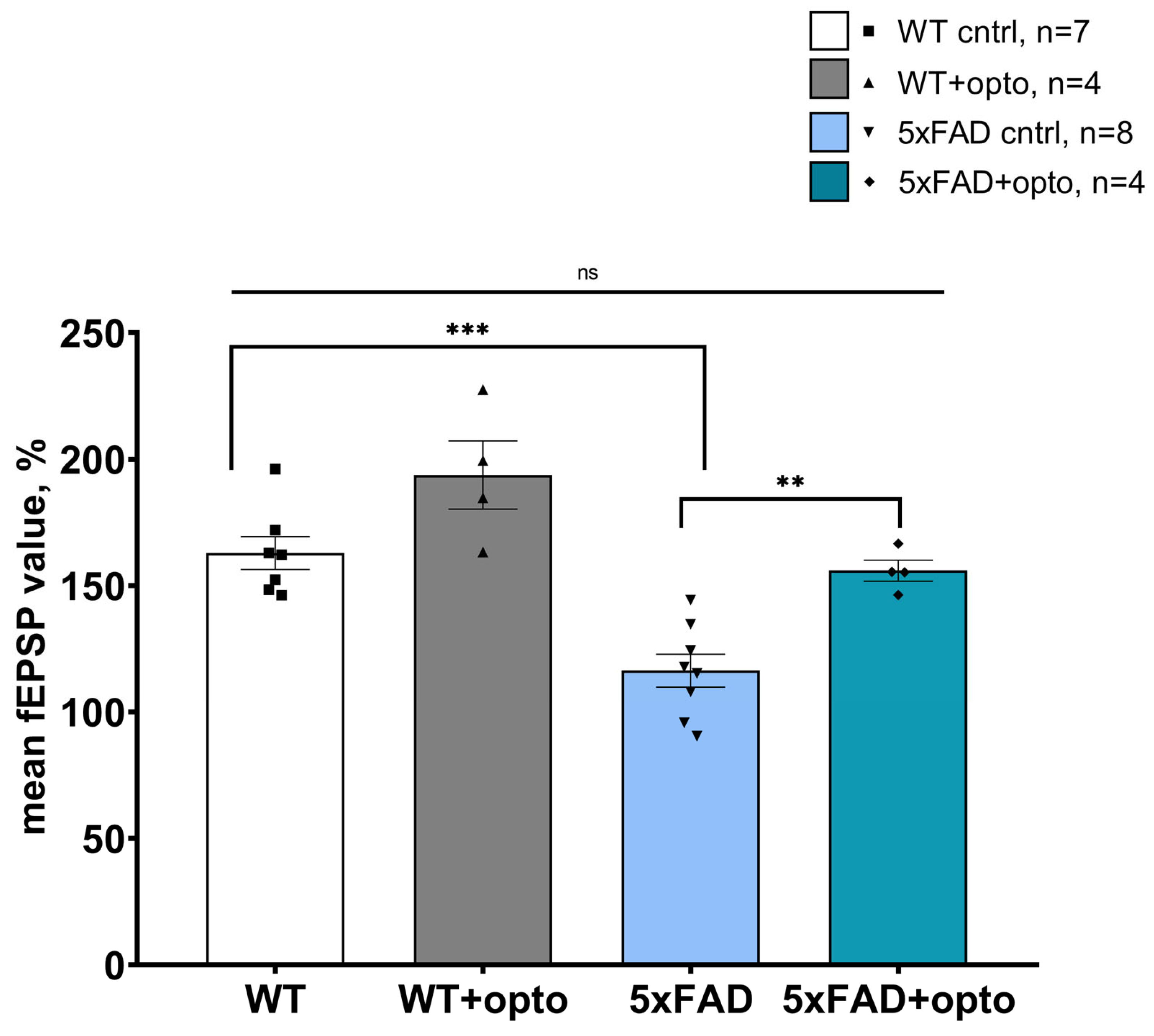
2.1. Optogenetic Activation of Astrocytes Expressing OPTO-α1AR-EYFP in Hippocampus Restores LTP Formation in 5xFAD Mice
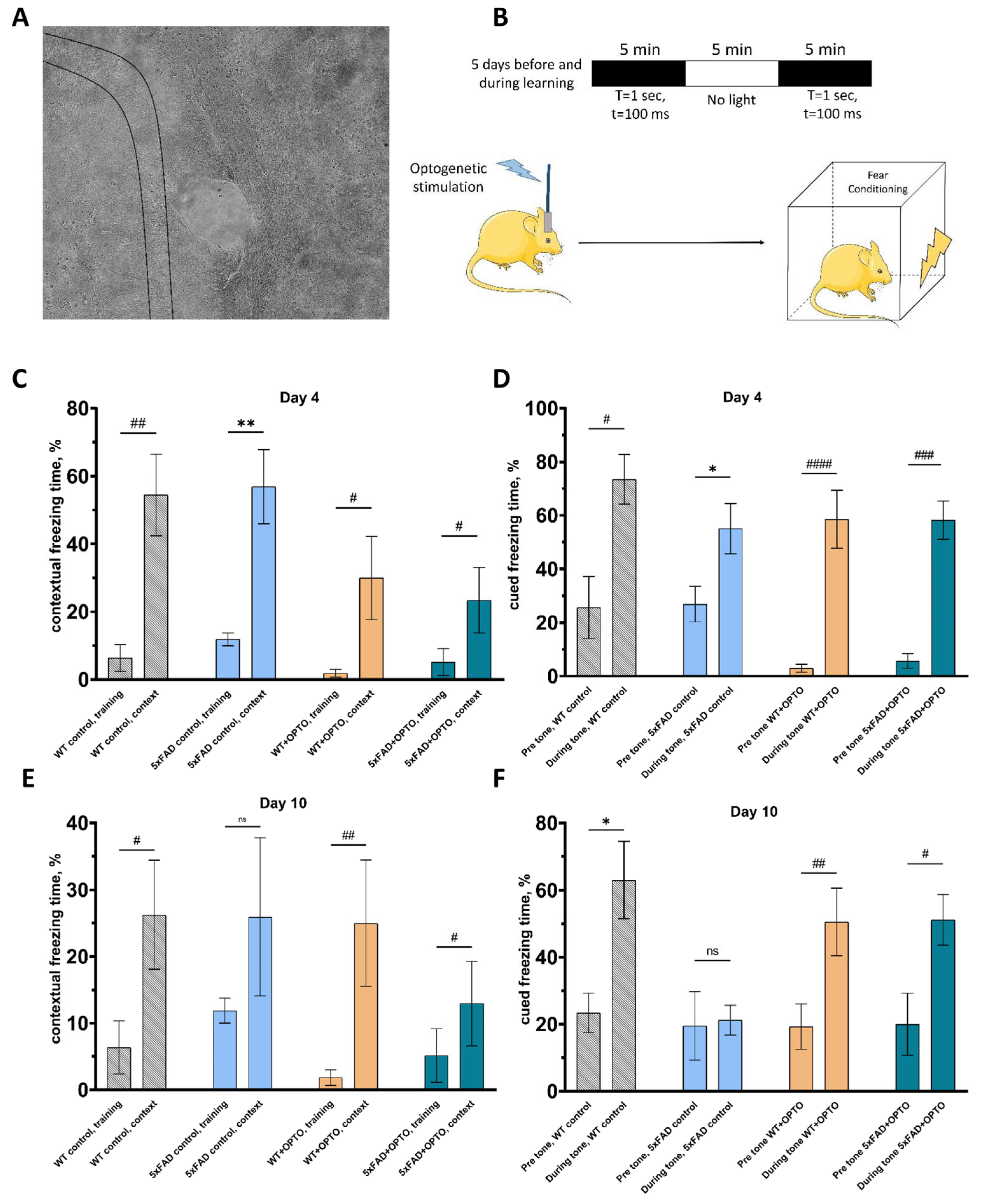
2.2. Modulation of Astrocytic Activity through Gq Pathway Beneficially Impacts on Cognitive Function of 5xFAD Mice
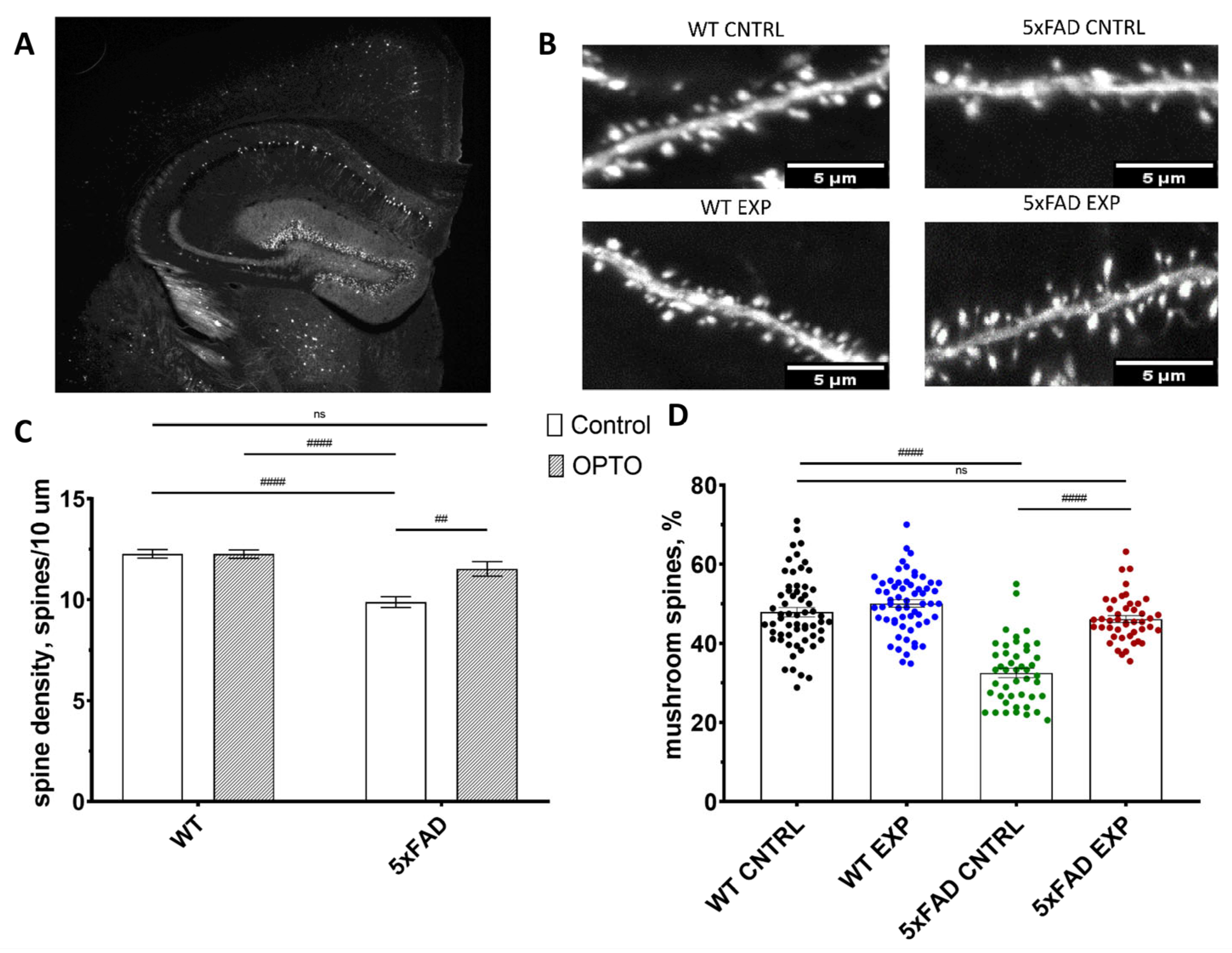
2.3. Optogenetic Stimulation of OPTO-α1AR Expressed in Astrocytes Increase Mushroom Spine Density in 5xFAD Mice
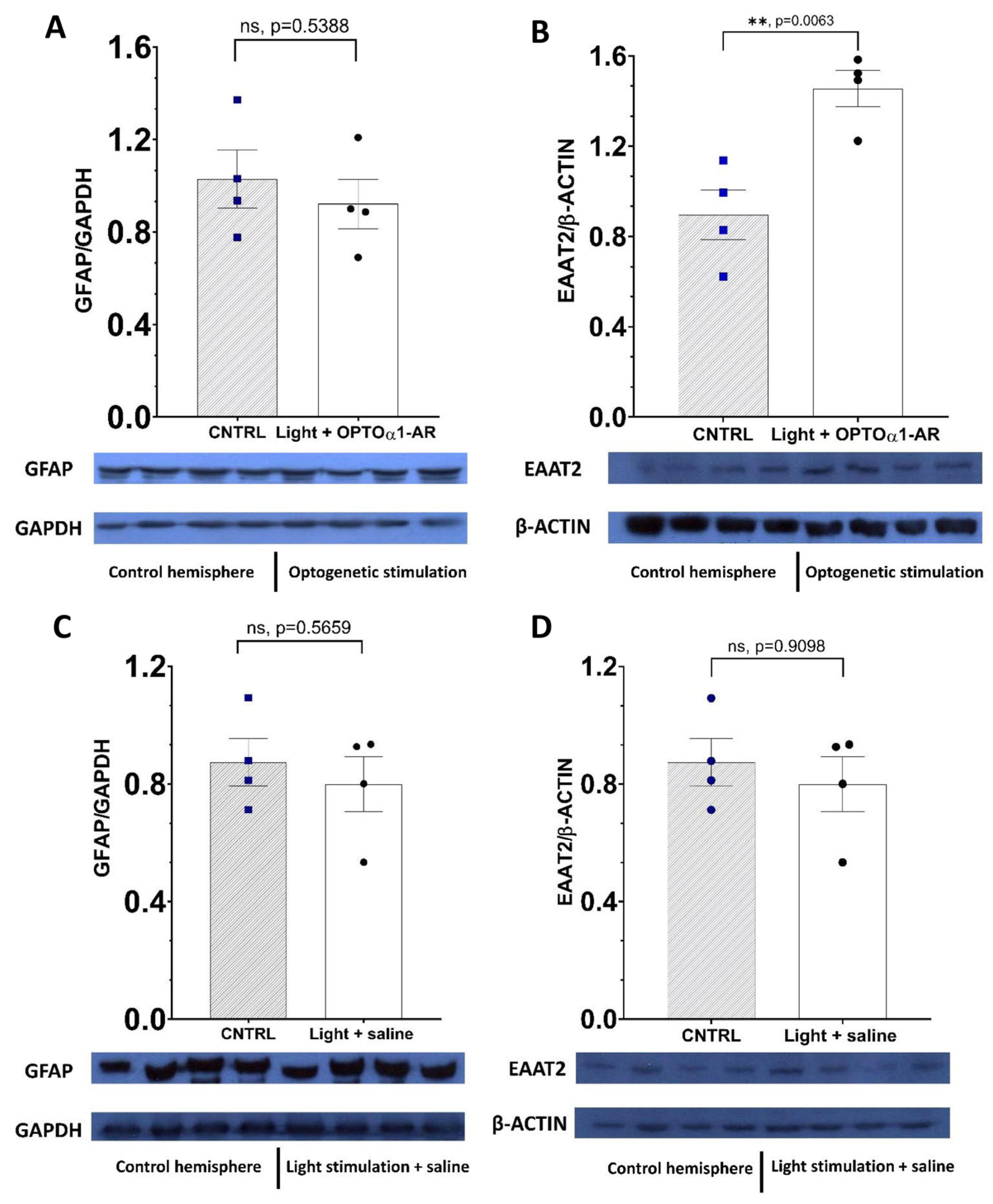
2.4. Chronic Optogenetic Stimulation of Hippocampal Astrocytes Doesn’t Transform Them into Reactive Ones
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Viral Constructs Delivery via Stereotaxic Surgery
4.3. Long-Term Potentiation Recording
4.4. Optogenetic Stimulation of Astrocytic Activity In Vivo
4.5. Fixed Slices Preparation and Evaluation of Density and Morphology of Dendritic Spines
4.6. Fear Conditioning Behavioral Testing
4.7. Morris Water Maze Behavioral Test
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simard, M.; Nedergaard, M. The Neurobiology of Glia in the Context of Water and Ion Homeostasis. Neuroscience 2004, 129, 877–896. [Google Scholar] [CrossRef]
- Pannasch, U.; Rouach, N. Emerging Role for Astroglial Networks in Information Processing: From Synapse to Behavior. Trends Neurosci. 2013, 36, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Semyanov, A.; Verkhratsky, A. Inclusive Brain: From Neuronal Doctrine to the Active Milieu. Function 2022, 3, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Song, D.; Xie, Z.; He, G.; Zhao, J.; Wang, Z.; Dong, Z.; Zhang, H.; Yang, L.; Jiang, M.; et al. Optogenetic Stimulation of CA3 Pyramidal Neurons Restores Synaptic Deficits to Improve Spatial Short-Term Memory in APP/PS1 Mice. Prog. Neurobiol. 2022, 209, 102209. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, E.; Erofeev, A.; Borodinova, A.; Bolshakova, A.; Balaban, P.; Bezprozvanny, I.; Vlasova, O.L. Optogenetic Activation of Astrocytes-Effects on Neuronal Network Function. Int. J. Mol. Sci. 2021, 22, 9613. [Google Scholar] [CrossRef]
- Haydon, P.G.; Nedergaard, M. How Do Astrocytes Participate in Neural Plasticity? Cold Spring Harb. Perspect. Biol. 2015, 7, a020438. [Google Scholar] [CrossRef] [Green Version]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite Synapses: Astrocytes Process and Control Synaptic Information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Guerra-Gomes, S.; Sousa, N.; Pinto, L.; Oliveira, J.F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Kuga, N.; Sasaki, T.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Large-Scale Calcium Waves Traveling through Astrocytic Networks in Vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Maekawa, S.; Morita, M. Astrocyte Calcium Waves Propagate Proximally by Gap Junction and Distally by Extracellular Diffusion of ATP Released from Volume-Regulated Anion Channels. Sci. Rep. 2017, 7, 13115. [Google Scholar] [CrossRef] [Green Version]
