Application of IRI Visualization to Terahertz Vibrational Spectroscopy of Hydroxybenzoic Acid Isomers
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Simulated THz Spectra of 2-, 3-, and 4-HA
2.2. Analysis of the Vibrational Modes
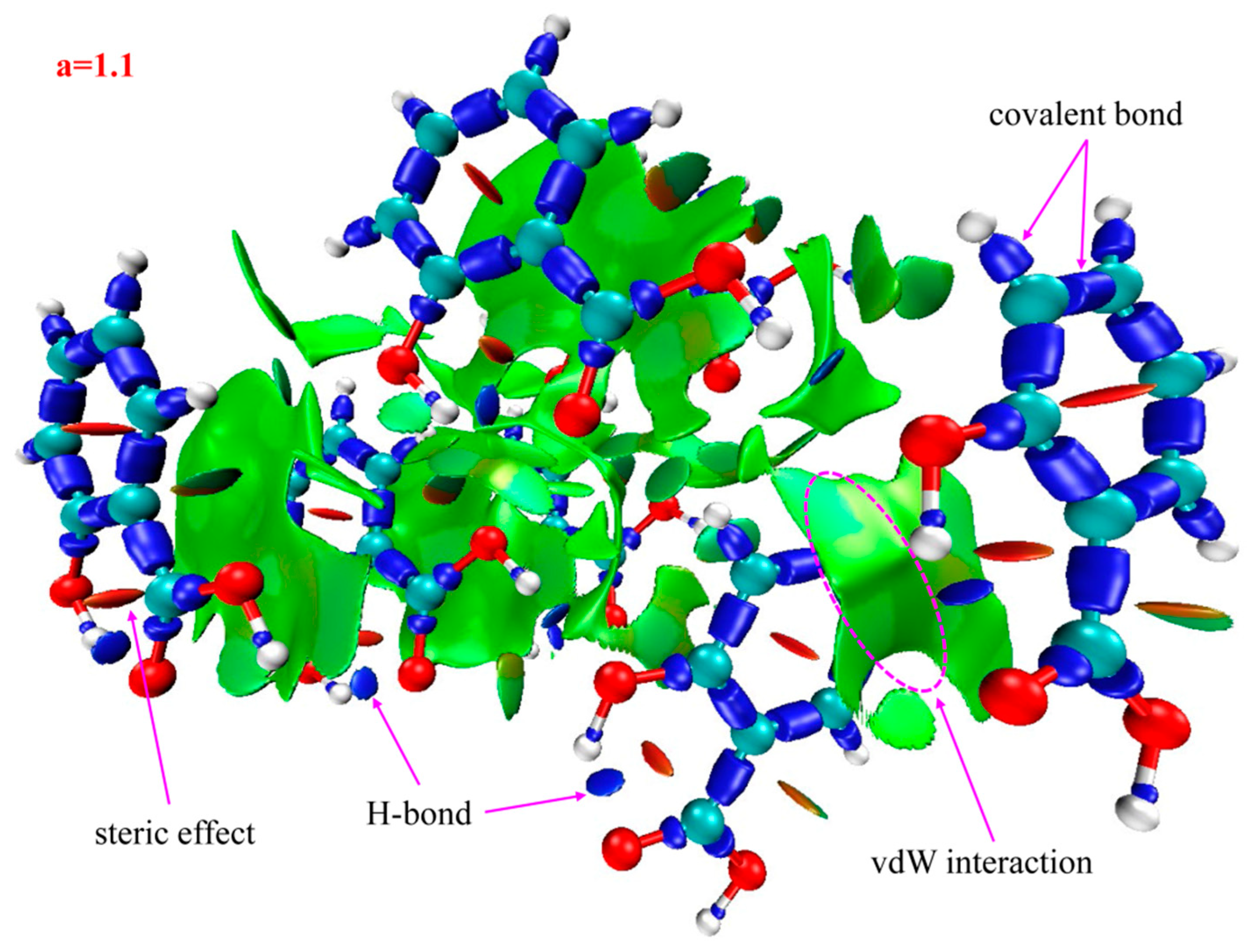
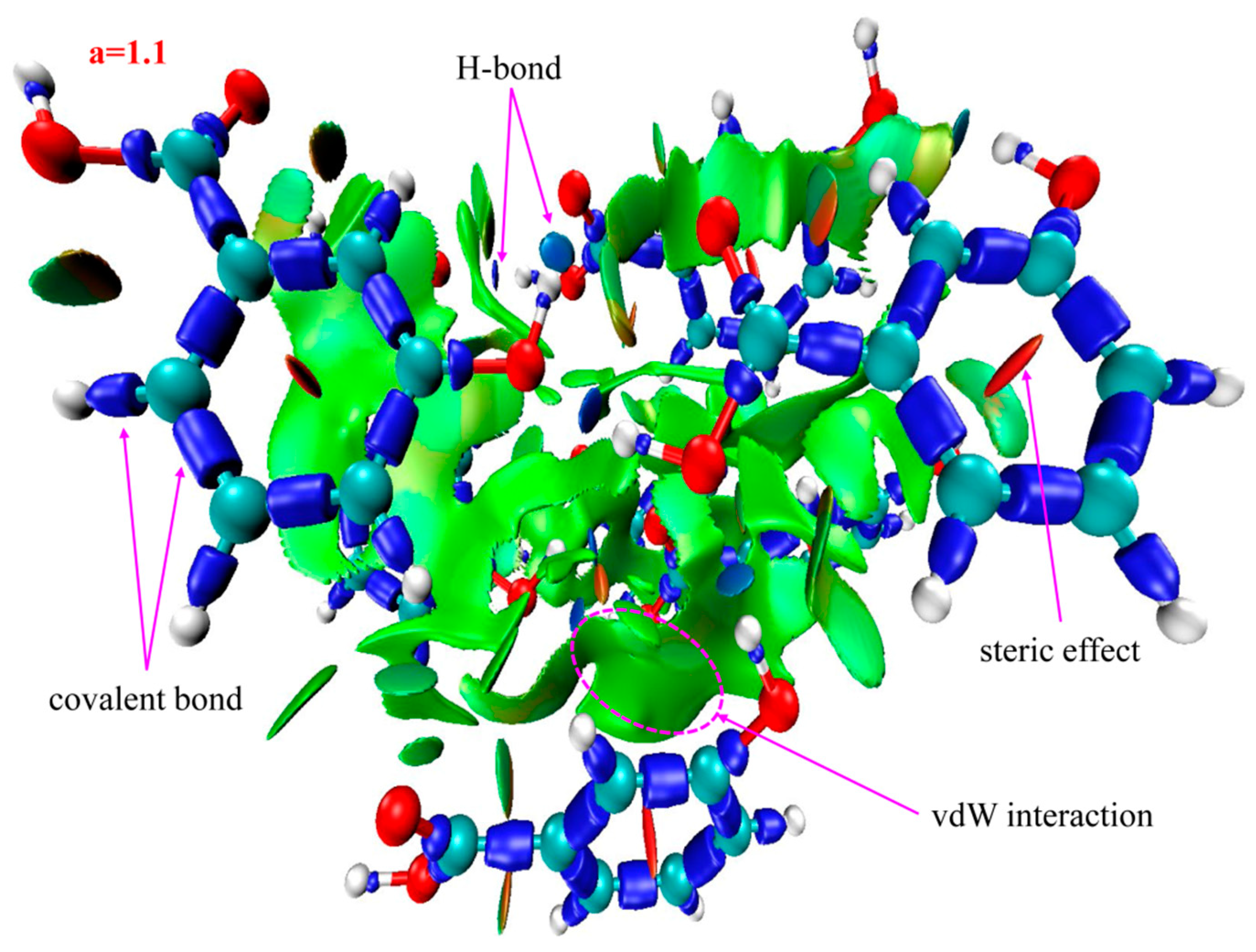
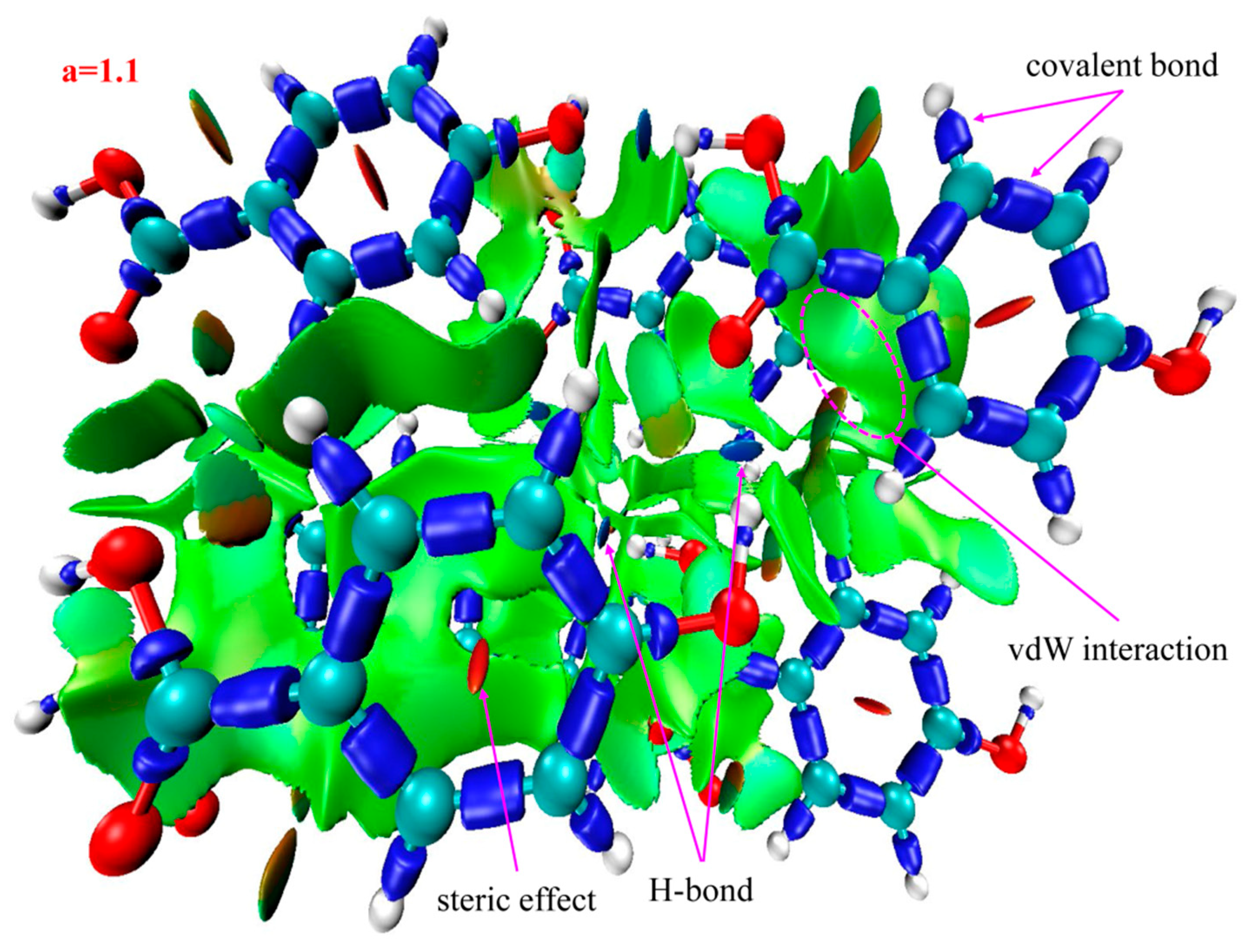
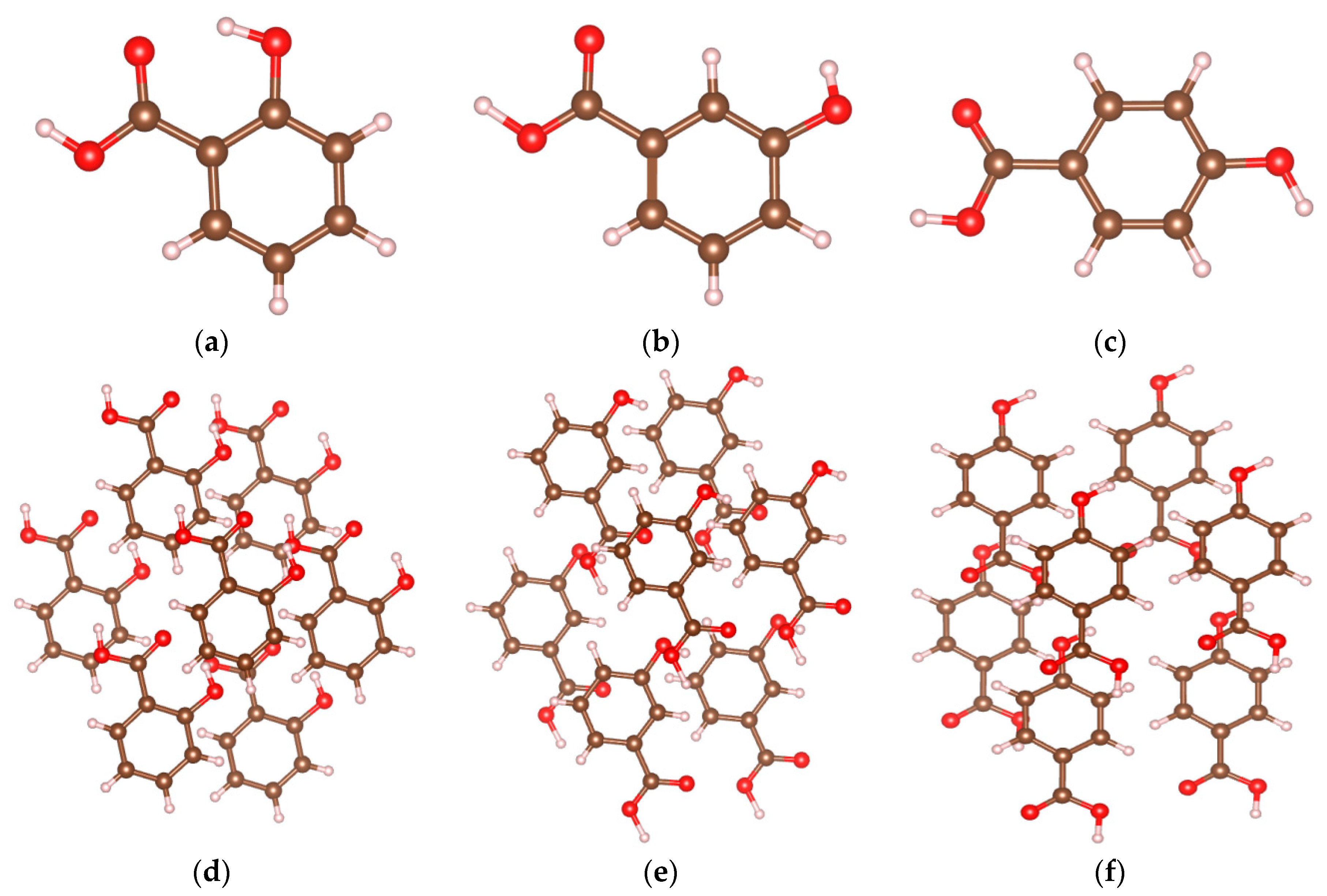
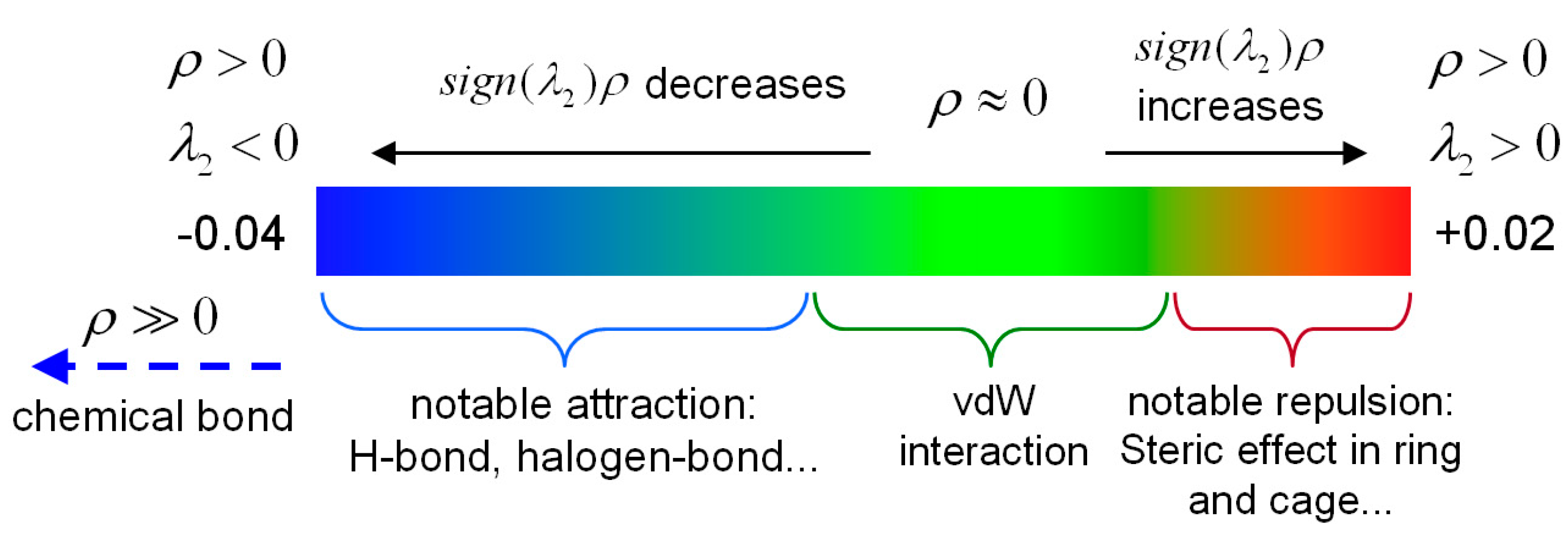
2.3. Identifying Weak Interactions with IRI
3. Materials and Methods
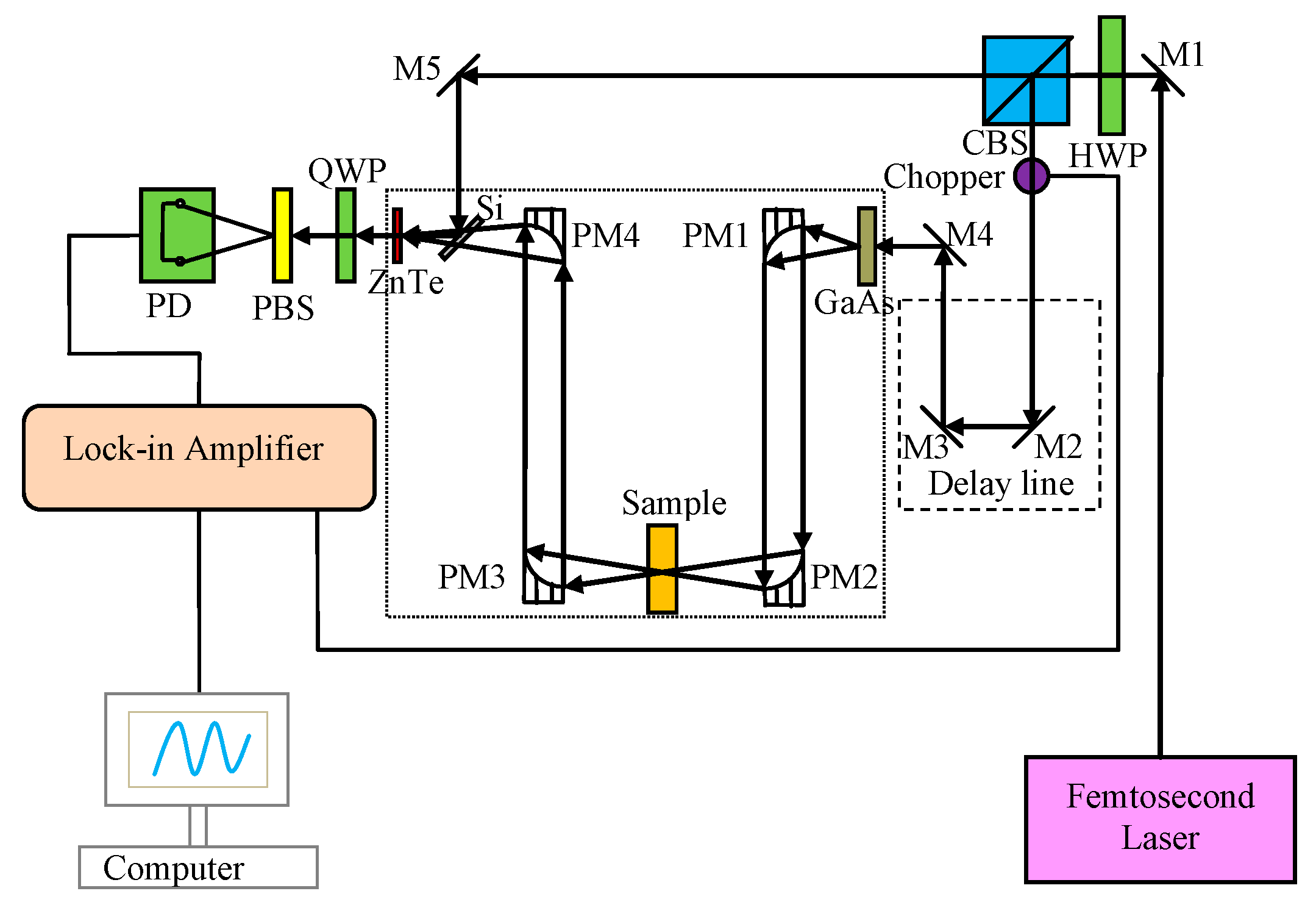
3.1. Experimental Apparatus
3.2. Sample Preparation
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heravi, M.M.; Behbahani, F.K.; Bamoharram, F.F. Acetylation of alcohols, phenols and salicylic acid by heteropoly acids in acetic anhydride: A green and eco-friendly protocol for synthesis of acetyl salicylic acid (Aspirin). Arkivoc 2007, XVI, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.T.; Teng, C.J.; Lee, Y.H.; Wu, J.Y.; Wang, K.L.; Lin, C.M. Design and Synthesis of a Long-Wavelength Latent Fluorogenic Substrate for Salicylate Hydroxylase: A Useful Fluorimetric Indicator for Analyte Determination by Dehydrogenase-Coupled Biosensors. Anal. Chem. 2010, 82, 7329–7334. [Google Scholar] [CrossRef] [PubMed]
