In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer
Abstract
:1. Introduction
2. Results
2.1. AR2011 In Vitro and In Vivo Lytic Activity
2.1.1. On Fresh Explants Obtained from Human Gynecological Cancers
2.1.2. On Malignant Ovarian Epithelial Cells Obtained from Ascites Fluid
2.1.3. In Vivo Efficacy of the Combination of AR2011 and Cisplatin
2.2. In Vitro and In Vivo Studies of AR2011(404), a Novel Oncolytic Vector Engineered to Express Immunomodulatory Genes
2.2.1. Vector Construction and In Vitro Studies
2.2.2. In Vivo Studies in Nude Mice
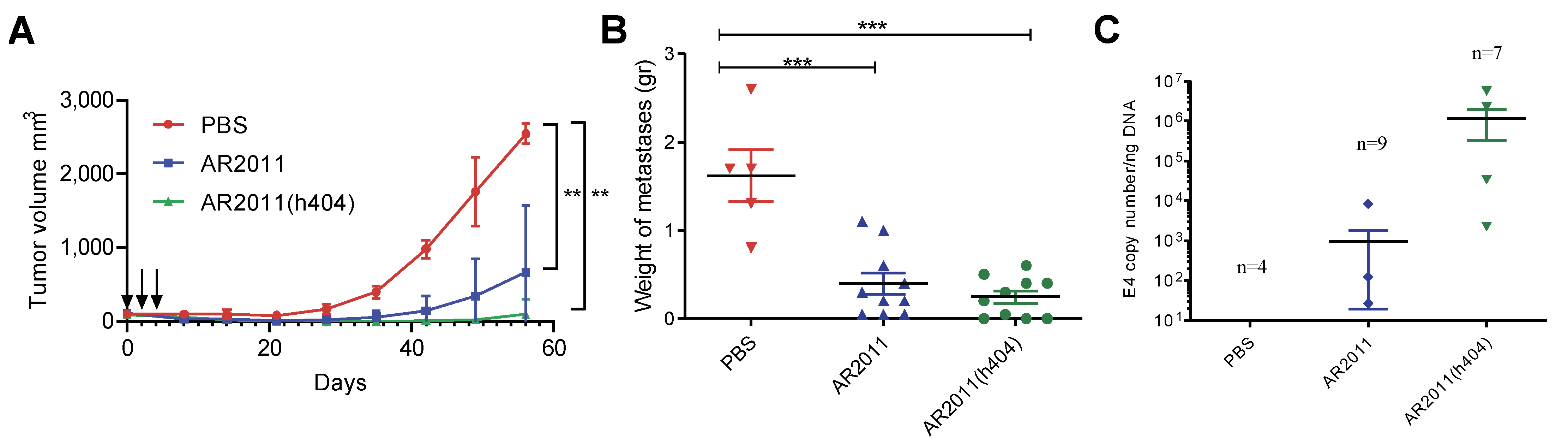
2.2.3. In Vivo Studies in Syngeneic Mice Models
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cell Lines and Cell Culture
4.3. Construction and Production of the Oncolytic Adenoviruses
4.4. In Vitro Cytotoxicity Assay
4.5. Isolation of Malignant Cells from Ovarian Cancer Liquid Ascites
4.6. In Vitro Lytic Assays in Combination with Cisplatin
4.7. Assessment of CD40L and 4-1BBL Expression
4.8. In Vivo Studies with Nude Mice
4.9. In Vivo Studies with Syngeneic Models
4.10. Statistical Analysis
4.11. Patents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Kryczek, I.; Dostal, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au Yeung, C.L.; Co, N.N.; Tsuruga, T.; Yeung, T.L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Leary, A.; Scott, C.L.; Serra, V.; Lord, C.J.; Bowtell, D.; Chang, D.K.; Garsed, D.W.; Jonkers, J.; Ledermann, J.A.; et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020, 31, 1606–1622. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Lokadasan, R.; James, F.V.; Narayanan, G.; Prabhakaran, P.K. Targeted agents in epithelial ovarian cancer: Review on emerging therapies and future developments. Ecancermedicalscience 2016, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Lou, E.; Vogel, R.I.; Hoostal, S.; Klein, M.; Linden, M.A.; Teoh, D.; Geller, M.A. Tumor-Stroma Proportion as a Predictive Biomarker of Resistance to Platinum-Based Chemotherapy in Patients With Ovarian Cancer. JAMA Oncol. 2019, 5, 1222–1224. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.Y.; Wu, N.; Chen, Y.C.; Cheng, Q.; Wang, J. PARP inhibitor resistance: The underlying mechanisms and clinical implications. Mol. Cancer 2020, 19, 107. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.Y.; Shan, W.L.; Chen, Y.; Zhu, Q.; Xia, B.R. Targeted therapy and immunotherapy: Diamonds in the rough in the treatment of epithelial ovarian cancer. Front. Pharmacol. 2023, 14, 1131342. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J., Jr.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.R.; Zhang, Z.C.; Zhang, Y.J.; Lou, G.; Jin, W.L. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front. Immunol. 2020, 11, 577869. [Google Scholar] [CrossRef]
- Heo, Y.A. Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef]
- Hajeri, P.B.; Sharma, N.S.; Yamamoto, M. Oncolytic Adenoviruses: Strategies for Improved Targeting and Specificity. Cancers 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.V.; Rivera, A.A.; Viale, D.L.; Benedetti, L.; Cuneo, N.; Kimball, K.J.; Wang, M.; Douglas, J.T.; Zhu, Z.B.; Bravo, A.I.; et al. A Tumor-stroma Targeted Oncolytic Adenovirus Replicated in Human Ovary Cancer Samples and Inhibited Growth of Disseminated Solid Tumors in Mice. Mol. Ther. 2012, 20, 2222–2233. [Google Scholar] [CrossRef] [Green Version]