- Scemes, E.; Giaume, C. Astrocyte Calcium Waves: What They Are and What They Do. Glia 2006, 54, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, H.; Ye, C.; Ge, W.; Chen, Y.; Jiang, Z.; Wu, C.; Poo, M.; Duan, S. ATP Released by Astrocytes Mediates Glutamatergic Activity-Dependent Heterosynaptic Suppression. Neuron 2003, 40, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Prolonged Febrile Seizures Impair Synaptic Plasticity and Alter Developmental Pattern of Glial Fibrillary Acidic Protein (GFAP) -Immunoreactive Astrocytes in the Hippocampus of Young Rats. Int. J. Mol. Sci. 2022, 23, 12224. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.; Ronco, V.; Grolla, A.A.; Verkhratsky, A.; Genazzani, A.A. Glial Calcium Signalling in Alzheimer’s Disease. Rev. Physiol. Biochem. Pharmacol. 2014, 167, 45–66. [Google Scholar] [CrossRef]
- Åbjørsbråten, K.S.; Skaaraas, G.H.E.S.; Cunen, C.; Bjørnstad, D.M.; Binder, K.M.G.; Bojarskaite, L.; Jensen, V.; Nilsson, L.N.G.; Rao, S.B.; Tang, W.; et al. Impaired Astrocytic Ca2+ Signaling in Awake-Behaving Alzheimer’s Disease Transgenic Mice. eLife 2022, 11, e75055. [Google Scholar] [CrossRef]
- Verkhratsky, A. Astroglial Calcium Signaling in Aging and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2019, 11, a035188. [Google Scholar] [CrossRef]
- Murphy, M.P.; Levine, H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics 2015, 12, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque Formation and the Intraneuronal Accumulation of β-Amyloid in Alzheimer’s Disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, Tangles, and Alzheimer’s Disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2005, 1739, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium Signaling and Molecular Mechanisms Underlying Neurodegenerative Diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef]
- Popugaeva, E.; Chernyuk, D.; Bezprozvanny, I. Reversal of Calcium Dysregulation as Potential Approach for Treating Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 344–354. [Google Scholar] [CrossRef]
- Homeostasis, C.; Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munaf, A.; Bernardini, R. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar]
- Bezprozvanny, I. Biochemical and Biophysical Research Communications Alzheimer’ s Disease e Where Do We Go from Here? Biochem. Biophys. Res. Commun. 2022, 633, 72–76. [Google Scholar] [CrossRef]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-Associated Astrocytes in Alzheimer’s Disease and Aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Monterey, M.D.; Wei, H.; Wu, X.; Wu, J.Q. The Many Faces of Astrocytes in Alzheimer’s Disease. Front. Neurol. 2021, 12, 619626. