Microvascular Skeletal-Muscle Crosstalk in Health and Disease
Abstract
1. Introduction
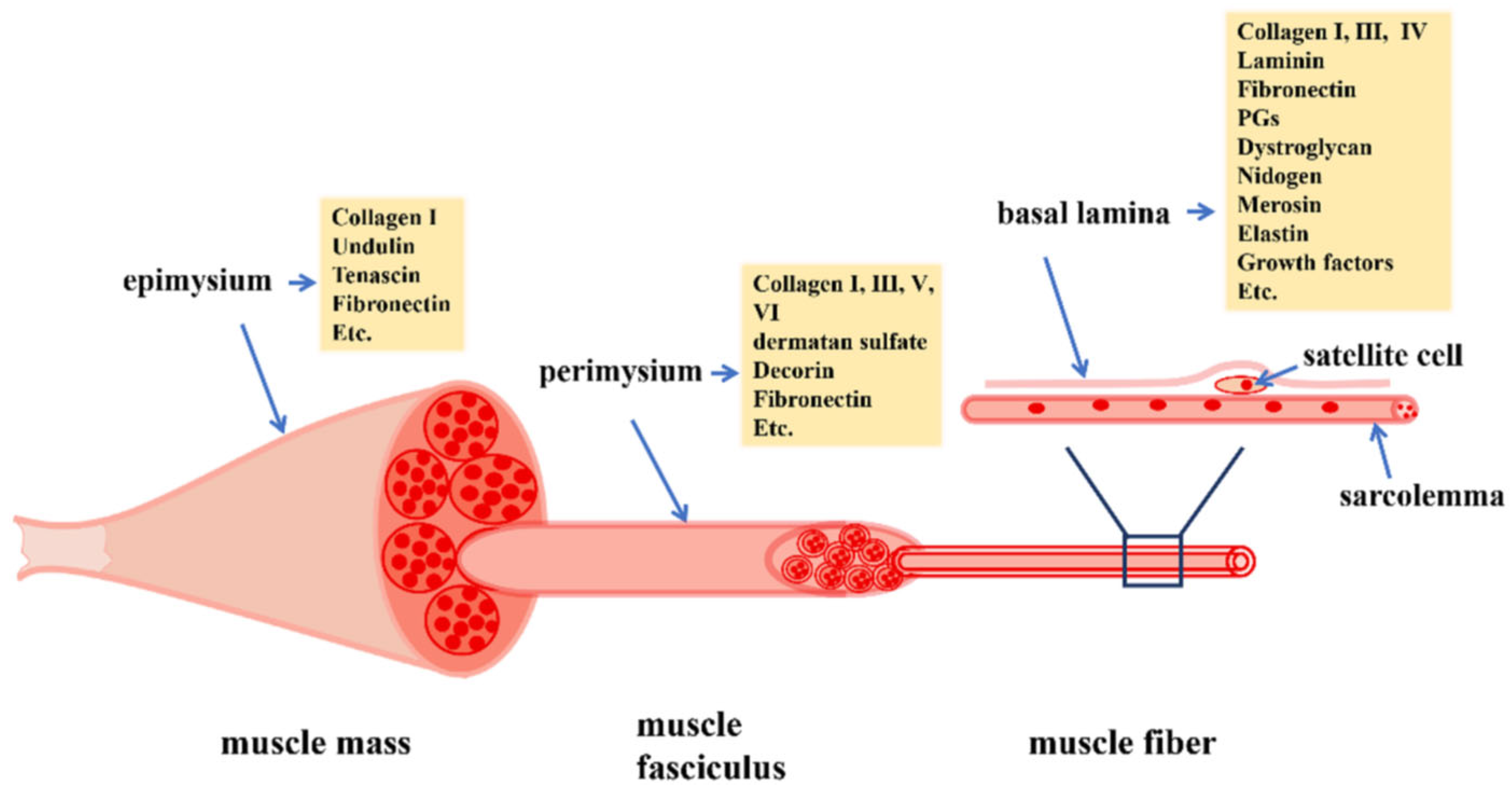
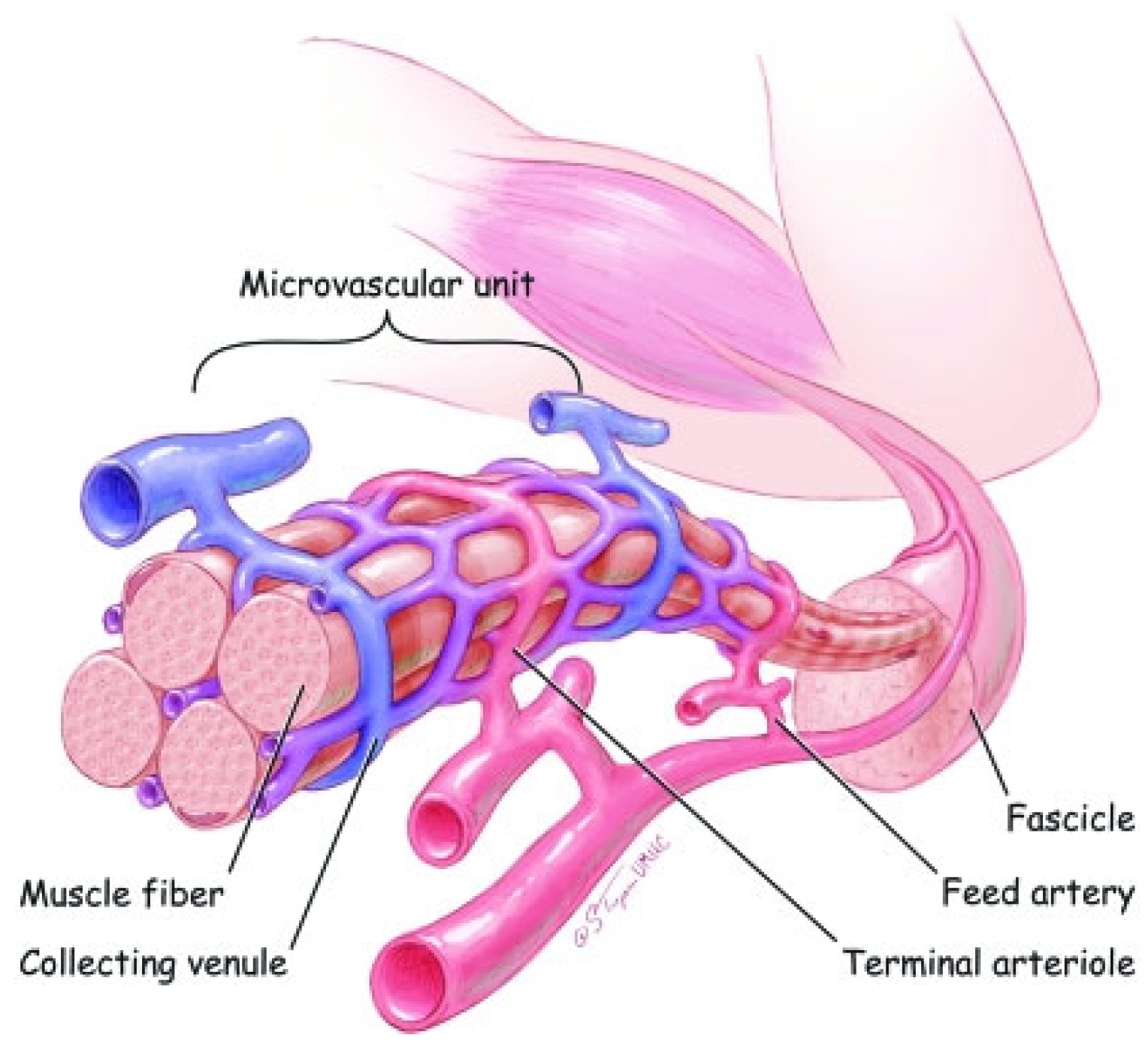
2. Development of Skeletal Muscle Myofibers and Microvasculature
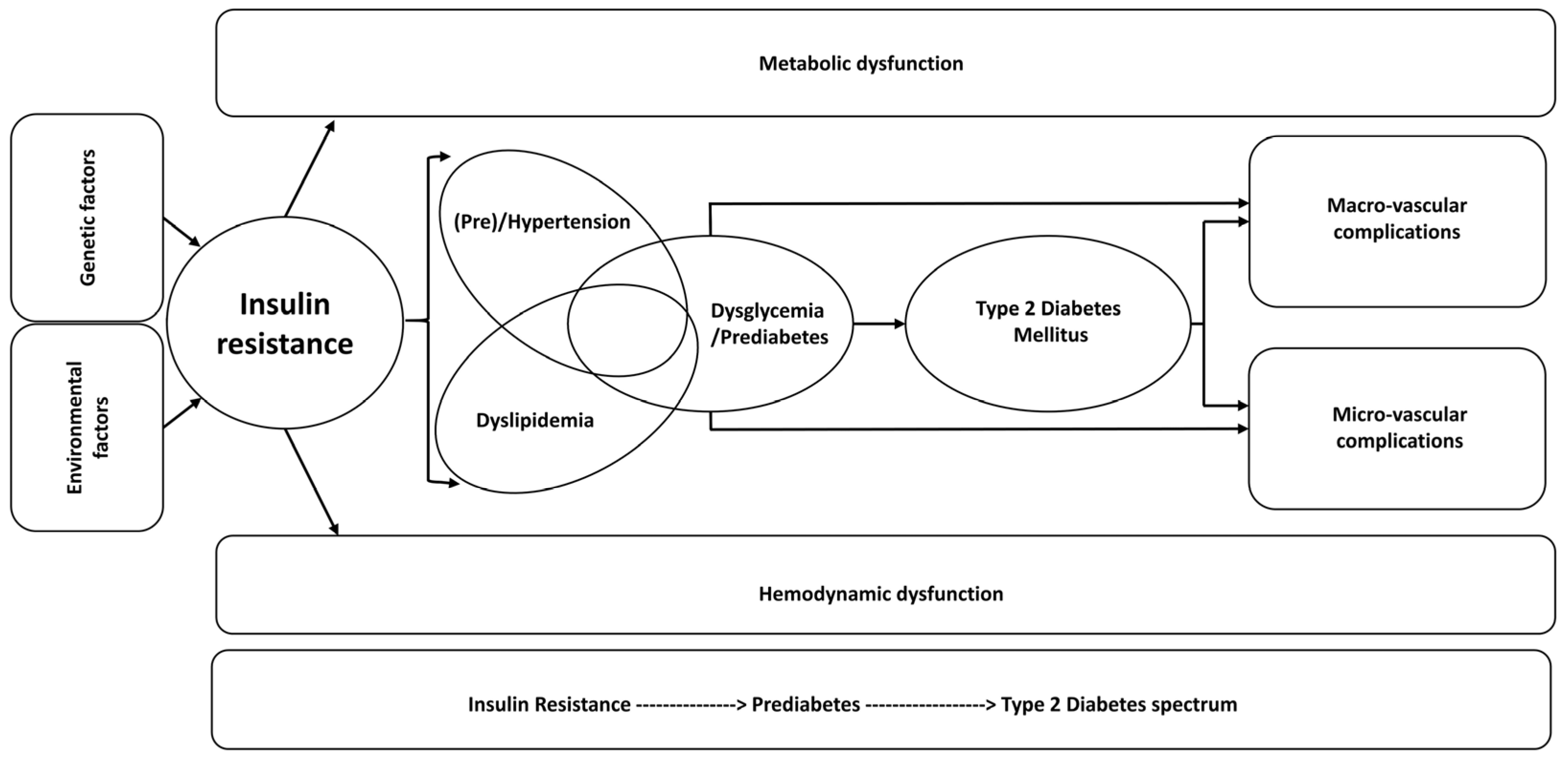
3. Vascular–Myocyte Interaction/Cross-Talk in Glucose and Vascular Homeostasis and Dysfunction in Diabetes and Hypertension
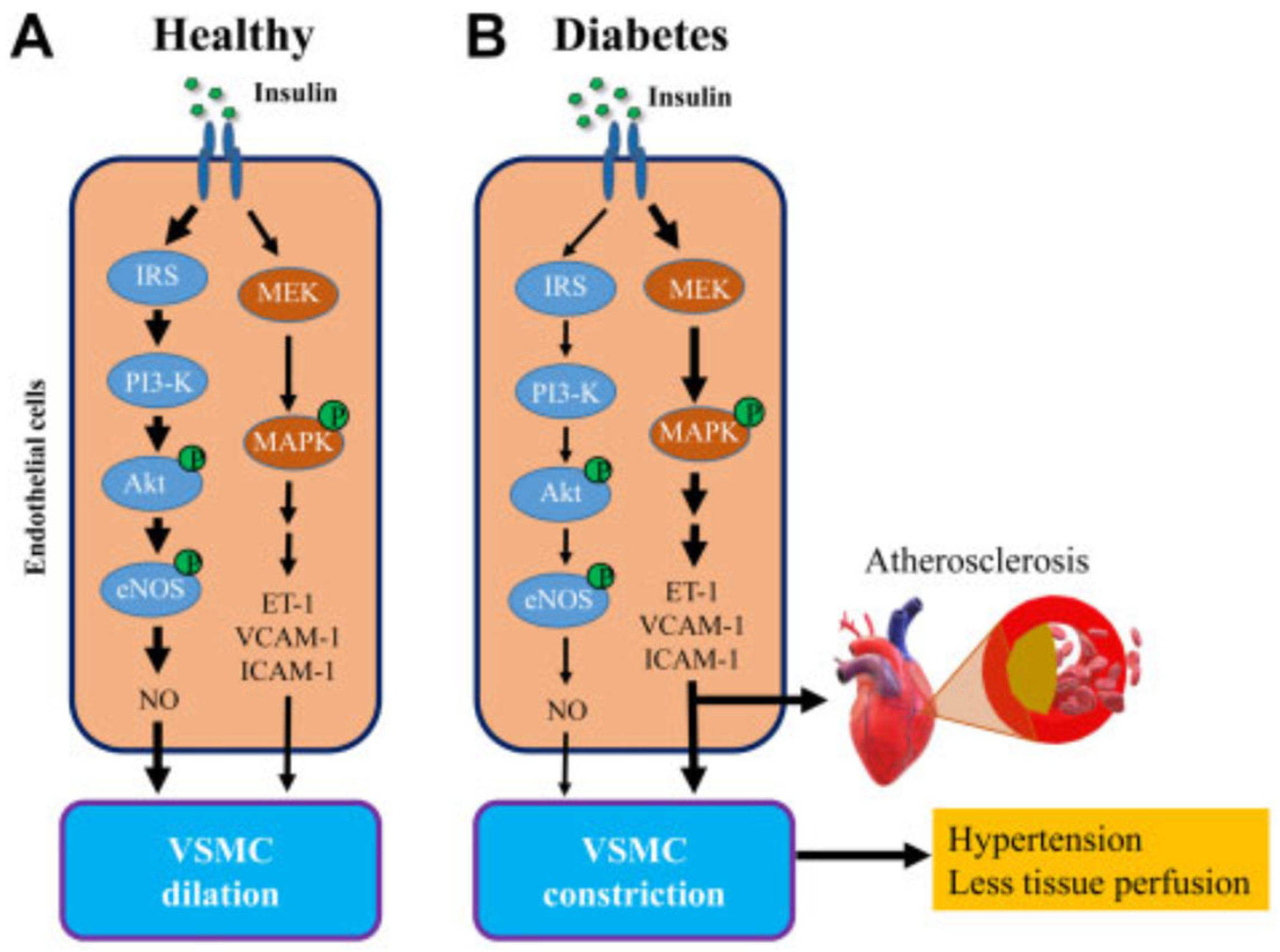
- [a]
- Action of insulin on myocyte metabolism:
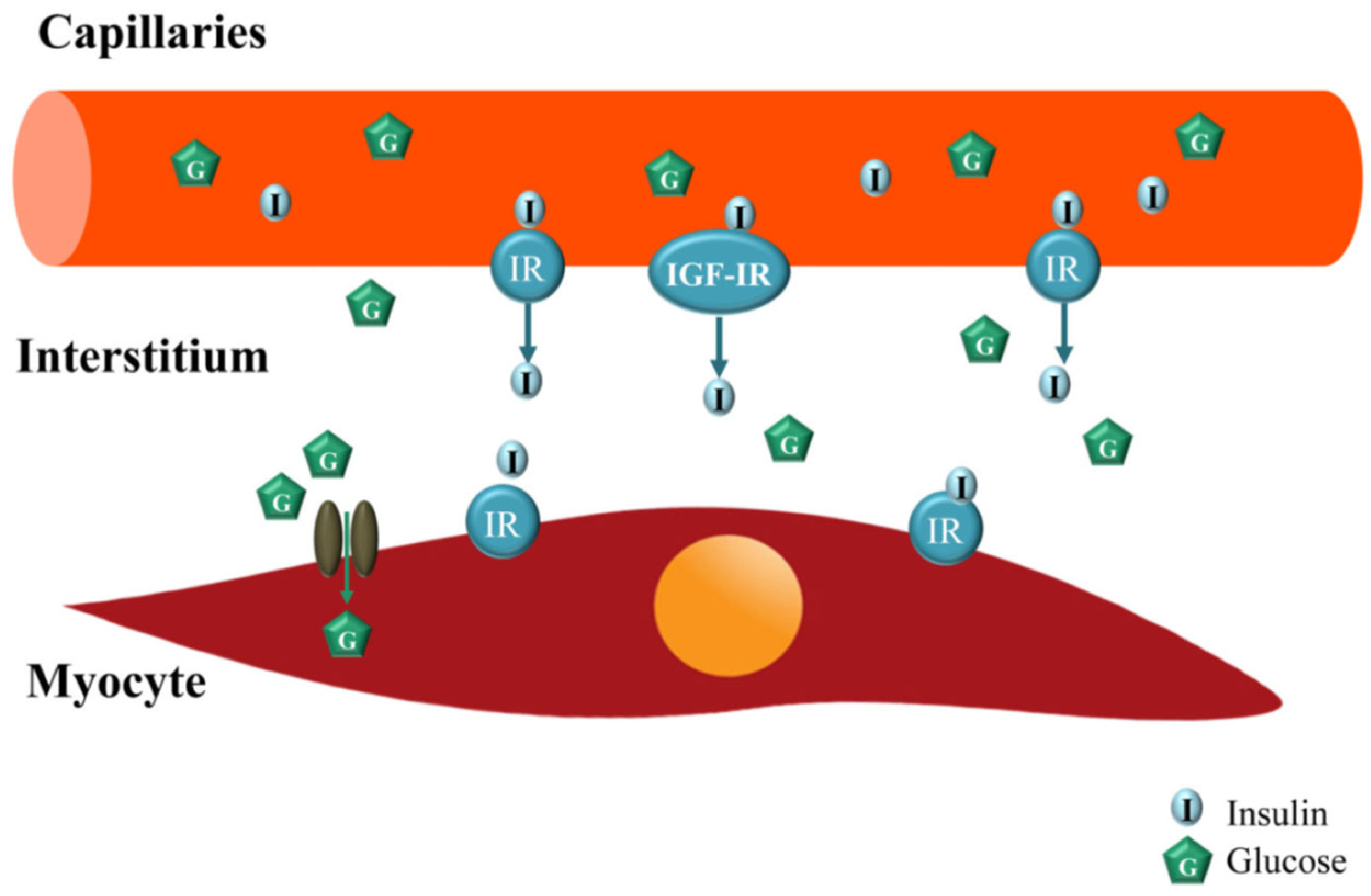
- [b]
- Action of insulin on MVU:
- [c]
- MVU myocyte dysfunction in type 2 diabetes:
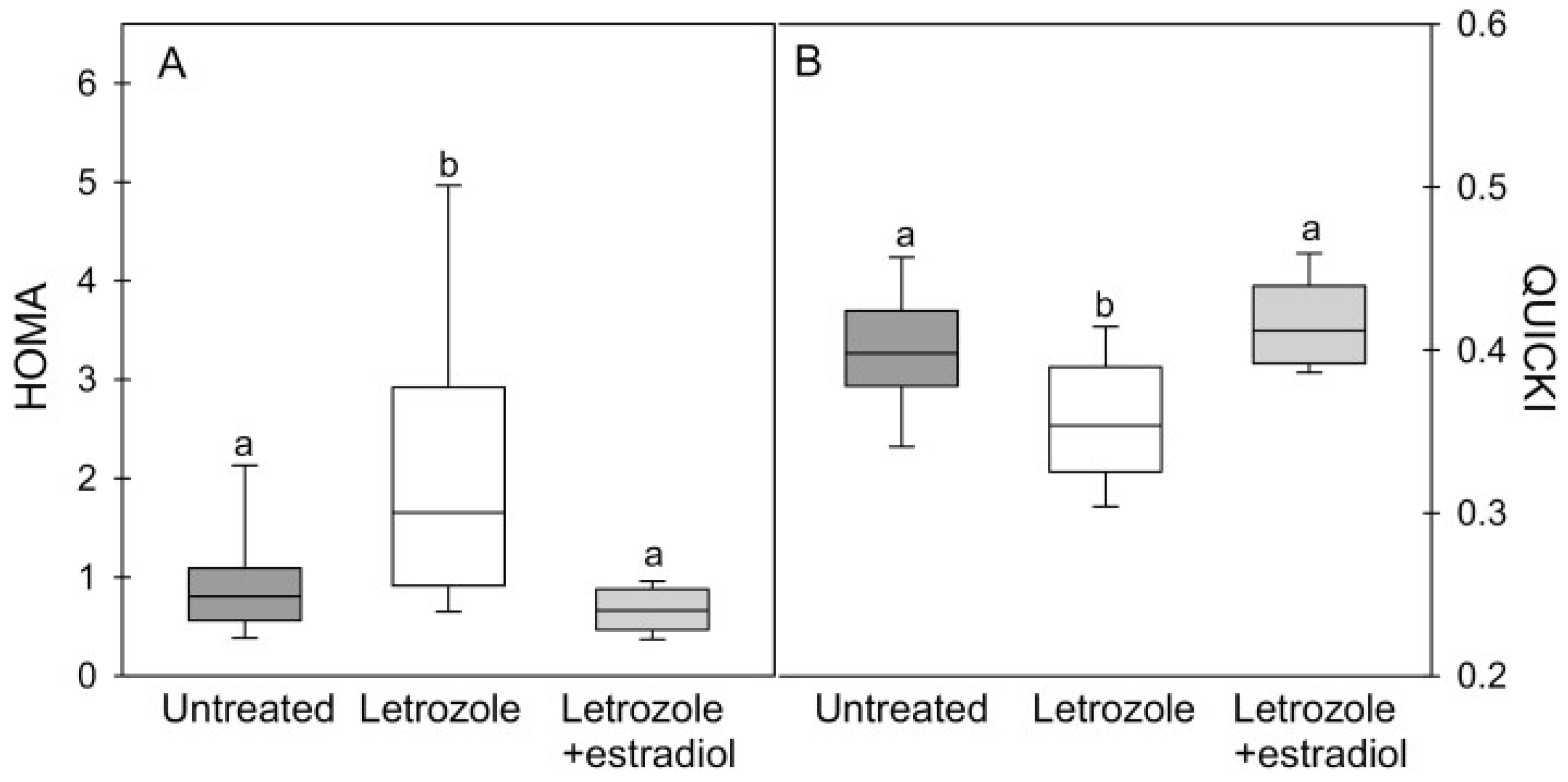
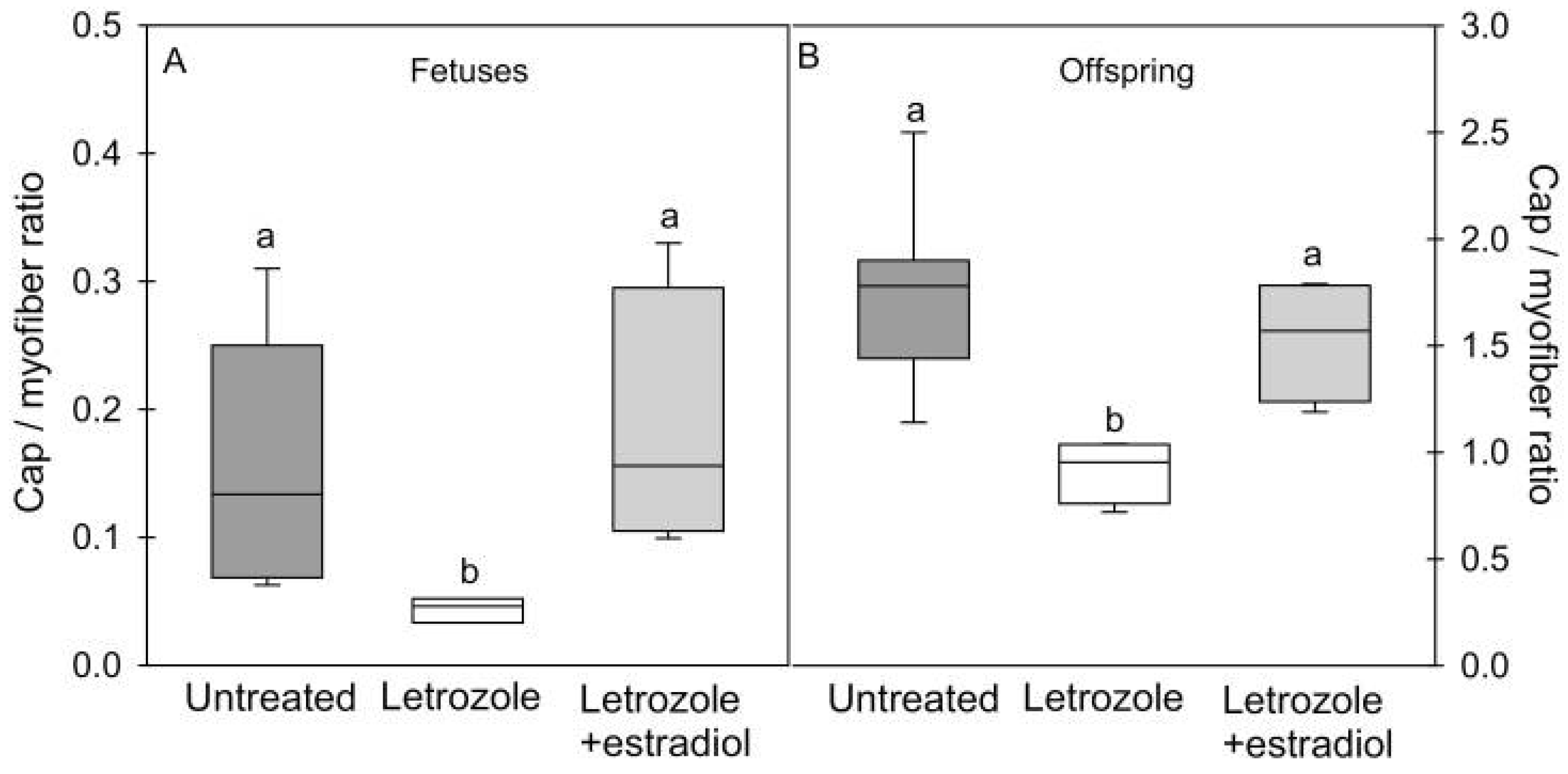
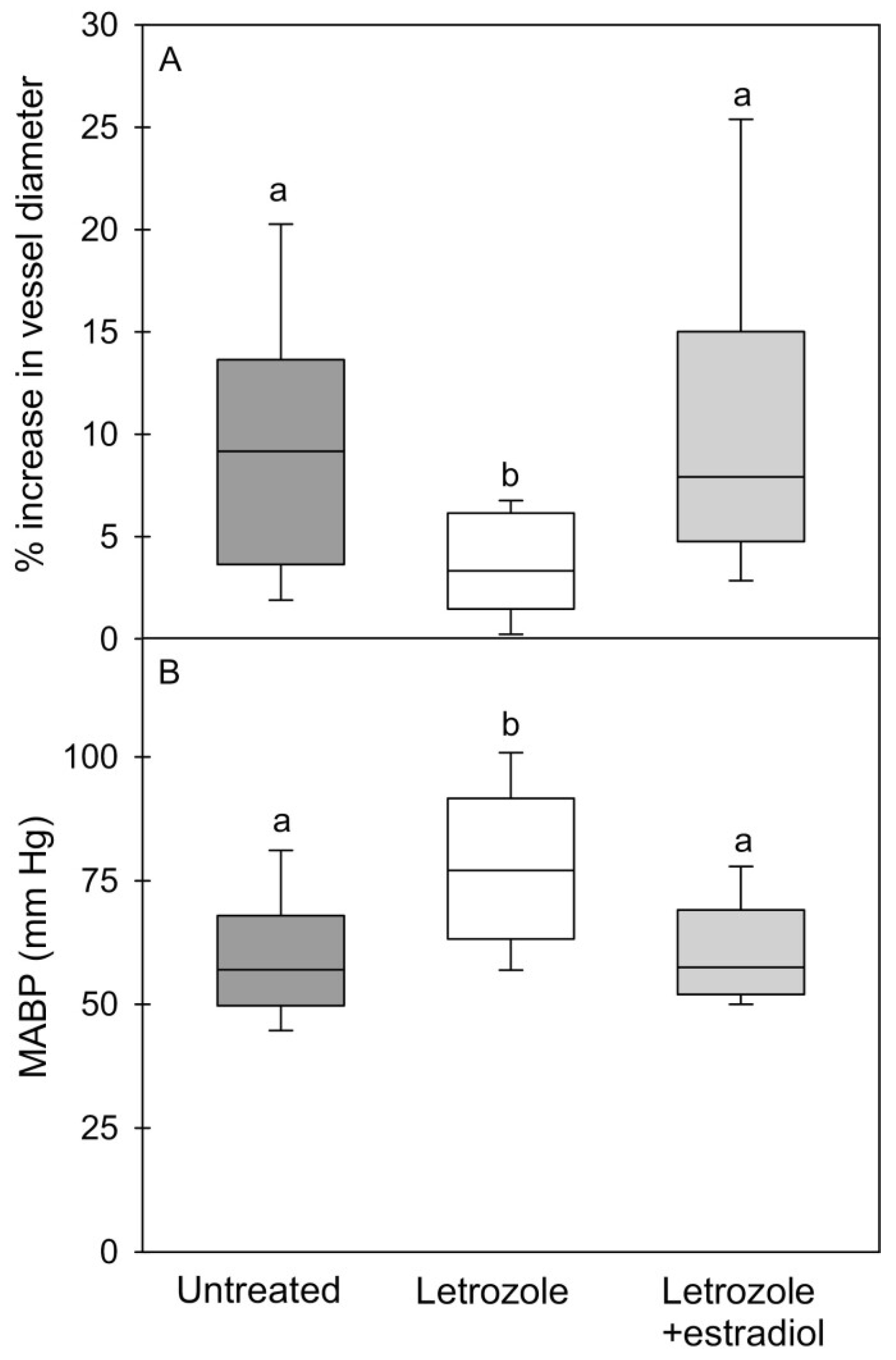
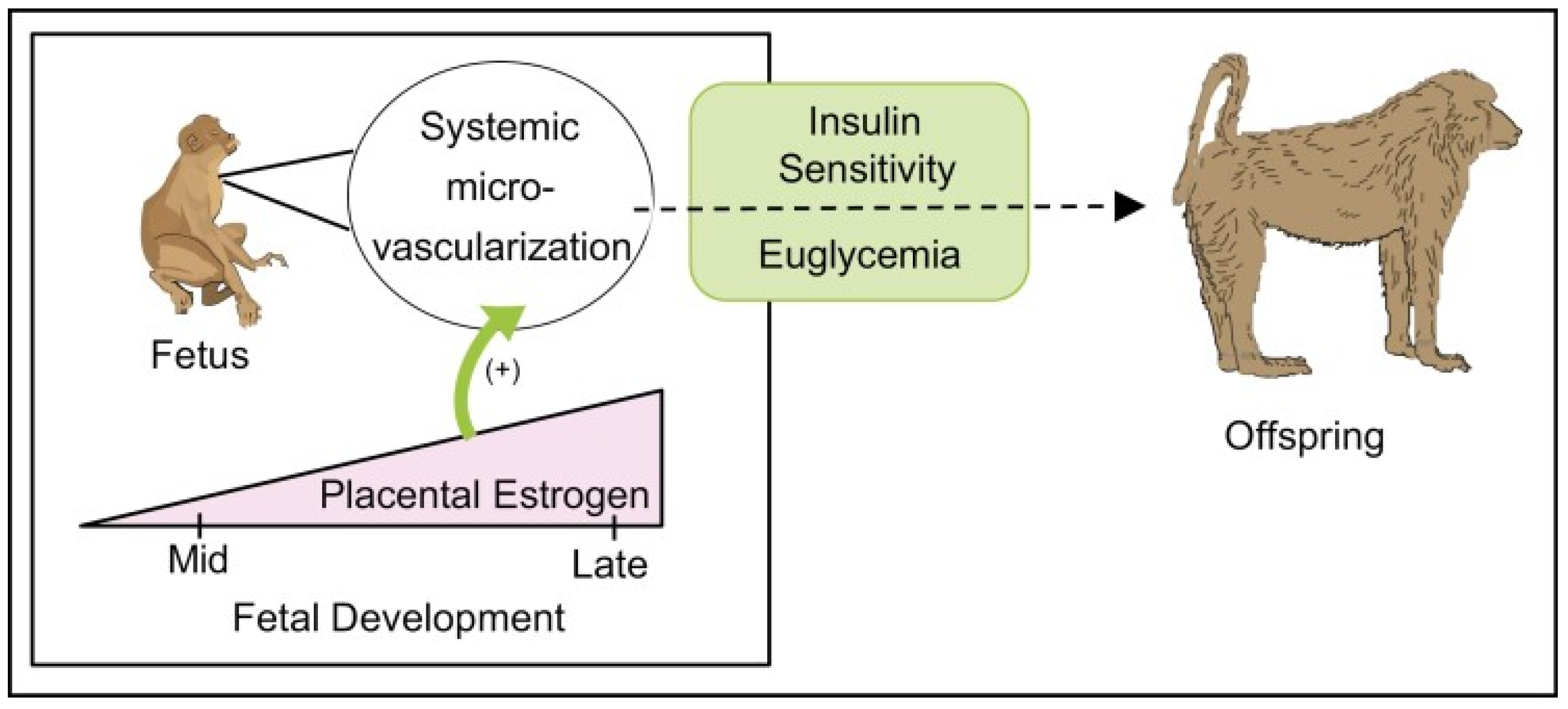
4. Estrogen Regulation of Fetal Skeletal Muscle Myogenesis and Capillarization In Utero: Impact on Glucose Homeostasis, Insulin Sensitivity and Vascular Function in Adulthood
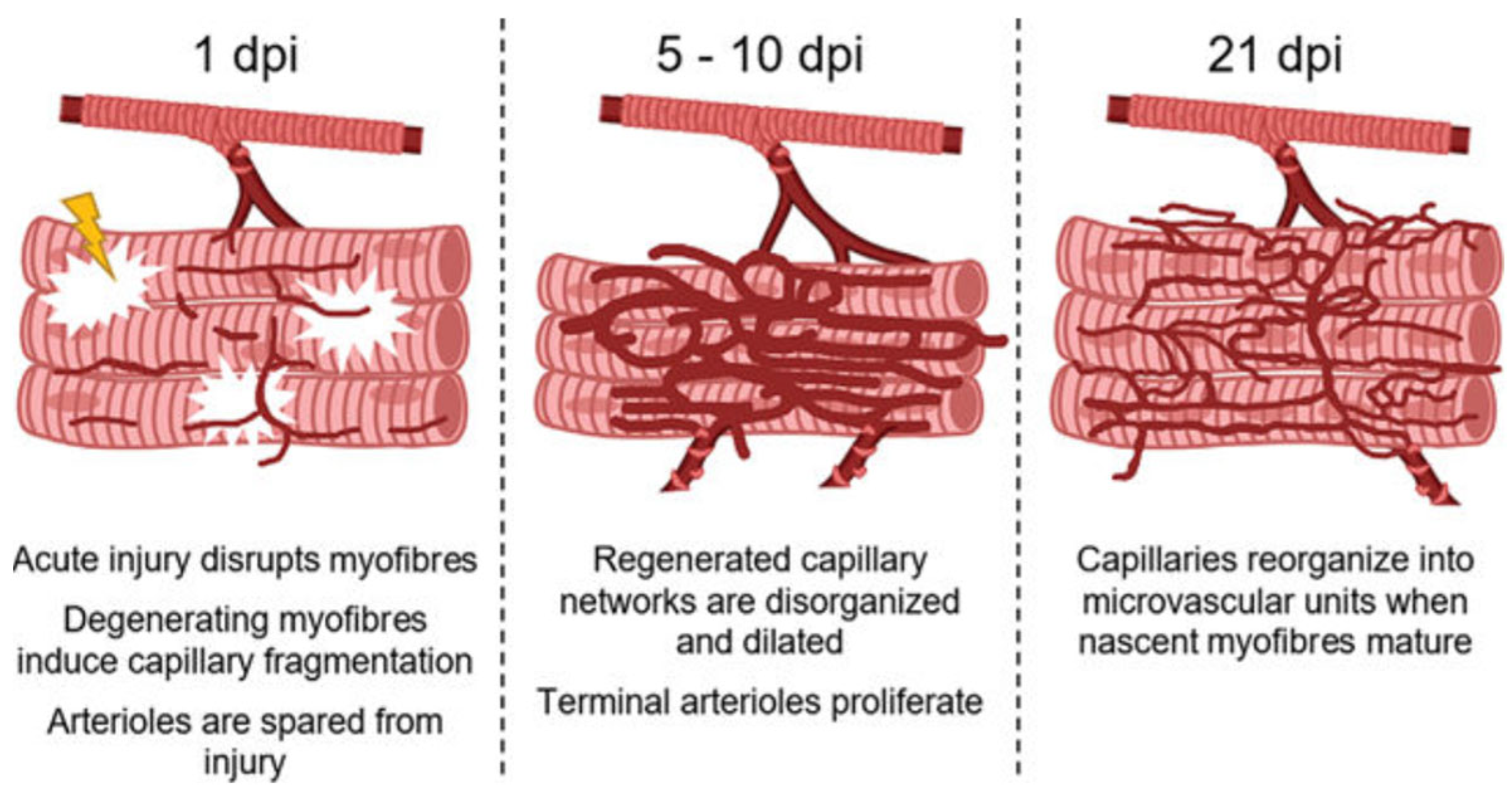
5. Vascular–Myocyte and EC–SC Interaction/Cross-Talk in Muscle Repair/Regeneration
6. Vascular- Myocyte Dysfunction in Duchenne Muscular Dystrophy (DMD)
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korthuis, R.J. Skeletal Muscle Circulation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Glancy, B.; Hsu, L.Y.; Dao, L.; Bakalar, M.; French, S.; Chess, D.J.; Taylor, J.L.; Picard, M.; Aponte, A.; Daniels, M.P.; et al. In vivo microscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers. Microcirculation 2014, 21, 131–147. [Google Scholar] [CrossRef]
- Egginton, S. Invited review: Activity-induced angiogenesis. Pflugers Arch. 2009, 457, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Nassari, S.; Duprez, D.; Fournier-Thibault, C. Non-myogenic contribution to muscle development and homeostasis: The role of connective tissues. Front. Cell Dev. Biol. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Dumont, N.A.; Rudnicki, M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013, 14, 1062–1072. [Google Scholar] [CrossRef]
- Christov, C.; Chretien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; Authier, F.J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol. Biol. Cell 2007, 18, 1397–1409. [Google Scholar] [CrossRef]
- Wong, A.; Chen, S.Q.; Halvorson, B.D.; Frisbee, J.C. Microvessel density: Integrating sex-based differences and elevated cardiovascular risks in metabolic syndrome. J. Vasc. Res. 2022, 59, 1–15. [Google Scholar] [CrossRef]
- Latroche, C.; Gitiaux, C.; Chretien, F.; Desguerre, I.; Mounier, R.; Chazaud, B. Skeletal muscle microvasculature: A highly dynamic lifeline. Physiology 2015, 30, 417–427. [Google Scholar] [CrossRef]