- Viale, D.L.; Cafferata, E.G.; Gould, D.; Rotondaro, C.; Chernajovsky, Y.; Curiel, D.T.; Podhajcer, O.L.; Lopez, M.V. Therapeutic improvement of a stroma-targeted CRAd by incorporating motives responsive to the melanoma microenvironment. J. Investig. Dermatol. 2013, 133, 2576–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.D.; Li, M.; Yuan, Y.; Mao, N.; Pan, L.Y. Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol. Rep. 2007, 17, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Wintzell, M.; Lofstedt, L.; Johansson, J.; Pedersen, A.B.; Fuxe, J.; Shoshan, M. Repeated cisplatin treatment can lead to a multiresistant tumor cell population with stem cell features and sensitivity to 3-bromopyruvate. Cancer Biol. Ther. 2012, 13, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Yin, X.; Languino, L.R.; Altieri, D.C. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat. Biopharm. Res. 2018, 10, 112–122. [Google Scholar] [CrossRef]
- Takakura, M.; Nakamura, M.; Kyo, S.; Hashimoto, M.; Mori, N.; Ikoma, T.; Mizumoto, Y.; Fujiwara, T.; Urata, Y.; Inoue, M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010, 17, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Short, J.J.; Rivera, A.A.; Wu, H.; Walter, M.R.; Yamamoto, M.; Mathis, J.M.; Curiel, D.T. Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol. Cancer Ther. 2010, 9, 2536–2544. [Google Scholar] [CrossRef] [Green Version]
- Young, A.M.; Archibald, K.M.; Tookman, L.A.; Pool, A.; Dudek, K.; Jones, C.; Williams, S.L.; Pirlo, K.J.; Willis, A.E.; Lockley, M.; et al. Failure of translation of human adenovirus mRNA in murine cancer cells can be partially overcome by L4-100K expression in vitro and in vivo. Mol. Ther. 2012, 20, 1676–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castells, M.; Thibault, B.; Delord, J.P.; Couderc, B. Implication of tumor microenvironment in chemoresistance: Tumor-associated stromal cells protect tumor cells from cell death. Int. J. Mol. Sci. 2012, 13, 9545–9571. [Google Scholar] [CrossRef] [PubMed]
- Tothill, R.W.; Tinker, A.V.; George, J.; Brown, R.; Fox, S.B.; Lade, S.; Johnson, D.S.; Trivett, M.K.; Etemadmoghadam, D.; Locandro, B.; et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 2008, 14, 5198–5208. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shu, J.; Chen, L.; Chen, X.; Zhao, J.; Li, S.; Mou, X.; Tong, X. Synergistic suppression effect on tumor growth of ovarian cancer by combining cisplatin with a manganese superoxide dismutase-armed oncolytic adenovirus. OncoTargets Ther. 2016, 9, 6381–6388. [Google Scholar] [CrossRef] [Green Version]