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhao, R.; Gao, K.; Wei, Z.; Yaoyao Yin, M.; Ting Lau, L.; Chui, D.; Cheung Hoi Yu, A. Astrocytes: Implications for Neuroinflammatory Pathogenesis of Alzheimers Disease. Curr. Alzheimer Res. 2011, 8, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Verkhratsky, A.; Olabarria, M.; Noristani, H.N.; Yeh, C.; Rodriguez, J.J. Astrocytes in Alzheimer’s Disease. Neurotherapeutics 2010, 7, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Mederos, S.; Hernández-Vivanco, A.; Ramírez-Franco, J.; Martín-Fernández, M.; Navarrete, M.; Yang, A.; Boyden, E.S.; Perea, G. Melanopsin for Precise Optogenetic Activation of Astrocyte-Neuron Networks. Glia 2019, 67, 915–934. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [Green Version]
- Maltsev, A.; Roshchin, M.; Bezprozvanny, I.; Smirnov, I.; Vlasova, O.; Balaban, P.; Borodinova, A. Bidirectional Regulation by “Star Forces”: Ionotropic Astrocyte’s Optical Stimulation Suppresses Synaptic Plasticity, Metabotropic One Strikes Back. Hippocampus 2022, 33, 18–36. [Google Scholar] [CrossRef]
- Erofeev, A.; Gerasimov, E.; Lavrova, A.; Bolshakova, A.; Postnikov, E.; Bezprozvanny, I.; Vlasova, O.L. Light Stimulation Parameters Determine Neuron Dynamic Characteristics. Appl. Sci. 2019, 9, 3673. [Google Scholar] [CrossRef] [Green Version]
- Levskaya, A.; Weiner, O.D.; Lim, W.A.; Voigt, C.A. Spatiotemporal Control of Cell Signalling Using a Light-Switchable Protein Interaction. Nature 2009, 461, 997–1001. [Google Scholar] [CrossRef] [Green Version]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma Frequency Entrainment Attenuates Amyloid Load and Modifies Microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Courtney, C.D.; Sobieski, C.; Ramakrishnan, C.; Ingram, R.J.; Wojnowski, N.M.; DeFazio, R.A.; Deisseroth, K.; Christian-Hinman, C.A. Optogenetic Activation of Gq Signaling in Astrocytes Yields Stimulation-Specific Effects on Basal Hippocampal Synaptic Excitation and Inhibition. bioRxiv 2021. [Google Scholar] [CrossRef]
- Berndt, A.; Schoenenberger, P.; Mattis, J.; Tye, K.M.; Deisseroth, K.; Hegemann, P.; Oertner, T.G. High-Efficiency Channelrhodopsins for Fast Neuronal Stimulation at Low Light Levels. Proc. Natl. Acad. Sci. USA 2011, 108, 7595–7600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Airan, R.D.; Thompson, K.R.; Fenno, L.E.; Bernstein, H.; Deisseroth, K. Temporally Precise in Vivo Control of Intracellular Signalling. Nature 2009, 458, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Popugaeva, E.; Chernyuk, D.; Zhang, H.; Postnikova, T.Y.; Pats, K.; Fedorova, E.; Poroikov, V.; Zaitsev, A.V.; Bezprozvanny, I. Derivatives of Piperazines as Potential Therapeutic Agents for Alzheimer’s Disease. Mol. Pharmacol. 2019, 95, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouzin, N.; Baranger, K.; Cavalier, M.; Marchalant, Y.; Cohen-Solal, C.; Roman, F.S.; Khrestchatisky, M.; Rivera, S.; Féron, F.; Vignes, M. Area-Specific Alterations of Synaptic Plasticity in the 5XFAD Mouse Model of Alzheimer’s Disease: Dissociation between Somatosensory Cortex and Hippocampus. PLoS ONE 2013, 8, 4–12. [Google Scholar] [CrossRef]