- He, Q.X.; Huang, X.C.; Chen, Z.L. Influence of organic acids, complexing agents and heavy metals on the bioleaching of iron from kaolin using Fe(III)-reducing bacteria. Appl. Clay Sci. 2011, 51, 478–483. [Google Scholar] [CrossRef]
- Kumar, D.; Mishra, D.S.; Chakraborty, B.; Kumar, P. Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J. Food Sci. Technol. Mysore 2013, 50, 797–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, M.; Ramage, G.; Edwards, C.A.; Combet, E. Evaluation of the antibacterial potential of polyphenols against periodontal pathogens. Proc. Nutr. Soc. 2013, 72, E193. [Google Scholar] [CrossRef] [Green Version]
- Rajasekharan, S.; Rajasekharan, R.; Vaidyanathan, C.S. Substrate-Mediated Purification and Characterizationof a 3-Hydroxybenzoic Acid-6-Hydroxylase Substrate-Mediated Purification and Characterizationof a 3-Hydroxybenzoic Acid-6-Hydroxylase from Micrococcus. Arch. Biocheistry Biophys. 1990, 278, 21–25. [Google Scholar] [CrossRef]
- de Oliveira, D.A.; de Rezende Rodovalho, V.; Flauzino, J.M.; da Silva, H.S.; Araujo, G.R.; Vaz, E.R.; Vieira, C.U.; Madurro, J.M.; Madurro, A.G.B. Serological Electrodetection of Rheumatoid Arthritis Using Mimetic Peptide. Protein Peptied Lett. 2018, 25, 873–883. [Google Scholar] [CrossRef]