- Nounamo, B.; Liem, J.; Cannon, M.; Liu, J. Myxoma Virus Optimizes Cisplatin for the Treatment of Ovarian Cancer In Vitro and in a Syngeneic Murine Dissemination Model. Mol. Ther. Oncolytics 2017, 6, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Pastor, R.; Ashshi, A.M.; El-Shemi, A.G.; Dmitriev, I.P.; Kashentseva, E.A.; Lu, Z.H.; Goedegebuure, S.P.; Podhajcer, O.L.; Curiel, D.T. Defining a murine ovarian cancer model for the evaluation of conditionally-replicative adenovirus (CRAd) virotherapy agents. J. Ovarian Res. 2019, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Qiu, H.; Zhang, M.; Zhang, F.; Yang, H.; Yang, L.; Jia, L.; Qin, K.; Jia, L.; Dou, X.; et al. Soluble CD40 ligands sensitize the epithelial ovarian cancer cells to cisplatin treatment. Biomed. Pharmacother. 2016, 79, 166–175. [Google Scholar] [CrossRef]
- Yi, L.; Zhao, Y.; Wang, X.; Dai, M.; Hellström, K.E.; Hellström, I.; Zhang, H. Human and Mouse CD137 Have Predominantly Different Binding CRDs to Their Respective Ligands. PLoS ONE 2014, 9, e86337. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cheng, D.; Xia, Z.; Luan, M.; Wu, L.; Wang, G.; Zhang, S. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J. Transl. Med. 2013, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoare, J.; Campbell, N.; Carapuça, E. Oncolytic virus immunotherapies in ovarian cancer: Moving beyond adenoviruses. Porto Biomed. J. 2018, 3, e7. [Google Scholar] [CrossRef] [PubMed]
- Vasey, P.A.; Shulman, L.N.; Campos, S.; Davis, J.; Gore, M.; Johnston, S.; Kirn, D.H.; O’Neill, V.; Siddiqui, N.; Seiden, M.V.; et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J. Clin. Oncol. 2002, 20, 1562–1569. [Google Scholar]
- Kim, K.H.; Dmitriev, I.P.; Saddekni, S.; Kashentseva, E.A.; Harris, R.D.; Aurigemma, R.; Bae, S.; Singh, K.P.; Siegal, G.P.; Curiel, D.T.; et al. A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 2013, 130, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Cerullo, V.; Pesonen, S.; Diaconu, I.; Escutenaire, S.; Arstila, P.T.; Ugolini, M.; Nokisalmi, P.; Raki, M.; Laasonen, L.; Sarkioja, M.; et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010, 70, 4297–4309. [Google Scholar] [CrossRef] [Green Version]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pastor, R.; Goedegebuure, P.S.; Curiel, D.T. Understanding and addressing barriers to successful adenovirus-based virotherapy for ovarian cancer. Cancer Gene Ther. 2020, 28, 375–389. [Google Scholar] [CrossRef]
- Fujiwara, T. Multidisciplinary oncolytic virotherapy for gastrointestinal cancer. Ann. Gastroenterol. Surg. 2019, 3, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Alfano, A.L.; Nicola Candia, A.; Cuneo, N.; Guttlein, L.N.; Soderini, A.; Rotondaro, C.; Sganga, L.; Podhajcer, O.L.; Lopez, M.V. Oncolytic Adenovirus-Loaded Menstrual Blood Stem Cells Overcome the Blockade of Viral Activity Exerted by Ovarian Cancer Ascites. Mol. Ther. Oncolytics 2017, 6, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Dmitriev, I.; Kashentseva, E.; Seki, T.; Wang, M.; Curiel, D.T. Construction and Characterization of Adenovirus Serotype 5 Packaged by Serotype 3 Hexon. J. Virol. 2002, 76, 12775–12782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.V.; Viale, D.L.; Cafferata, E.G.A.; Bravo, A.I.; Carbone, C.; Gould, D.; Chernajovsky, Y.; Podhajcer, O.L. Tumor Associated Stromal Cells Play a Critical Role on the Outcome of the Oncolytic Efficacy of Conditionally Replicative Adenoviruses. PLoS ONE 2009, 4, e5119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, T.O.; Rivera, A.; Rein, D.; Wang, M.; Ulasov, I.; Breidenbach, M.; Kataram, M.; Contreras, J.L.; Krumdieck, C.; Yamamoto, M.; et al. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin. Cancer Res. 2004, 10, 8697–8703. [Google Scholar] [CrossRef] [Green Version]