- Hwang, K.D.; Bak, M.S.; Kim, S.J.; Rhee, S.; Lee, Y.S. Restoring Synaptic Plasticity and Memory in Mouse Models of Alzheimer’s Disease by PKR Inhibition. Mol. Brain 2017, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jo, J.; Song, J. Adiponectin Improves Long-Term Potentiation in the 5XFAD Mouse Brain. Sci. Rep. 2019, 9, 8918. [Google Scholar] [CrossRef] [Green Version]
- Kimura, R.; Ohno, M. Impairments in Remote Memory Stabilization Precede Hippocampal Synaptic and Cognitive Failures in 5XFAD Alzheimer Mouse Model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.J.; Mahmood, U.; Kim, H.; Choi, M.; Choi, Y.; Lee, J.P.; Chang, M.J.; Kim, H.S. Alterations in Protein Phosphorylation in the Amygdala of the 5XFamilial Alzheimer’s Disease Animal Model. J. Pharmacol. Sci. 2017, 133, 261–267. [Google Scholar] [CrossRef]
- Gür, E.; Fertan, E.; Alkins, K.; Wong, A.A.; Brown, R.E.; Balcı, F. Interval Timing Is Disrupted in Female 5xFAD Mice: An Indication of Altered Memory Processes. J. Neurosci. Res. 2019, 97, 817–827. [Google Scholar] [CrossRef]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef]
- Frankfurt, M.; Luine, V. The Evolving Role of Dendritic Spines and Memory: Interaction(s) with Estradiol. Horm. Behav. 2015, 74, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Pchitskaya, E.; Bezprozvanny, I. Dendritic Spines Shape Analysis—Classification or Clusterization? Perspective. Front. Synaptic Neurosci. 2020, 12, 31. [Google Scholar] [CrossRef]
- Popugaeva, E.; Pchitskaya, E.; Speshilova, A.; Alexandrov, S.; Zhang, H.; Vlasova, O.; Bezprozvanny, I. STIM2 Protects Hippocampal Mushroom Spines from Amyloid Synaptotoxicity. Mol. Neurodegener. 2015, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, L.; Pchitskaya, E.; Zakharova, O.; Saito, T.; Saido, T.; Bezprozvanny, I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-in Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 13275–13286. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.L.; Pozueta, J.; Gong, B.; Arancio, O.; Shelanski, M. Reversal of Long-Term Dendritic Spine Alterations in Alzheimer Disease Models. Proc. Natl. Acad. Sci. USA 2009, 106, 16877–16882. [Google Scholar] [CrossRef] [Green Version]
- Vorhees, C.V.; Williams, M.T. Morris Water Maze: Procedures for Assessing Spatial and Related Forms of Learning and Memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Vorhees, C.V.; Williams, M.T. Assessing Spatial Learning and Memory in Rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef] [Green Version]