- Liu, J.S.; Sun, S.; Han, Y.L.; Meng, J.; Chen, Y.; Yu, H.; Zhang, X.; Ma, F. Lignin waste as co-substrate on decolorization of azo dyes by Ganoderma lucidum. J. Taiwan Inst. Chem. Eng. 2021, 122, 85–92. [Google Scholar] [CrossRef]
- Fernández-del-Río, L.; Clarke, C.F. Coenzyme Q biosynthesis: An update on the origins of the benzenoid ring and discovery of new ring precursors. Metabolites 2021, 11, 385. [Google Scholar] [CrossRef]
- Wang, S.W.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. 4-Hydroxybenzoic acid-a versatile platform intermediate for value-added compounds. Appl. Microbiol. Biotechnol. 2018, 102, 3561–3571. [Google Scholar] [CrossRef]
- Boczar, M.; Boda, L.; Wojcik, M.J. Theoretical modeling of infrared spectra of hydrogen-bonded crystals of salicylic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Nie, J.Y.; Tu, S. Study on identification methods in the detection of transgenic material based on terahertz time domain spectroscopy. Opt. Quantum Electron. 2015, 47, 3533–3543. [Google Scholar]
- Tu, S.; Zhang, W.T.; Xiong, X.M.; Chen, T. Principal Component Analysis for Transgenic Cotton Seeds Based on Terahertz Time Domain Spectroscopy System. Acta Photonica Sin. 2015, 44, 176–181. [Google Scholar]
- Ding, L.; Fan, W.H.; Song, C.; Chen, X.; Chen, Z.Y. Terahertz Spectroscopic Investigation of Salicylic Acid and Sodium Salicylate. J. Appl. Spectrosc. 2019, 85, 1143–1150. [Google Scholar] [CrossRef]
