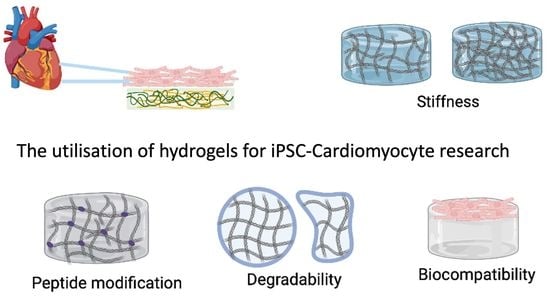
The Utilisation of Hydrogels for iPSC-Cardiomyocyte Research
Abstract
1. Introduction
1.1. Cardiac Extracellular Matrix
1.2. Hydrogels as Synthetic Biomaterials Scaffolds
1.3. Functionalisation of Hydrogels
- Application of hydrogels in cardiovascular research
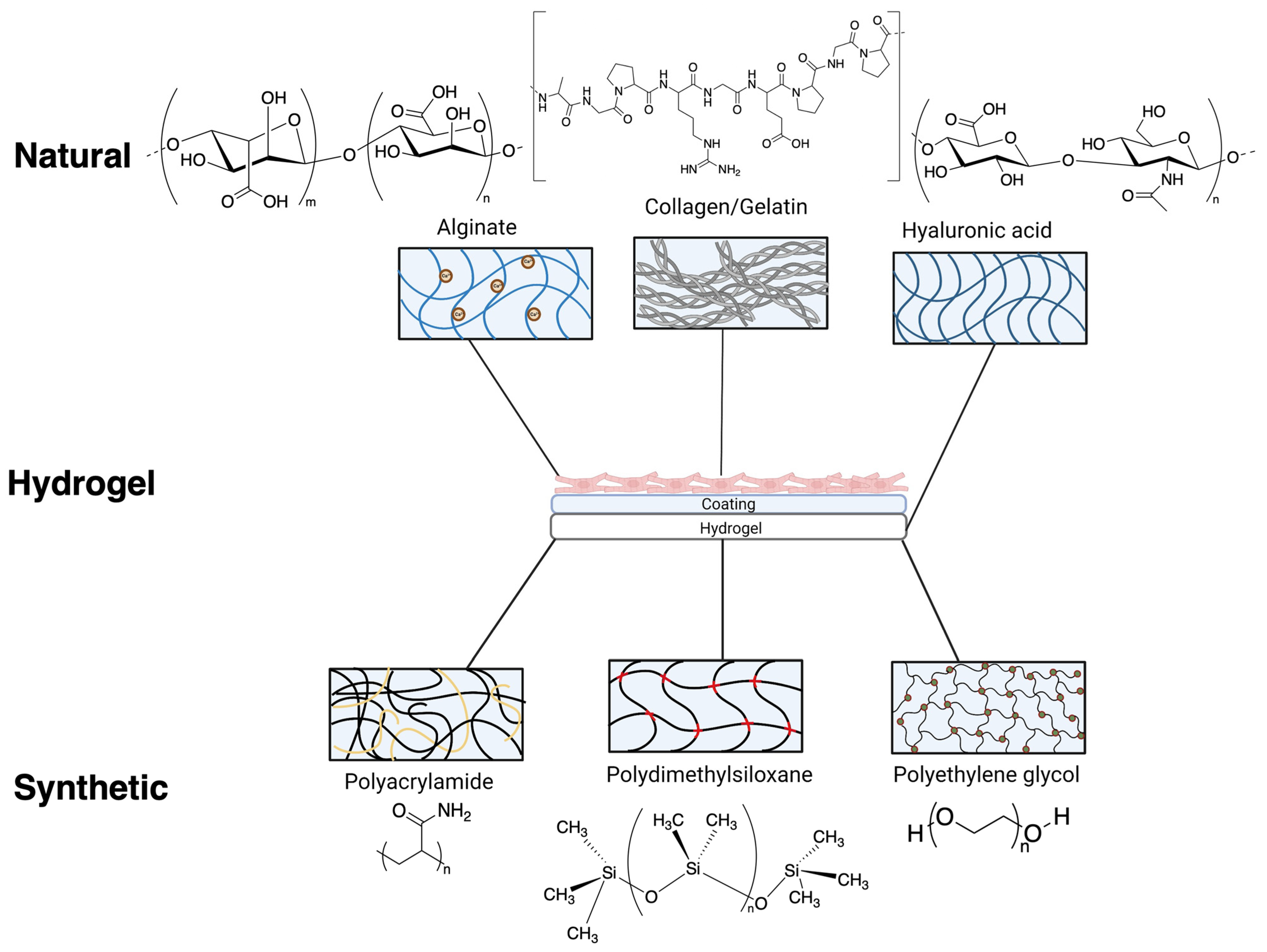
2. Hydrogels from Natural Polymers
2.1. Collagen/Gelatin Based Hydrogels
2.2. Alginate Gels
2.3. Hyaluronic Acid-Based Hydrogels
3. Synthetic Hydrogels
3.1. Polydimethylsiloxane
3.2. Polyacrylamide
3.3. Polyethylene Glycol (PEG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Li, P.H.; Chen, H.Z. Cardiomyocyte Senescence and Cellular Communications Within Myocardial Microenvironments. Front. Endocrinol. 2020, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Meschiari, C.A.; Ero, O.K.; Pan, H.; Finkel, T.; Lindsey, M.L. The impact of aging on cardiac extracellular matrix. Geroscience 2017, 39, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B. Aging, exercise, and extracellular matrix in the heart. J. Exerc. Rehabil. 2013, 9, 338–347. [Google Scholar] [CrossRef]
- Cho, N.; Razipour, S.E.; McCain, M.L. TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp. Biol. Med. 2018, 243, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.-Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121 Pt 22, 3794–3802. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Seth, A.; McCulloch, C.A. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1871–H1881. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Jang, Y.; Park, Y.; Kim, J. Engineering Biomaterials to Guide Heart Cells for Matured Cardiac Tissue. Coatings 2020, 10, 925. [Google Scholar] [CrossRef]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.G.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem. Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef]
- Yoon, S.J.; Fang, Y.H.; Lim, C.H.; Kim, B.S.; Son, H.S.; Park, Y.; Sun, K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 163–171. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Yigit, S.; Sanyal, R.; Sanyal, A. Fabrication and functionalization of hydrogels through “click” chemistry. Chem. Asian J. 2011, 6, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Deforest, C.A.; Sims, E.A.; Anseth, K.S. Peptide-Functionalized Click Hydrogels with Independently Tunable Mechanics and Chemical Functionality for 3D Cell Culture. Chem. Mater. 2010, 22, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Plenderleith, R.A.; Pateman, C.J.; Rodenburg, C.; Haycock, J.W.; Claeyssens, F.; Sammon, C.; Rimmer, S. Arginine-glycine-aspartic acid functional branched semi-interpenetrating hydrogels. Soft Matter. 2015, 11, 7567–7578. [Google Scholar] [CrossRef]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011, 7, 152–162. [Google Scholar] [CrossRef]