- Gitiaux, C.; Kostallari, E.; Lafuste, P.; Authier, F.J.; Christov, C.; Gherardi, R.K. Whole microvascular unit deletions in dermatomyositis. Ann. Rheum. Dis. 2012, 72, 445–452. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Buckley, A.F.; Bossen, E.H. Skeletal muscle microvasculature in the diagnosis of neuromuscular disease. J. Neuropathol. Exp. Neurol. 2013, 72, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Kassar-Duchossoy, L.; Giacone, E.; Gayraud-Morel, B.; Jory, A.; Gomes, D.; Tajbakhsh, S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005, 19, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Lagha, M.; Sato, T.; Bajard, L.; Daubas, P.; Esner, M.; Montarras, D.; Relaix, F.; Buckingham, M. Regulation of skeletal muscle stem cell behavior by Pax3 and Pax7. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lagha, M.; Brunelli, S.; Messina, G.; Cumano, A.; Kume, T.; Relaix, F.; Buckingham, M.E. Pax3: Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev. Cell 2009, 17, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Mayeuf-Louchart, A.; Lagha, M.; Danckaert, A.; Rocancourt, D.; Relaix, F.; Vincent, S.D.; Buckingham, M. Notch regulation of myogenic versus endothelial fates of cells that migrate from the somite to the limb. Proc. Natl. Acad. Sci. USA 2014, 111, 8844–8849. [Google Scholar] [CrossRef]
- De Ramirez, A.F.; Hernandez-Torres, F.; Rodriguez-Outeirino, L.; Dominguez, J.N.; Matias-Valiente, L.; Sanchez-Fernandez, C.; Franco, D.; Aranega, A.E. Pitx2 differentially regulates the distinct phases of myogenic program and delineates satellite cell lineages during muscle Development. Front. Cell Dev. Biol. 2022, 10, 940622. [Google Scholar] [CrossRef]
- Della Gaspera, B.; Weill, L.; Chanoine, C. Evolution of somite compartmentalization: A view from Xenopus. Front. Cell Dev. Biol. 2022, 9, 790847. [Google Scholar] [CrossRef]
- Ben-Yair, R.; Kalcheim, C. Notch and bone morphogenetic protein differentially act on dermomyotome cells to generate endothelium, smooth, and striated muscle. J. Cell Biol. 2008, 180, 607–618. [Google Scholar] [CrossRef]
- Nowotschin, S.; Xenopoulos, P.; Schrode, N.; Hadjantonakis, A.K. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev. Biol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999, 56, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Oota, I.; Arakawa, T.; Takuma, T. Differential gene expression of vascular endothelial growth factor isoforms and their receptors in the development of the rat masseter muscle. Arch. Oral Biol. 2002, 47, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Breen, E.C.; Gerber, H.P.; Ferrara, N.M.; Wagner, P.D. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol. Genom. 2004, 18, 63–69. [Google Scholar] [CrossRef]
- Huey, K.A.; Smith, S.A.; Sulaeman, A.; Breen, E.C. Skeletal myofiber VEGF is necessary for myogenic and contractile adaptations to functional overload of the plantaris in adult mice. J. Appl. Physiol. 2016, 120, 188–195. [Google Scholar] [CrossRef]