- Evangelisti, L.; Tang, S.; Velino, B.; Caminati, W. Microwave spectrum of salicylic acid. J. Mol. Struct. 2009, 921, 285–288. [Google Scholar] [CrossRef]
- Vulpius, D.; Geipel, G.; Bernhard, G. Excited-state proton transfer of 3-hydroxybenzoic acid and 4-hydroxybenzoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Xue, J.D.; Hong, Z.; Du, Y. Pharmaceutical Cocrystal Formation of Pyrazinamide with 3-Hydroxybenzoic Acid: A Terahertz and Raman Vibrational Spectroscopies Study. Molecules 2019, 24, 488. [Google Scholar] [CrossRef] [Green Version]
- Lepodise, L.M.; Horvat, J.; Lewis, R.A. Terahertz (6–15 THz) Spectroscopy and Numerical Modeling of Intermolecular Vibrations in Benzoic Acid and Its Derivatives. Appl. Spectrosc. 2015, 69, 590–596. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, S.W. Effect of 4-hydroxybenzoic acid on CO2 separation performance of poly (ethylene oxide) membrane. Macromol. Res. 2016, 24, 1111–1114. [Google Scholar] [CrossRef]
- Brandan, S.A.; Lopez, F.M.; Montejo, M.; González, J.L.; Altabef, A.B. Theoretical and experimental vibrational spectrum study of 4-hydroxybenzoic acid as monomer and dimer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1422–1434. [Google Scholar] [CrossRef]