- Zhou, H.X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, B.; Kang, T.W.; Noh, J.H.; Kim, M.J.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. Electrostatically Interactive Injectable Hydrogels for Drug Delivery. Tissue Eng. Regen. Med. 2018, 15, 513–520. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert. Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Fisher, S.A.; Baker, A.E.G.; Shoichet, M.S. Designing Peptide and Protein Modified Hydrogels: Selecting the Optimal Conjugation Strategy. J. Am. Chem. Soc. 2017, 139, 7416–7427. [Google Scholar] [CrossRef] [PubMed]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Bhana, B.; Iyer, R.K.; Chen, W.L.K.; Zhao, R.; Sider, K.L.; Likhitpanichkul, M.; Simmons, C.A.; Radisic, M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010, 105, 1148–1160. [Google Scholar] [CrossRef]
- Hall, M.L.; Ogle, B.M. Cardiac Extracellular Matrix Modification as a Therapeutic Approach. Adv. Exp. Med. Biol. 2018, 1098, 131–150. [Google Scholar]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Farè, S. History and Applications of Hydrogels. J. Biomed. Sci. 2016, 4, 1–23. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Alonzo, M.; Kumar, S.A.; Allen, S.; Delgado, M.; Alvarez-Primo, F.; Suggs, L.; Joddar, B. Hydrogel scaffolds with elasticity-mimicking embryonic substrates promote cardiac cellular network formation. Prog. Biomater. 2020, 9, 125–137. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing injectable gelatin hydrogels for localized therapeutic cell delivery. J. Biomed. Mater. Res. A 2020, 108, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Galie, P.A.; Khalid, N.; Carnahan, K.E.; Westfall, M.V.; Stegemann, J.P. Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc. Pathol. 2013, 22, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Heras-Bautista, C.O.; Mikhael, N.; Lam, J.; Shinde, V.; Katsen-Globa, A.; Dieluweit, S.; Molcanyi, M.; Uvarov, V.; Jütten, P.; Sahito, R.G.; et al. Cardiomyocytes facing fibrotic conditions re-express extracellular matrix transcripts. Acta Biomater. 2019, 89, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Crocini, C.; Walker, C.J.; Anseth, K.S.; Leinwand, L.A. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog. Biophys. Mol. Biol. 2020, 154, 71–79. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2009, 30, 4094–4103. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef]