- Widdowson, E.M.; Crabb, D.E.; Milner, R.D. Cellular development of some human organs before birth. Arch. Dis. Child. 1972, 47, 652–655. [Google Scholar] [CrossRef]
- Grunewald, M.; Avraham, I.; Dorm, Y.; Bachar-Lustig, E.; Itin, A.; Jung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Romero, N.B.; Mezmezian, M.; Fidzianska, A. Main steps of skeletal muscle development in the human: Morphological analysis and ultrastructural characteristics of developing human muscle. Handb. Clin. Neurol. 2013, 113, 1299–1310. [Google Scholar] [CrossRef]
- Brown, L.D. Endocrine regulation of fetal skeletal muscle growth: Impact on future metabolic health. J. Endocrinol. 2014, 221, R13–R29. [Google Scholar] [CrossRef]
- Verma, M.; Asakura, Y.; Murakonda, B.S.R.; Pengo, T.; Latroche, C.; Chazaud, B.; McLoon, L.K.; Asakura, A. Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and notch signaling. Cell Stem Cell 2018, 23, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Helmbacher, F.; Stricker, S. Tissue cross talks governing limb muscle development and regeneration. Semin. Cell Dev. Biol. 2020, 104, 14–30. [Google Scholar] [CrossRef]
- Kostallari, E.; Baba-Amer, Y.; Alonso-Martin, S.; Ngoh, P.; Relaix, F.; Lafuste, P.; Gherardi, R.K. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development 2015, 142, 1242–1253. [Google Scholar] [CrossRef]
- Abou-Khalil, R.; Partridge, T.; Chazaud, B. Les cellules du muscle chantent en choeur une berceuse pour cellules souches [How muscle environmental cells induce stem cells quiescence]. Med. Sci. 2010, 5, 454–456. (In French) [Google Scholar] [CrossRef]
- Mounier, R.; Chrétien, F.; Chazaud, B. Blood vessels and the satellite cell niche. Curr. Top Dev. Biol. 2011, 96, 121–138. [Google Scholar] [CrossRef]
- Dellavalle, A.; Maroli, G.; Covarello, D.; Azzoni, E.; Innocenzi, A.; Perani, L.; Antonini, S.; Sambasivan, R.; Brunelli, S.; Tajbakhsh, S.; et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2011, 2, 499. [Google Scholar] [CrossRef]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. 2014, 163, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Pepe, G.J. Placental steroid hormone biosynthesis in primate pregnancy. Endocr. Rev. 1990, 11, 124–150. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Aberdeen, G.W.; Babischkin, J.S.; Prior, S.J.; Lynch, T.J.; Baranyk, I.A.; Pepe, G.J. Estrogen promotes microvascularization in the fetus and thus vascular function and insulin sensitivity in offspring. Endocrinology 2022, 163, ebqac037. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Climie, R.E.; van Sloten, T.T.; Bruno, R.M.; Taddei, S.; Empana, J.P.; Stehouwer, C.D.A.; Sharman, J.E.; Boutouyrie, P.; Laurent, S. Macrovasculature and microvasculature at the crossroads between type 2 diabetes mellitus and hypertension. Hypertension 2019, 73, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Unnikrishnan, A.G.; Baruah, M.P.; Sahay, R.; Bantwal, G. Metabolic and energy imbalance in dysglycemia-based chronic disease. Diabetes Metab. Syndr. Obes. 2021, 14, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Song, Y.; Ford, E.S.; Liu, S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch. Intern. Med. 2004, 164, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Gress, T.W.; Nieto, F.J.; Shahar, E.; Wofford, M.R.; Brancati, F.L. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis risk in communities study. N. Engl. J. Med. 2000, 342, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Garber, A.J.; Grunberger, G.; Handelsman, Y.; Garvey, W.T. Dysglemia-based chronic disease: An American Association of Clinical Endocrinologists Position Statement. Endocr. Pract. 2018, 24, 995–1011. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R.; Bjorkman, O.; Olsson, M.; Wahren, J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Olfert, I.M.; Baum, O.; Hellsten, Y.; Egginton, S. Advances and challenges in skeletal muscle angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H326–H336. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Dubinin, M.V. Diabetes mellitus, mitochondrial dysfunction and Ca(2+)-dependent permeability transition pore. Int. J. Mol. Sci. 2020, 21, 6559. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z. Vascular function, insulin action, and exercise: An intricate interplay. Trends Endocrinol. Metab. 2015, 26, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef] [PubMed]
- Love, K.M.; Barrett, E.J.; Malin, S.K.; Reusch, J.E.B.; Regensteiner, J.G.; Liu, Z. Diabetes pathogenesis and management: The endothelium comes of age. J. Mol. Cell Biol. 2021, 13, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Bogardus, C.; Bergman, R.; Thuillez, P.; Lillioja, S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J. Clin. Investig. 1994, 1, 10–16. [Google Scholar] [CrossRef]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Wang, H.; Upchurch, C.T.; Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E252–E263. [Google Scholar] [CrossRef]
- Barrett, E.J.; Liu, Z. The endothelial cell: An “early responder” in the development of insulin resistance. Rev. Endocr. Metab. Disord. 2013, 14, 21–27. [Google Scholar] [CrossRef]
- Clark, M.G.; Wallis, M.G.; Barrett, E.J.; Vincent, M.A.; Richards, S.M.; Clerk, L.H.; Rattigan, S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E241–E258. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hope, I.D.; Ader, M.; Bergman, R.N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J. Clin. Investig. 1989, 84, 1620–1628. [Google Scholar] [CrossRef]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: Evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007, 56, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Wang, W.; Liu, J.; Barrett, E.J.; Carey, R.M.; Cao, W.; Liu, Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 2010, 55, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J. Clin. Endocrinol. Metab. 