- Heath, E.A.; Singh, P.; Ebisuzaki, Y. Structure of p-hydroxybenzoic acid and p-hydroxybenzoic acid-acetone complex (2/1). Acta Crystallogr. 1992, 48, 1960–1965. [Google Scholar] [CrossRef]
- Tu, S.; Wang, Z.G.; Zhang, W.T.; Xiao, H.; She, Y.; Tang, Y.; Yi, C.; Liang, G. Study of Weak Intermolecular Interactions and Vibrational Modes in Hydroxybenzoic Acid Isomers using Terahertz Spectroscopy and Density Functional Theory Techniques. Infrared Phys. Technol. 2021, 119, 103950. [Google Scholar] [CrossRef]
- Yi, C.C.; Tuo, S.; Tu, S.; Zhang, W.T. Improved fuzzy C-means clustering algorithm based on t-SNE for terahertz spectral recognition. Infrared Phys. Technol. 2021, 117, 103856. [Google Scholar] [CrossRef]
- Tu, S.; Wang, Z.G.; Liang, G.L.; Zhang, W.; Tang, Y.; She, Y.; Yi, C.; Bi, X. A novel approach to discriminate transgenic soybean seeds based on terahertz spectroscopy. Optik 2021, 242, 167089. [Google Scholar] [CrossRef]
- Ma, J.L.; Xu, K.J.; Li, Z.; Jin, B.B.; Fu, R.; Zhang, C.H.; Ji, Z.M.; Zhang, C.; Chen, Z.X.; Chen, J.; et al. Temperature-dependent terahertz spectroscopy of D-, L- and DL-ornidazole. Acta Phys. Sin. 2009, 58, 6101–6107. [Google Scholar]
- Jamróz, M.H. Vibrational Energy Distribution Analysis (VEDA): Scopes and limitations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 220–230. [Google Scholar] [CrossRef]