- Oyama, T.G.; Oyama, K.; Kimura, A.; Yoshida, F.; Ishida, R.; Yamazaki, M.; Miyoshi, H.; Taguchi, M. Collagen hydrogels with controllable combined cues of elasticity and topography to regulate cellular processes. Biomed. Mater. 2021, 16, 045037. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, K.; Li, X.; Liu, C.; Ortiz, L.S.; Wu, K.; Huang, N. Gelatin-based hydrogels combined with electrical stimulation to modulate neonatal rat cardiomyocyte beating and promote maturation. Bio-Des. Manuf. 2021, 4, 100–110. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Michelini, L.; Probo, L.; Fare, S.; Negrini, C. Characterization of gelatin hydrogels derived from different animal sources. Mater. Lett. 2020, 272, 127865. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.D.; Brown, M.A.; Iyer, R.K.; Radisic, M.; Murthy, S.K. Controlled capture and release of cardiac fibroblasts using peptide-functionalized alginate gels in microfluidic channels. Lab Chip 2009, 9, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.S.; Slemming-Adamsen, P.; Hanif, M.; Zhang, Z.; Wang, Z.; Chen, M. Wet electrospun alginate/gelatin hydrogel nanofibers for 3D cell culture. Int. J. Biol. Macromol. 2018, 118 Pt B, 1648–1654. [Google Scholar] [CrossRef]
- Schulz, A.; Gepp, M.M.; Stracke, F.; von Briesen, H.; Neubauer, J.C.; Zimmermann, H. Tyramine-conjugated alginate hydrogels as a platform for bioactive scaffolds. J. Biomed. Mater. Res. A 2019, 107, 114–121. [Google Scholar] [CrossRef]
- Hunt, N.C.; Shelton, R.M.; Henderson, D.J.; Grover, L.M. Calcium-alginate hydrogel-encapsulated fibroblasts provide sustained release of vascular endothelial growth factor. Tissue Eng. Part A 2013, 19, 905–914. [Google Scholar] [CrossRef]
- Abecasis, B.; Canhão, P.G.M.; Almeida, H.V.; Calmeiro, T.; Fortunato, E.; Gomes-Alves, P.; Serra, M.; Alves, P.M. Toward a Microencapsulated 3D hiPSC-Derived In vitro cardiac micro tissue for recapitulation of human heart microenvironment features. Front. Bioeng. Biotechnol. 2020, 8, 580744. [Google Scholar] [CrossRef]
- Shu, X.Z.; Ghosh, K.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Clark, R.A.; Prestwich, G.D. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J. Biomed. Mater. Res. Part A 2003, 68, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Lin, V.; McCollough, A.; Atzet, S.; Prestwich, G.D.; Wechsler, A.S.; Murray, M.E.; Oake, S.A.; Kresh, J.Y.; Janmey, P.A. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J. Biomech. 2012, 45, 824–831. [Google Scholar] [CrossRef]
- Derkus, B. Human cardiomyocyte-derived exosomes induce cardiac gene expressions in mesenchymal stromal cells within 3D hyaluronic acid hydrogels and in dose-dependent manner. J. Mater. Sci. Mater. Med. 2021, 32, 2. [Google Scholar] [CrossRef]