| Sample | Pathology | Observation |
|---|---|---|
| 1 | Epithelial ovarian cancer | First cytoreduction |
| 2 | Epithelial ovarian cancer | Relapse |
| 3 | Epithelial ovarian cancer | Bilateral tumor Neoadjuvant chemotherapy C, P and Bevacizumab |
| 4 | Epithelial ovarian cancer | Neoadjuvant chemotherapy |
| 5 | Krukenberg tumor | First cytoreduction |
| 6 | Cervical cancer | Conization |
| 7 | Cervical cancer | Conization |
| 8 | Cervical cancer | Simple hysterectomy |
| 9 | Cervical cancer | Simple hysterectomy |
| 10 | Cervical cancer | Conization |
| 11 | Cervical cancer | Simple hysterectomy |
| 12 | Uterus cancer | Radical Hysterectomy |
| 13 | Uterus cancer | Normal uterus tissue after radical hysterectomy * |
| 14 | Uterus cancer | Normal uterus tissue obtained after radical hysterectomy * |
| 15 | Uterus cancer | Hysterectomy |
| 16 | Uterus cancer | Hysterectomy |
| OC-AF | Y(P) 1 | Y(O) 1 |
|---|---|---|
| 8 | 0.30 | 0.60 |
| 10 | 0.71 | 0.81 |
| 14 | 0.66 | 0.64 |
| 15 | 0.66 | 0.70 |
| 18 | 0.81 | 0.77 |
| 19 | 0.57 | 0.66 |
| 20 | 0.80 | 0.85 |
| 21 | 0.88 | 0.87 |
| 24 | 0.78 | 0.80 |
| 28 | 0.80 | 0.87 |
| 30 | 0.88 | 0.83 |
| 38 | 0.77 | 0.74 |
| 39 | 0.61 | 0.72 |
| 40 | 0.90 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfano, A.; Cafferata, E.G.A.; Gangemi, M.; Nicola Candia, A.; Malnero, C.M.; Bermudez, I.; Lopez, M.V.; Ríos, G.D.; Rotondaro, C.; Cuneo, N.; et al. In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer. Int. J. Mol. Sci. 2023, 24, 9992. https://doi.org/10.3390/ijms24129992
Alfano A, Cafferata EGA, Gangemi M, Nicola Candia A, Malnero CM, Bermudez I, Lopez MV, Ríos GD, Rotondaro C, Cuneo N, et al. In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer. International Journal of Molecular Sciences. 2023; 24(12):9992. https://doi.org/10.3390/ijms24129992
Chicago/Turabian StyleAlfano, Ana, Eduardo G. A. Cafferata, Mariela Gangemi, Alejandro Nicola Candia, Cristian M. Malnero, Ismael Bermudez, Mauricio Vargas Lopez, Gregorio David Ríos, Cecilia Rotondaro, Nicasio Cuneo, and et al. 2023. "In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer" International Journal of Molecular Sciences 24, no. 12: 9992. https://doi.org/10.3390/ijms24129992
APA StyleAlfano, A., Cafferata, E. G. A., Gangemi, M., Nicola Candia, A., Malnero, C. M., Bermudez, I., Lopez, M. V., Ríos, G. D., Rotondaro, C., Cuneo, N., Curiel, D. T., Podhajcer, O. L., & Lopez, M. V. (2023). In Vitro and In Vivo Efficacy of a Stroma-Targeted, Tumor Microenvironment Responsive Oncolytic Adenovirus in Different Preclinical Models of Cancer. International Journal of Molecular Sciences, 24(12), 9992. https://doi.org/10.3390/ijms24129992