2009, 94, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J.; Cao, W.; Liu, Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J. Clin. Endocrinol. Metab. 2011, 96, 438–446. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.X.; Liu, Z.; Barrett, E.J. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 2008, 57, 540–547. [Google Scholar] [CrossRef]
- Chiu, J.D.; Richey, J.M.; Harrison, L.N.; Zuniga, E.; Kolka, C.M.; Kirkman, E.; Ellmerer, M.; Bergman, R.N. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes 2008, 57, 828–835. [Google Scholar] [CrossRef]
- Honig, C.R.; Odoroff, C.L.; Frierson, J.L. Active and passive capillary control in red muscle at rest and in exercise. Am. J. Physiol. 1982, 243, H196–H206. [Google Scholar] [CrossRef]
- Keske, M.A.; Dwyer, R.M.; Russell, R.D.; Blackwood, S.J.; Brown, A.A.; Hu, D.; Premilovac, D.; Richards, S.M.; Rattigan, S. Regulation of microvascular flow and metabolism: An overview. Clin. Exp. Pharmacol. Physiol. 2017, 44, 143–149. [Google Scholar] [CrossRef]
- Clerk, L.H.; Vincent, M.A.; Lindner, J.R.; Clark, M.G.; Rattigan, S.; Barrett, E.J. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab. Res. Rev. 2004, 20, 3–12. [Google Scholar] [CrossRef]
- Vincent, M.A.; Barrett, E.J.; Lindner, J.R.; Clark, M.G.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E123–E129. [Google Scholar] [CrossRef]
- Vincent, M.A.; Dawson, D.; Clark, A.D.; Lindner, J.R.; Rattigan, S.; Clark, M.G.; Barrett, E.J. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 2002, 51, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Price, W.J.; Jahn, L.A.; Leong-Poi, H.; Barrett, E.J. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1191–E1197. [Google Scholar] [CrossRef]
- Dawson, D.; Vincent, M.A.; Barrett, E.J.; Kaul, S.; Clark, A.; Leong-Poi, H.; Lindner, J.R. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E714–E720. [Google Scholar] [CrossRef]
- Sörensen, B.M.; Houben, A.J.; Berendschot, T.T.; Schouten, J.S.; Kroon, A.A.; van der Kallen, C.J.; Henry, R.M.; Koster, A.; Sep, S.J.; Dagnelie, P.C.; et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The Maastricht Study. Circulation 2016, 134, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.M.; Ferreira, I.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Kamp, O.; Bouter, L.M.; Stehouwer, C.D. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis 2004, 174, 49–56. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Brechtel, G.; Johnson, A.; Fineberg, N.; Baron, A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994, 94, 1172–1179. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Naka, K.K.; Papathanassiou, K.; Bechlioulis, A.; Kazakos, N.; Pappas, K.; Tigas, S.; Makriyiannis, D.; Tsatsoulis, A.; Michalis, L.K. Determinants of vascular function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 127. [Google Scholar] [CrossRef]