- Young, J.L.; Kretchmer, K.; Ondeck, M.G.; Zambon, A.C.; Engler, A.J. Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci. Rep. 2014, 4, 6425. [Google Scholar] [CrossRef] [PubMed]
- McCain, M.L.; Sheehy, S.P.; Grosberg, A.; Goss, J.A.; Parker, K.K. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. Sci. USA 2013, 110, 9770–9775. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Ang, Y.-S.; Fu, J.-D.; Rivas, R.N.; Mohamed, T.M.A.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef]
- Bouhrira, N.; Galie, P.A.; Janmey, P.A. Hyaluronan Disrupts Cardiomyocyte Organization within 3D Fibrin-Based Hydrogels. Biophys. J. 2019, 116, 1340–1347. [Google Scholar] [CrossRef]
- Young, J.L.; Tuler, J.; Braden, R.; Schüp-Magoffin, P.; Schaefer, J.; Kretchmer, K.; Christman, K.L.; Engler, A.J. In vivo response to dynamic hyaluronic acid hydrogels. Acta Biomater. 2013, 9, 7151–7157. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid. Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.S.; Tien, J.; Chen, C.S. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J. Biomed. Mater. Res. A 2003, 66, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, M.; Ahmadi, E.; Khodabandeh Shahraki, M.; Aliakbari, F.; Morshedi, D. Temperature-dependent formulation of a hydrogel based on Hyaluronic acid-polydimethylsiloxane for biomedical applications. Heliyon 2020, 6, e03494. [Google Scholar] [CrossRef]
- Cui, J.; Lackey, M.A.; Tew, G.N.; Crosby, A.J. Mechanical Properties of End-Linked PEG/PDMS Hydrogels. Macromolecules 2012, 45, 6104–6110. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Corbin, E.A.; Caliari, S.R.; Ouyang, L.; Vega, S.L.; Truitt, R.; Han, L.; Margulies, K.B.; Burdick, J.A. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 2017, 145, 23–32. [Google Scholar] [CrossRef]
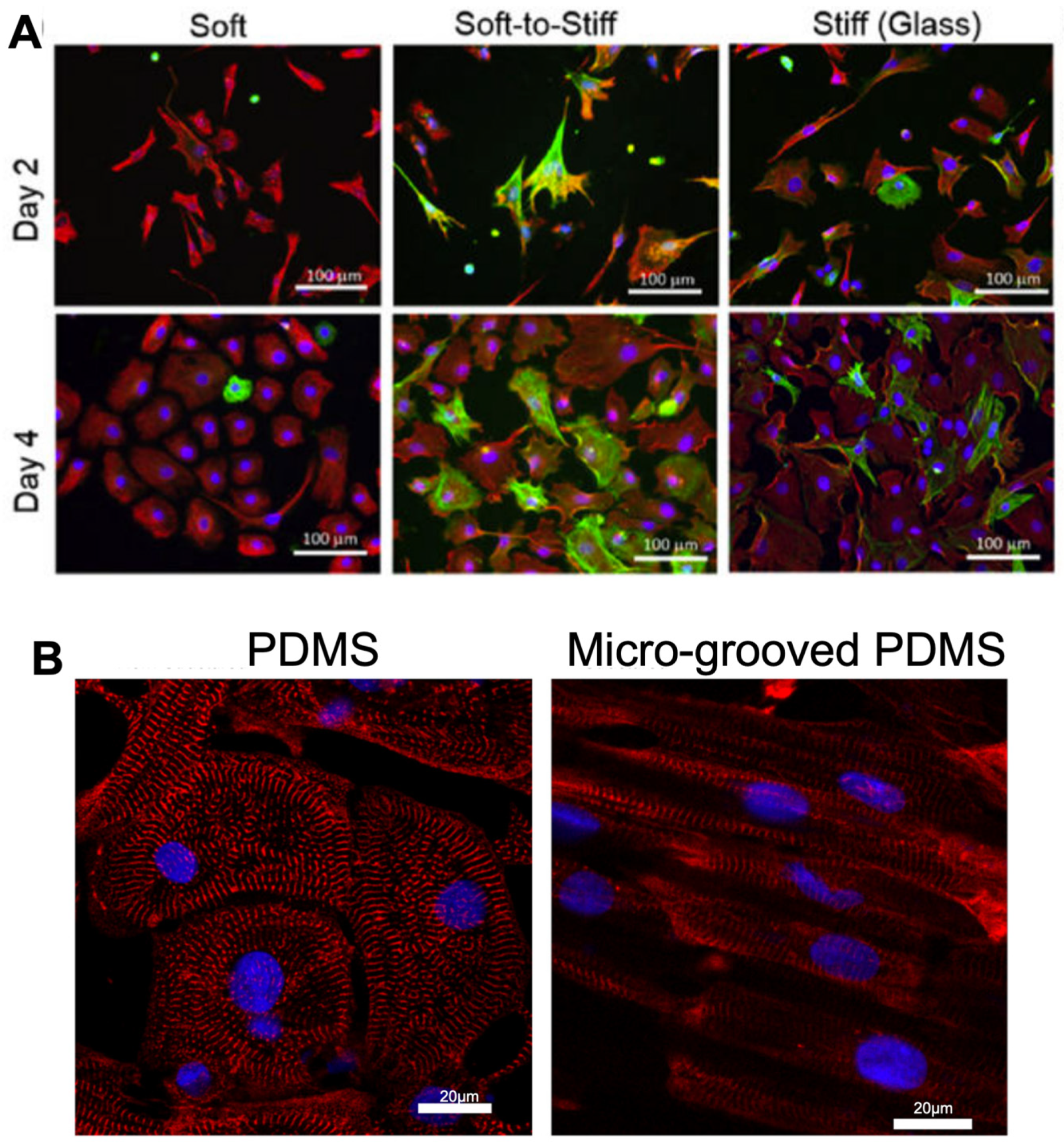
- Petersen, A.P.; Lyra-Leite, D.M.; Ariyasinghe, N.R.; Cho, N.; Goodwin, C.M.; Kim, J.Y.; McCain, M.L. Microenvironmental Modulation of Calcium Wave Propagation Velocity in Engineered Cardiac Tissues. Cell Mol. Bioeng. 2018, 11, 337–352. [Google Scholar] [CrossRef]
- Korner, A.; Mosqueira, M.; Hecker, M.; Ullrich, N.D. Substrate Stiffness Influences Structural and Functional Remodeling in Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Physiol. 2021, 12, 710619. [Google Scholar] [CrossRef]
- Rao, C.; Prodromakis, T.; Kolker, L.; Chaudhry, U.A.; Trantidou, T.; Sridhar, A.; Weekes, C.; Camelliti, P.; Harding, S.; Darzi, A.; et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 2013, 34, 2399–2411. [Google Scholar] [CrossRef]
- Herron, T.J.; Da Rocha, A.M.; Campbell, K.F.; Ponce-Balbuena, D.; Willis, C.; Guerrero-Serna, G.; Liu, Q.; Klos, M.; Musa, H.; Zarzoso, M.; et al. Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circ. Arrhythm Electrophysiol. 2016, 9, e003638. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectronic. Engineering 2009, 86, 1321–1324. [Google Scholar]