- Chen, T.; Zhong, X.; Li, Z.; Hu, F. Analysis of Intermolecular Weak Interactions and Vibrational Characteristics for Vanillin and Ortho-Vanillin by Terahertz Spectroscopy and Density Functional Theory. IEEE Trans. Terahertz Sci. Technol. 2020, 11, 318–329. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Chen, Z.; Kang, K.; Feng, B.; Zhang, Y. Terahertz absorbance spectrum fitting method for quantitative detection of concealed contraband. J. Appl. Phys. 2007, 102, 113108. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, H.; Zhou, H. Quantitative Determination of Cyfluthrin in N-Hexane by Terahertz Time-Domain Spectroscopy With Chemometrics Methods. IEEE Trans. Instrum. Meas. 2010, 59, 1414–1423. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R. Data-Driven High-Throughput Prediction of the 3-D Structure of Small Molecules: Review and Progress. A Response from The Cambridge Crystallographic Data Centre. J. Chem. Inf. Model. 2011, 51, 2787. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.M.; Joe, L.V.; Howard, A.A.; Tschumper, G.S.; Hammer, N.I. Spectroscopic and computational insight into weak noncovalent interactions in crystalline pyrimidine. Chem. Phys. Lett. 2011, 501, 319–323. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Chen, Q.X. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions. Chem.-Methods 2007, 1, 231–239. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energy and SAPT theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