- Lillioja, S.; Young, A.A.; Culter, C.L.; Ivy, J.L.; Abbott, W.G.; Zawadzki, J.K.; Yki-Jarvinen, H.; Christin, L.; Secomb, T.W.; Bogardus, C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J. Clin. Investig. 1987, 80, 415–424. [Google Scholar] [CrossRef]
- Williams, S.B.; Cusco, J.A.; Roddy, M.A.; Johnstone, M.T.; Creager, M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1996, 27, 567–574. [Google Scholar] [CrossRef]
- Gudbjornsdottir, S.; Sjostrand, M.; Strindberg, L.; Lonnroth, P. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Rattigan, S. Muscle perfusion: Its measurement and role in metabolic regulation. Diabetes 2012, 61, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Torgan, C.E.; Brozinick JTJr Kastello, G.M.; Ivy, J.L. Muscle morphological and biochemical adaptations to training in obese Zucker rats. J. Appl. Physiol. 1989, 67, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.L.; Fernandes, T.; Soci, U.P.; Silveira, A.C.; Barretti, D.L.; Negrão, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxidative Med. Cell Longev. 2017, 2017, 2415246. [Google Scholar] [CrossRef]
- Bonner, J.S.; Lantier, L.; Hasenour, C.M.; James, F.D.; Bracy, D.P.; Wasserman, D.H. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 2013, 62, 572–580. [Google Scholar] [CrossRef]
- Feihl, F.; Liaudet, L.; Waeber, B.; Levy, B.I. Hypertension: A disease of the microcirculation? Hypertension 2006, 48, 1012–1017. [Google Scholar] [CrossRef]
- Feihl, F.; Liaudet, L.; Levy, B.I.; Waeber, B. Hypertension and microvascular remodelling. Cardiovasc. Res. 2008, 78, 274–285. [Google Scholar] [CrossRef]
- Horton, W.B.; Barrett, E.J. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr. Rev. 2021, 42, 29–55. [Google Scholar] [CrossRef]
- Eriksson, K.F.; Saltin, B.; Lindgarde, F. Increased skeletal muscle capillary density precedes diabetes development in men with impaired glucose tolerance. A 15-year follow-up. Diabetes 1994, 43, 805–808. [Google Scholar] [CrossRef]
- Umek, N.; Horvat, S.; Cvetko, E.; Kreft, M.; Janacek, J.; Kubinova, L.; Stopar, P.T.; Erzen, I. 3D analysis of capillary network in skeletal muscle of obese insulin-resistant mice. Histochem. Cell Biol. 2019, 152, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Messa, G.A.M.; Piasecki, M.; Hurst, J.; Hill, C.; Tallis, J.; Degens, H. The impact of a high-fat diet in mice is dependent on duration and age, and differs between muscles. J. Exp. Biol. 2020, 223, 217117. [Google Scholar] [CrossRef] [PubMed]
- Cebasek, V.; Erzen, I.; Vyhnal, A.; Janácek, J.; Ribaric, S.; Kubínová, L. The estimation error of skeletal muscle capillary supply is significantly reduced by 3D method. Microvasc. Res. 2010, 79, 40–46. [Google Scholar] [CrossRef]