- Lyra-Leite, D.; Andres, A.M.; Cho, N.; Petersen, A.P.; Ariyasinghe, N.R.; Kim, S.S.; Gottlieb, R.A.; McCain, M.L. Matrix-guided control of mitochondrial function in cardiac myocytes. Acta Biomater. 2019, 97, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.S.; Myers, K.A.; Gardel, M.L.; Waterman, C.M. Stiffness-controlled three-dimensional extracellular matrices for high-resolution imaging of cell behavior. Nat. Protoc. 2012, 7, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010, 47, 10.16.1–10.16.16. [Google Scholar] [CrossRef] [PubMed]
- Boothe, S.D.; Myers, J.D.; Pok, S.; Sun, J.; Xi, Y.; Nieto, R.M.; Cheng, J.; Jacot, J.G. The Effect of Substrate Stiffness on Cardiomyocyte Action Potentials. Cell Biochem. Biophys. 2016, 74, 527–535. [Google Scholar] [CrossRef]
- Morishima, M.; Horikawa, K.; Funaki, M. Cardiomyocytes cultured on mechanically compliant substrates, but not on conventional culture devices, exhibit prominent mitochondrial dysfunction due to reactive oxygen species and insulin resistance under high glucose. PLoS ONE 2018, 13, e0201891. [Google Scholar] [CrossRef]
- Gilles, G.; McCulloch, A.D.; Brakebusch, C.H.; Herum, K.M. Maintaining resting cardiac fibroblasts in vitro by disrupting mechanotransduction. PLoS ONE 2020, 15, e0241390. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, I.; Humphrey, E.J.; Dinelli, F.; Terracciano, C.M.; Prodromakis, T. Effect of patterned polyacrylamide hydrogel on morphology and orientation of cultured NRVMs. Sci. Rep. 2018, 8, 11991. [Google Scholar] [CrossRef]
- Torres-García, R.; Flores-Estrada, J.; Cauich-Rodríguez, J.V.; Flores-Reyes, M.; Flores-Merino, M.V. Design of a polyacrylamide and gelatin hydrogel as a synthetic extracellular matrix. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 266–277. [Google Scholar] [CrossRef]
- Dehbari, N.; Tavakoli, J.; Singh Khatrao, S.; Tang, Y. In situ polymerized hyperbranched polymer reinforced poly(acrylic acid) hydrogels. Mater. Chem. Front. 2017, 1, 1995–2004. [Google Scholar] [CrossRef]
- Kim, D.; Park, K. Swelling and mechanical properties of superporous hydrogels of poly(acrylamide-co-acrylic acid)/polyethylenimine interpenetrating polymer networks. Polymer 2004, 45, 189–196. [Google Scholar] [CrossRef]