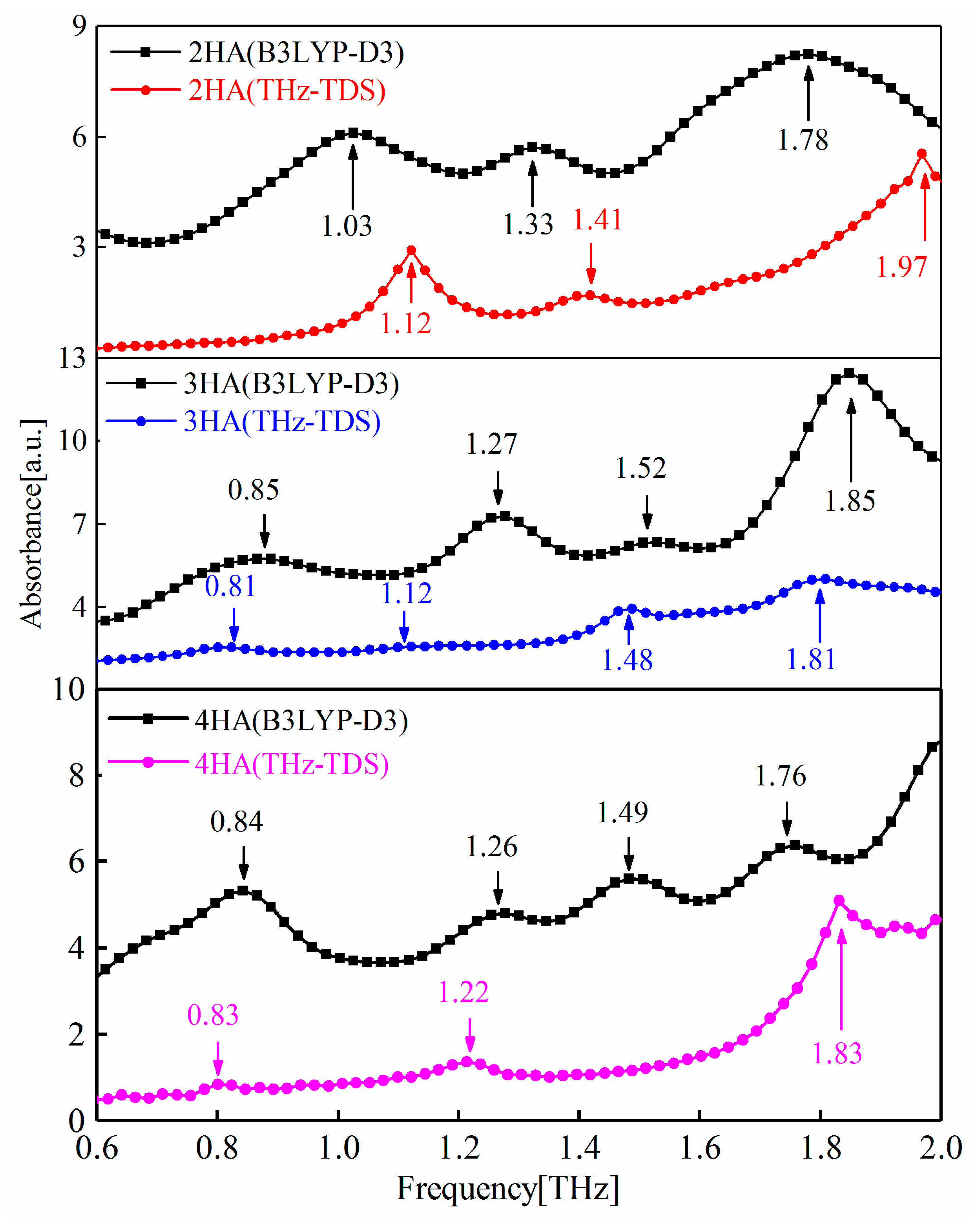
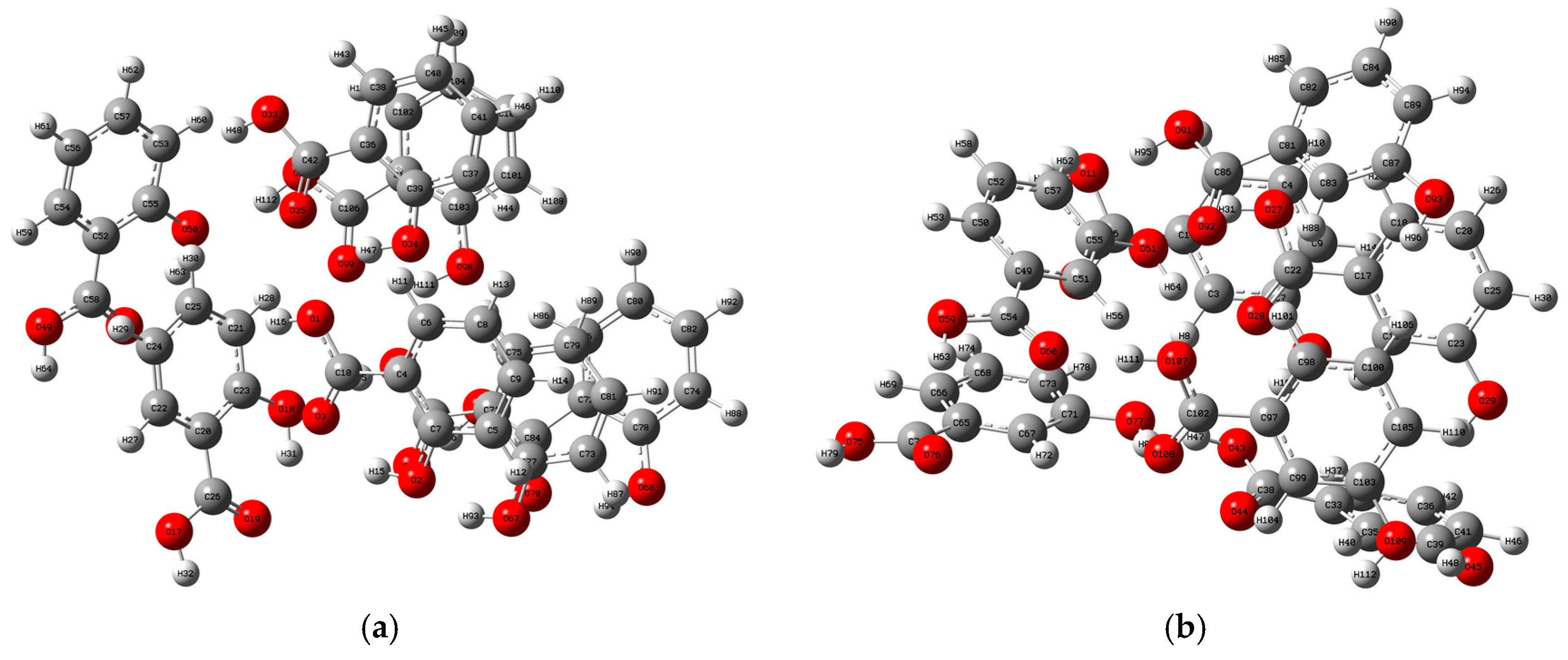
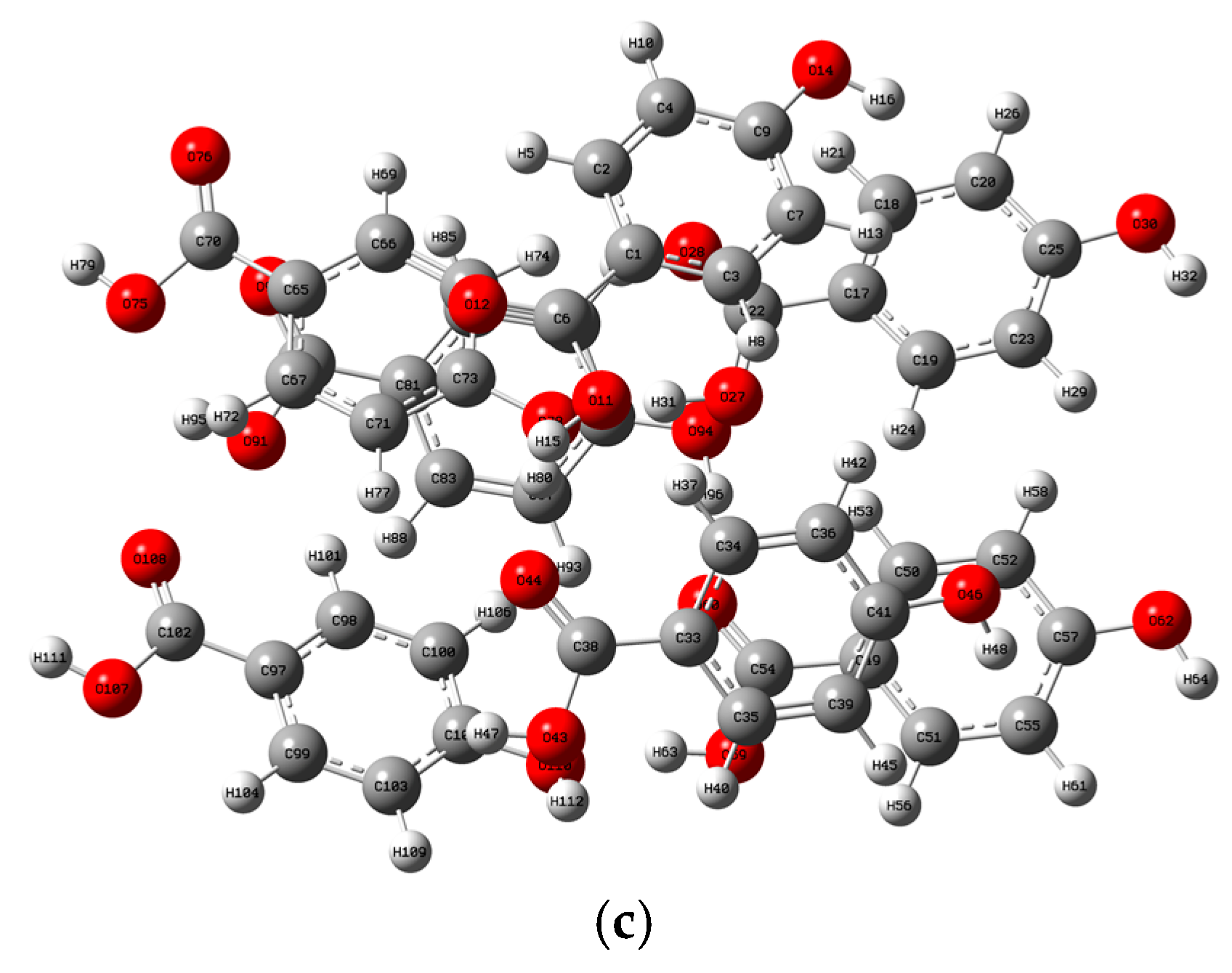
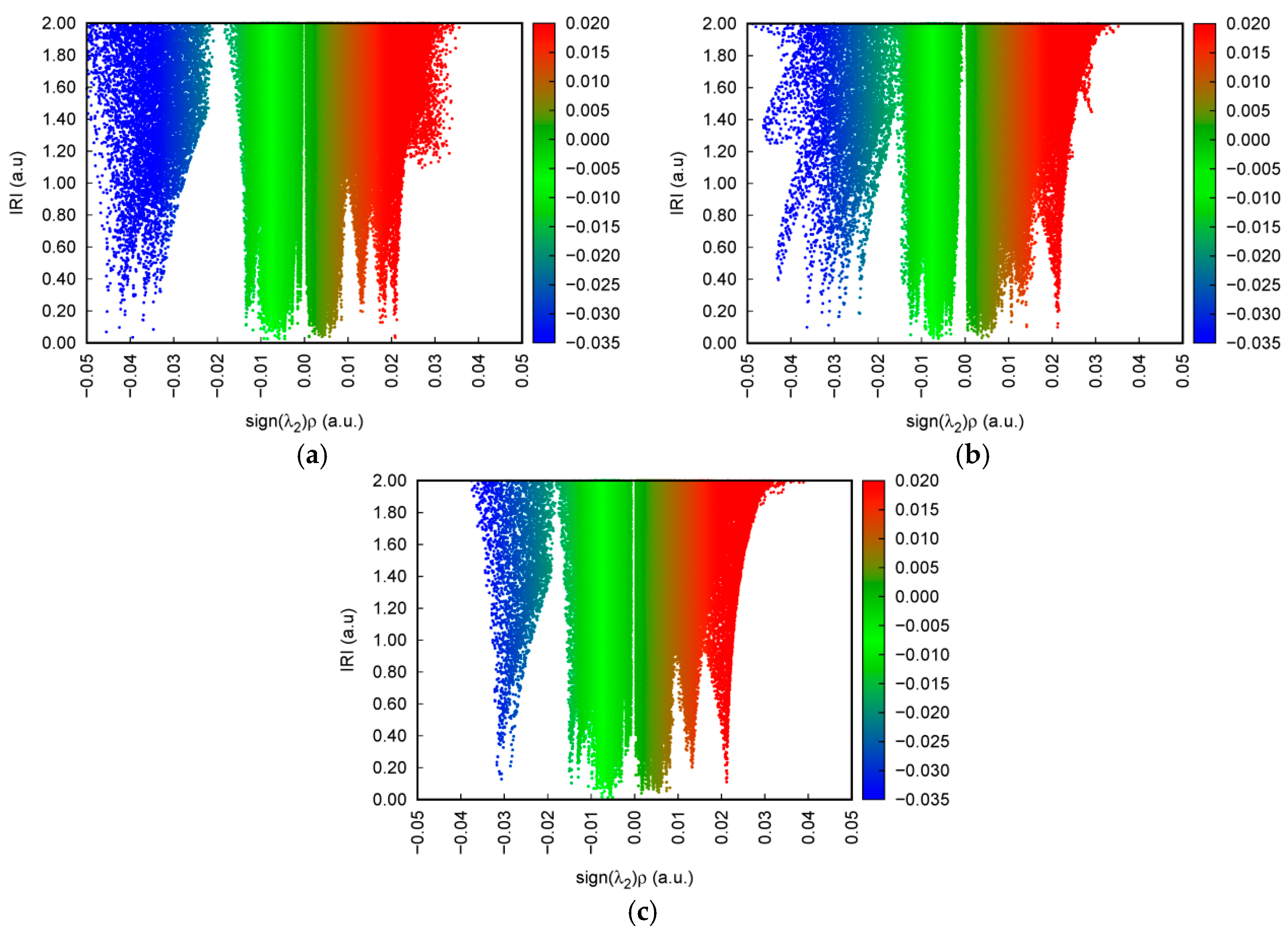
| Samples | Experimentally Obtained/THz | Calculated/THz | Vibrational Modes |
|---|---|---|---|
| 2-HA | 1.12 | 1.03 | O: O(1)H(16) and C(25)C(21)H(16) |
| 1.41 | 1.33 | B: H(48)C(53)C(57); T: C(10)C(103)O(98)H(87) | |
| – | 1.78 | Intermolecular interaction | |
| 1.97 | – | Intermolecular interaction | |
| 3-HA | 0.81 | 0.85 | Intermolecular interaction |
| 1.12 | – | Intermolecular interaction | |
| – | 1.27 | S: C(55)H(95) | |
| 1.48 | 1.52 | T: H(16)O(13)C(7)C(3) | |
| 1.81 | 1.85 | Intermolecular interaction | |
| 4-HA | 0.83 | 0.84 | T: C(1)C(6)O(78)C(73) |
| 1.22 | 1.26 | B: H(96)O(60)C(54) | |
| – | 1.49 | T: C(3)C(1)C(6)O(78) | |
| 1.83 | 1.76 | Intermolecular interaction |
| Samples | Spikes/a.u. | Isosurfaces | Weak Interaction Categories |
|---|---|---|---|
| 2-HA | sign(λ2)ρ = −0.043 sign(λ2)ρ = −0.035 | blue | H-bonds |
| sign(λ2)ρ = −0.014 sign(λ2)ρ = −0.009 sign(λ2)ρ = −0.007 sign(λ2)ρ = −0.005 | green | vdW interactions | |