- Ugwoke, C.K.; Cvetko, E.; Umek, N. Skeletal muscle microvascular dysfunction in obesity-related insulin resistance: Pathophysiological mechanisms and therapeutic perspectives. Int. J. Mol. Sci. 2022, 23, 847. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Bairey, M.N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- O’Reilly, J.; Ono-Moore, K.D.; Chintapalli, S.V.; Rutkowsky, J.M.; Tolentino, T.; Lloyd, K.C.; Olfert, I.M.; Adams, S.H. Sex differences in skeletal muscle revealed through fiber type, capillarity, and transcriptomics profiling in mice. Physiol. Rep. 2021, 9, e15031. [Google Scholar] [CrossRef]
- Cullinan-Bove, K.; Koos, R.D. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: Rapid stimulation by estrogen correlates with estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 1993, 133, 829–837. [Google Scholar] [CrossRef]
- Nayak, N.R.; Brenner, R.M. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 1845–1855. [Google Scholar] [CrossRef]
- Higgins, K.J.; Liu, S.; Abdelrahim, M.; Yoon, K.; Vanderlaag, K.; Porter, W.; Metz, R.P.; Safe, S. Vascular endothelial growth factor receptor-2 expression is induced by 17β-estradiol in ZR-75 breast cancer cells by estrogen receptor α/Sp proteins. Endocrinology 2006, 147, 3285–3295. [Google Scholar] [CrossRef]
- Aberdeen, G.W.; Wiegand, S.J.; Bonagura, T.W., Jr.; Pepe, G.W.; Albrecht, E.D. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology 2008, 149, 6076–6083. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Shaul, P.W. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002, 23, 665–686. [Google Scholar] [CrossRef]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Ekman, M.; Johansson, O.; Jansson, E.; Esbjornsson, M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem. Cell Biol. 2009, 131, 181–189. [Google Scholar] [CrossRef]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Habibi, J.; Mugerfeld, I.; Garro, M.; Sowers, J.R. Loss of estrogen receptor α signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal. Med. 2012, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Thorburn, A.W.; Britt, K.L.; Hewitt, K.N.; Wreford, N.G.; Proietto, J.; Oz, O.K.; Leury, B.J.; Robertson, K.M.; Yao, S.; et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA 2000, 97, 12735–12740. [Google Scholar] [CrossRef]
- Rochira, V.; Madeo, B.; Zirilli, L.; Caffagni, G.; Maffei, L.; Carani, C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: Evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet. Med. 2007, 24, 1491–1495. [Google Scholar] [CrossRef]
- Takeda, K.; Toda, K.; Saibara, T.; Nakagawa, M.; Saika, K.; Onishi, T.; Sugiura, T.; Shizuta, Y. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J. Endocrinol. 2003, 176, 237–246. [Google Scholar] [CrossRef]