- Diaz-Bello, B.; Monroy-Romero, A.X.; Perez-Calixto, D.; Zamarron-Hernandez, D.; Serna-Marquez, N.; Vazquez-Victorio, G.; Hautefeuille, M. Method for the Direct Fabrication of Polyacrylamide Hydrogels with Controlled Stiffness in Polystyrene Multiwell Plates for Mechanobiology Assays. ACS Biomater. Sci. Eng. 2019, 5, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Denisin, A.K.; Pruitt, B.L. Tuning the Range of Polyacrylamide Gel Stiffness for Mechanobiology Applications. ACS Appl. Mater. Interfaces 2016, 8, 21893–21902. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wei, F.; Li, W.; Liu, P.; Wu, Y.; Dai, M.; Chen, J. Mechanism of Polyacrylamide Hydrogel Instability on High-Temperature Conditions. ACS Omega 2018, 3, 10716–10724. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Krsko, P.; Libera, M. Biointeractive hydrogels. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Jain, E.; Hill, L.; Canning, E.; Sell, S.A.; Zustiak, S.P. Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker. J. Mater. Chem. B 2017, 5, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, M.R.; Ardalani, H.; Zhang, J.; Hou, Z.; Nguyen, E.H.; Swanson, S.; Nguyen, B.K.; Bolin, J.; Elwell, A.; Bischel, L.L.; et al. Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater. 2016, 35, 32–41. [Google Scholar] [CrossRef]
- Khang, A.; Rodriguez, A.G.; Schroeder, M.E.; Sansom, J.; Lejeune, E.; Anseth, K.S.; Sacks, M.S. Quantifying heart valve interstitial cell contractile state using highly tunable poly(ethylene glycol) hydrogels. Acta Biomater. 2019, 96, 354–367. [Google Scholar] [CrossRef]
- Abecasis, B.; Gomes-Alves, P.; Rosa, S.; Gouveia, P.J.; Ferreira, L.; Serra, M.; Alves, P.M. Unveiling the molecular crosstalk in a human induced pluripotent stem cell-derived cardiac model. Biotechnol. Bioeng. 2019, 116, 1245–1252. [Google Scholar] [CrossRef]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, e019338. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Li, D.; Guo, X.; Kasper, F.K.; Duan, C.; Zhou, J.; Mikos, A.G.; Wang, C. Injectable biodegradable hydrogels for embryonic stem cell transplantation: Improved cardiac remodelling and function of myocardial infarction. J. Cell Mol. Med. 2012, 16, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhu, Y.; Liu, G.; Yuan, Z.; Li, H.; Zhao, Q. Local intramyocardial delivery of bioglass with alginate hydrogels for post-infarct myocardial regeneration. Biomed. Pharm. 2020, 129, 110382. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.E.; Cao, Y.; Lin, Y.-H.; Chi, C.; Bai, B.; Sparagna, G.C.; Zhao, Y.; Du, Y.; Londono, P.; Reisz, J.A.; et al. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes Enables Modeling of Human Hypertrophic Cardiomyopathy. Stem. Cell Rep. 2021, 16, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.R.; Joo, H.J.; Kim, D.H.; Cui, L.H.; Choi, S.C.; Kim, J.H.; Lim, D.S. Nanopillar Surface Topology Promotes Cardiomyocyte Differentiation through Cofilin-Mediated Cytoskeleton Rearrangement. ACS Appl. Mater. Interfaces 2017, 9, 16803–16812. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; McGuire, A.F.; Burridge, P.W.; Matsa, E.; Lou, H.-Y.; Wu, J.C.; Cui, B. Accurate nanoelectrode recording of human pluripotent stem cell-derived cardiomyocytes for assaying drugs and modeling disease. Microsyst. Nanoeng. 2017, 3, 16080. [Google Scholar] [CrossRef]