- Redish, A.D.; Touretzky, D.S. The Role of the Hippocampus in Solving the Morris Water Maze. Neural Comput. 1998, 10, 73–111. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Chang, K.A. Spatial Memory Deficiency Early in 6xTg Alzheimer’s Disease Mouse Model. Sci. Rep. 2021, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ali, G.; Ahmad, N.; Akram, M.; Kumari, G.; Amin, M.U.; Umar, M.N. Attenuation of Spatial Memory in 5xfad Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals 2020, 13, 318. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. Visuo-Spatial Learning and Memory Impairments in the 5xFAD Mouse Model of Alzheimer’s Disease: Effects of Age, Sex, Albinism, and Motor Impairments. Genes Brain Behav. 2022, 21, e12794. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.C.; Hon, W.; Tyan, Y.M.; Liao, W.L. Involvement of Hippocampal NMDA and AMPA Receptors in Acquisition, Formation and Retrieval of Spatial Memory in the Morris Water Maze. Chin. J. Physiol. 1994, 37, 201–212. [Google Scholar] [PubMed]
- Alexander, G.M.; Rogan, S.C.; Abbas, A.I.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron 2009, 63, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Zhang, H.; Liu, J.; Popugaeva, E.; Xu, N.-J.; Feske, S.; White, C.L.; Bezprozvanny, I. Reduced Synaptic STIM2 Expression and Impaired Store-Operated Calcium Entry Cause Destabilization of Mature Spines in Mutant Presenilin Mice. Neuron 2014, 82, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial Fibrillary Acidic Protein as a Biomarker in Severe Traumatic Brain Injury Patients: A Prospective Cohort Study. Crit. Care 2015, 19, 362. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Sun, Q.; Fan, J.; Jiang, Y.; Yang, W.; Cui, Y.; Yu, Z.; Jiang, H.; Li, B. Role of Astrocytes in Post-Traumatic Epilepsy. Front. Neurol. 2019, 10, 1149. [Google Scholar] [CrossRef]
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive Astrocyte-Driven Epileptogenesis Is Induced by Microglia Initially Activated Following Status Epilepticus. JCI Insight 2021, 6, e135391. [Google Scholar] [CrossRef]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive Roles for Reactive Astrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Leszczyniecka, M.; Kang, D.; Sarkar, D.; Chao, W.; Volsky, D.J.; Fisher, P.B. Insights into Glutamate Transport Regulation in Human Astrocytes: Cloning of the Promoter for Excitatory Amino Acid Transporter 2 (EAAT2). Proc. Natl. Acad. Sci. USA 2003, 100, 1955–1960. [Google Scholar] [CrossRef] [Green Version]
- Covelo, A.; Araque, A. Neuronal Activity Determines Distinct Gliotransmitter Release from a Single Astrocyte. eLife 2018, 7, e32237. [Google Scholar] [CrossRef]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate Exocytosis from Astrocytes Controls Synaptic Strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Perea, G.; Yang, A.; Boyden, E.S.; Sur, M. Optogenetic Astrocyte Activation Modulates Response Selectivity of Visual Cortex Neurons in Vivo. Nat. Commun. 2014, 5, 3262. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Reddy, H. Role of Glutamate and NMDA in Alzheimer’s Desease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-Serine Is an Endogenous Ligand for the Glycine Site of the N-Methyl-D-Aspartate Receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.I.