| sign(λ2)ρ = +0.007 sign(λ2)ρ = +0.022 | brown | steric effects | |
| red | |||
| 3-HA | sign(λ2)ρ = −0.044 sign(λ2)ρ = −0.036 sign(λ2)ρ = −0.024 | blue | H-bonds |
| sign(λ2)ρ = −0.012 sign(λ2)ρ = −0.006 | green | vdW interactions | |
| sign(λ2)ρ = +0.004 sign(λ2)ρ = +0.021 | brown red | steric effects | |
| 4-HA | sign(λ2)ρ = −0.03 | blue | H-bonds |
| sign(λ2)ρ = −0.014 sign(λ2)ρ = −0.006 | green | vdW interaction | |
| sign(λ2)ρ = +0.006 sign(λ2)ρ = +0.013 sign(λ2)ρ = +0.022 | brown | steric effects | |
| red |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.; Zhang, W.; Tang, Y.; Li, Y.; Hu, J. Application of IRI Visualization to Terahertz Vibrational Spectroscopy of Hydroxybenzoic Acid Isomers. Int. J. Mol. Sci. 2023, 24, 10417. https://doi.org/10.3390/ijms241310417
Tu S, Zhang W, Tang Y, Li Y, Hu J. Application of IRI Visualization to Terahertz Vibrational Spectroscopy of Hydroxybenzoic Acid Isomers. International Journal of Molecular Sciences. 2023; 24(13):10417. https://doi.org/10.3390/ijms241310417
Chicago/Turabian StyleTu, Shan, Wentao Zhang, Yuan Tang, Yuanpeng Li, and Junhui Hu. 2023. "Application of IRI Visualization to Terahertz Vibrational Spectroscopy of Hydroxybenzoic Acid Isomers" International Journal of Molecular Sciences 24, no. 13: 10417. https://doi.org/10.3390/ijms241310417