- Coulombe, K.L.; Bajpai, V.K.; Andreadis, S.T.; Murry, C.E. Heart regeneration with engineered myocardial tissue. Annu. Rev. Biomed. Eng. 2014, 16, 1–28. [Google Scholar] [CrossRef]


| Hydrogel (Type) | Stiffnesses | Degradability | Applications | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Alginate (Natural) | 200–500 kPa | Degrades within 2 weeks | 3D scaffold for ECM [39] Assessing electrical conductivity [40] | Electrical conductivity Low cost | Poor cell attachment |
| Hyaluronan (Natural) | 1.5–8 kPa | 5–7 days | Regenerative drug delivery [20] | Component of the ECM Fast gelation | Disruption of cell structure |
| Collagen/Gelatin (Natural) | 1–236 kPa | Days-weeks, depending on temperature | Self-healing injectable therapeutic delivery [41] | Low cost Easily modified | Limited range of stiffness |
| Polydimethylsiloxane (PDMS) (Synthetic) | 12 kPa–2 MPa | Can last up to several months | Assessing cell contractility [42] | Easy to produce Biocompatible | Hydrophobic |
| Polyacrylamide (PAA) (Synthetic) | 1–200 kPa | Up to 10 days | Electrophysiology of iPSC-CMs [43] | Easily modifiable Optically transparent | Cell adhesion issues & lower stiffness range |
| Polyethylene glycol (PEG) (Synthetic) | 10 kPa–1 MPa | Can be chemically modified to last months | Structural investigation of ventricular CMs [44] Injectable hydrogels improving cardiac function post MI [19] | Hydrophilic Non-toxic | Lack of cell specific adhesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, L.; Worch, J.C.; Dove, A.P.; Gehmlich, K. The Utilisation of Hydrogels for iPSC-Cardiomyocyte Research. Int. J. Mol. Sci. 2023, 24, 9995. https://doi.org/10.3390/ijms24129995
Patel L, Worch JC, Dove AP, Gehmlich K. The Utilisation of Hydrogels for iPSC-Cardiomyocyte Research. International Journal of Molecular Sciences. 2023; 24(12):9995. https://doi.org/10.3390/ijms24129995
Chicago/Turabian StylePatel, Leena, Joshua C. Worch, Andrew P. Dove, and Katja Gehmlich. 2023. "The Utilisation of Hydrogels for iPSC-Cardiomyocyte Research" International Journal of Molecular Sciences 24, no. 12: 9995. https://doi.org/10.3390/ijms24129995
APA StylePatel, L., Worch, J. C., Dove, A. P., & Gehmlich, K. (2023). The Utilisation of Hydrogels for iPSC-Cardiomyocyte Research. International Journal of Molecular Sciences, 24(12), 9995. https://doi.org/10.3390/ijms24129995