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K.; et al. Possible Role of D-Serine in the Pathophysiology of Alzheimer’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 385–388. [Google Scholar] [CrossRef]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived L-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517.e8. [Google Scholar] [CrossRef]
- Bado, P.; Madeira, C.; Vargas-Lopes, C.; Moulin, T.C.; Wasilewska-Sampaio, A.P.; Maretti, L.; De Oliveira, R.V.; Amaral, O.B.; Panizzutti, R. Effects of Low-Dose d-Serine on Recognition and Working Memory in Mice. Psychopharmacology 2011, 218, 461–470. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA Receptor Antagonist That Improves Memory by Restoration of Homeostasis in the Glutamatergic System—Too Little Activation Is Bad, Too Much Is Even Worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef] [PubMed]
- Salmina, A.B.; Gorina, Y.V.; Erofeev, A.I.; Balaban, P.M.; Bezprozvanny, I.B.; Vlasova, O.L. Optogenetic and Chemogenetic Modulation of Astroglial Secretory Phenotype. Rev. Neurosci. 2021, 32, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Gorina, Y.V.; Salmina, A.B.; Erofeev, A.I.; Gerasimov, E.I.; Bolshakova, A.V.; Balaban, P.M.; Bezprozvanny, I.B.; Vlasova, O.L. Astrocyte Activation Markers. Biochemistry 2022, 87, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Prieto, J.A.; Araque, A. Sensing and Regulating Synaptic Activity by Astrocytes at Tripartite Synapse. Neurochem. Res. 2021, 46, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Annu. Rev. Physiol. 2009, 72, 335–355. [Google Scholar] [CrossRef] [Green Version]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramár, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic Phenotyping and Characterization of the 5xFAD Mouse Model of Alzheimer’s Disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef]
- Oblak, A.L.; Lin, P.B.; Kotredes, K.P.; Pandey, R.S.; Garceau, D.; Williams, H.M.; Uyar, A.; O’Rourke, R.; O’Rourke, S.; Ingraham, C.; et al. Comprehensive Evaluation of the 5XFAD Mouse Model for Preclinical Testing Applications: A MODEL-AD Study. Front. Aging Neurosci. 2021, 13, 713726. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for Early Cognitive Impairment Related to Frontal Cortex in the 5XFAD Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Curzon, P.; Rustay, N.R.; Browman, K.E. Cued and Contextual Fear Conditioning for Rodents; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-42005-234-3. [Google Scholar]
- Xiao, C.; Liu, Y.; Xu, J.; Gan, X.; Xiao, Z. Septal and Hippocampal Neurons Contribute to Auditory Relay and Fear Conditioning. Front. Cell. Neurosci. 2018, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Zernov, N.; Veselovsky, A.V.; Poroikov, V.V.; Melentieva, D.; Bolshakova, A.; Popugaeva, E. New Positive TRPC6 Modulator Penetrates Blood–Brain Barrier, Eliminates Synaptic Deficiency and Restores Memory Deficit in 5xFAD Mice. Int. J. Mol. Sci. 2022, 23, 13552. [Google Scholar] [CrossRef]
- Marinina, K.S.; Bezprozvanny, I.B.; Egorova, P.A. Cognitive Decline and Mood Alterations in the Mouse Model of Spinocerebellar Ataxia Type 2. Cerebellum 2023. [Google Scholar] [CrossRef]
- Parsaei, L.; Torkaman-Boutorabi, A.; Asadi, F.; Zarrindast, M.R. Interaction between Dorsal Hippocampal NMDA Receptors and Lithium on Spatial Learning Consolidation in Rats. Brain Res. Bull. 2016, 127, 1–10. [Google Scholar] [CrossRef]
- Whissell, P.D.; Tohyama, S.; Martin, L.J. The Use of DREADDs to Deconstruct Behavior. Front. Genet. 2016, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.J.; Marchant, N.J. The Use of Chemogenetics in Behavioural Neuroscience: Receptor Variants, Targeting Approaches and Caveats. Br. J. Pharmacol. 2018, 175, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Urban, D.J.; Roth, B.L. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–417. [Google Scholar] [CrossRef]
- Roth, B.L. Use of DREADDS. Neuron 2017, 89, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Sala, C.; Segal, M. Dendritic Spines: The Locus of Structural and Functional Plasticity. Physiol. Rev. 2014, 94, 141–188. [Google Scholar] [CrossRef]
- Koch, C.; Zador, A. The Function of Dendritic Spines: Devices Subserving Biochemical Rather than Electrical Compartmentalization. J. Neurosci. 1993, 13, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Bourne, J.; Harris, K.M. Do Thin Spines Learn to Be Mushroom Spines That Remember? Curr. Opin. Neurobiol. 2007, 17, 381–386. [Google Scholar] [CrossRef]
- Mahmmoud, R.R.; Sase, S.; Aher, Y.D.; Sase, A.; Gröger, M.; Mokhtar, M.; Höger, H.; Lubec, G. Spatial and Working Memory Is Linked to Spine Density and Mushroom Spines. PLoS ONE 2015, 10, e0139739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul, H.M.; Sama, M.A.; Furman, J.L.; Mathis, D.M.; Beckett, T.L.; Weidner, A.M.; Patel, E.S.; Baig, I.; Murphy, M.P.; LeVine, H.; et al. Cognitive Decline in Alzheimer’s Disease Is Associated with Selective Changes in Calcineurin/NFAT Signaling. J. Neurosci. 2009, 29, 12957–12969. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Yang, L.; Du, H.; Sun, Q.; Wang, X.; Cong, L.; Liu, X.; Yin, L.; Li, S.; Du, Y. Insulin Attenuates Beta-Amyloid-Associated Insulin/Akt/EAAT Signaling Perturbations in Human Astrocytes. Cell. Mol. Neurobiol. 2016, 36, 851–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, G.O.; Yeung, Y.J.; Kwakowsky, A. Is EAAT2 a Potential Therapeutic Intervention Target for Alzheimer’s Disease? Neural Regen. Res. 2022, 18, 1721–1722. [Google Scholar] [CrossRef]
- Takahashi, K.; Kong, Q.; Lin, Y.; Stouffer, N.; Schulte, D.A.; Lai, L.; Liu, Q.; Chang, L.C.; Dominguez, S.; Xing, X.; et al. Restored Glial Glutamate Transporter EAAT2 Function as a Potential Therapeutic Approach for Alzheimer’s Disease. J. Exp. Med. 2015, 212, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Greenamyre, J.T.; Maragos, W.F.; Albin, R.L.; Penney, J.B.; Young, A.B. Glutamate Transmission and Toxicity in Alzheimer’s Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 1988, 12, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Anggono, V.; Tsai, L.H.; Götz, J. Glutamate Receptors in Alzheimer’s Disease: Mechanisms and Therapies. Neural Plast. 2016, 2016, 8256196. [Google Scholar] [CrossRef]
- Choi, D.W. Ionic Dependence of Glutamate Neurotoxicity. J. Neurosci. 1987, 7, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.Y.; Choi, D.W. Selective Blockade of Non-NMDA Receptors Does Not Block Rapidly Triggered Glutamate-Induced Neuronal Death. Brain Res. 1991, 548, 318–321. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate Neurotoxicity and Diseases of the Nervous System. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Wood, O.W.G.; Yeung, J.H.Y.; Faull, R.L.M.; Kwakowsky, A. EAAT2 as a Therapeutic Research Target in Alzheimer’s Disease: A Systematic Review. Front. Neurosci. 2022, 16, 952096. [Google Scholar] [CrossRef]
- Osten, P.; Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H. Stereotaxic Gene Delivery in the Rodent Brain. Nat. Protoc. 2007, 1, 3166–3173. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ehlenberger, D.B.; Dickstein, D.L.; Hof, P.R.; Wearne, S.L. Automated Three-Dimensional Detection and Shape Classification of Dendritic Spines from Fluorescence Microscopy Images. PLoS ONE 2008, 3, e1997. [Google Scholar] [CrossRef] [Green Version]
- Zernov, N.; Bezprozvanny, I.; Popugaeva, E. CaMKIIβ Knockdown Decreases Store-Operated Calcium Entry in Hippocampal Dendritic Spines. IBRO Neurosci. Rep. 2022, 12, 90–97. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, E.; Bezprozvanny, I.; Vlasova, O.L. Activation of Gq-Coupled Receptors in Astrocytes Restores Cognitive Function in Alzheimer’s Disease Mice Model. Int. J. Mol. Sci. 2023, 24, 9969. https://doi.org/10.3390/ijms24129969
Gerasimov E, Bezprozvanny I, Vlasova OL. Activation of Gq-Coupled Receptors in Astrocytes Restores Cognitive Function in Alzheimer’s Disease Mice Model. International Journal of Molecular Sciences. 2023; 24(12):9969. https://doi.org/10.3390/ijms24129969
Chicago/Turabian StyleGerasimov, Evgenii, Ilya Bezprozvanny, and Olga L. Vlasova. 2023. "Activation of Gq-Coupled Receptors in Astrocytes Restores Cognitive Function in Alzheimer’s Disease Mice Model" International Journal of Molecular Sciences 24, no. 12: 9969. https://doi.org/10.3390/ijms24129969
APA StyleGerasimov, E., Bezprozvanny, I., & Vlasova, O. L. (2023). Activation of Gq-Coupled Receptors in Astrocytes Restores Cognitive Function in Alzheimer’s Disease Mice Model. International Journal of Molecular Sciences, 24(12), 9969. https://doi.org/10.3390/ijms24129969