- Belgorosky, A.; Guercio, G.; Pepe, C.; Saraco, N.; Rivarola, M.A. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Horm. Res. 2009, 72, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bryzgalova, G.; Hedman, E.; Khan, A.; Efendic, S.; Gustafsson, J.A.; Dahlman-Wright, K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: A possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol. Endocrinol. 2006, 20, 1287–1299. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, A.; Paassilta, M.; Heikkinen, J.; Bäckström, A.C.; Savolainen, M.; Kesäniemi, Y.A. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin. Endocrinol. 2001, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.L.; Howard, B.V.; Thomson, C.A.; Lacroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Margolis, K.L.; Bonds, D.E.; Rodabough, R.J.; Tinker, L.; Phillips, L.S.; Allen, C.; Bassford, T.; Burke, G.; Torrens, J.; Howard, B.V. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004, 47, 1175–1187. [Google Scholar] [CrossRef]
- Wagner, J.D.; Thomas, M.J.; Williams, J.K.; Zhang, L.; Greaves, K.A.; Cefalu, W.T. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. J. Clin. Endocrinol. Metab. 1998, 83, 896–901. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen receptor beta controls muscle growth and regeneration in young female mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ono, Y. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 2016, 229, 267–275. [Google Scholar] [CrossRef]
- Kobori, M.; Yamamuro, T. Effects of gonadectomy and estrogen administration on rat skeletal muscle. Clin. Orthop. Relat. Res. 1989, 243, 306–311. [Google Scholar] [CrossRef]
- Diel, P. The role of the estrogen receptor in skeletal muscle mass homeostasis and regeneration. Acta Physiol. 2014, 212, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.; Schleipen, B.; Fritzemeier, K.H.; Zierau, O.; Diel, P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012, 26, 1909–1920. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Greising, S.M.; Warren, G.L.; Lowe, D.A. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS ONE 2010, 5, e10164. [Google Scholar] [CrossRef] [PubMed]
- Vasconsuelo, A.; Milanesi, L.; Boland, R. 17β-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Endocrinol. 2008, 196, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.H.; Witczak, C.A.; Hirshman, M.F.; Goodyear, L.J.; Greenberg, A.S. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem. Biophys. Res. Commun. 2009, 382, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.-A. Muscle GLUT4 regulation by estrogen receptors Erβ and ERα. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl. S6), 588S–595S. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of insulin resistance. Horm. Res. 2005, 64 (Suppl. S3), 2–7. [Google Scholar] [CrossRef]
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef]
- Maniu, A.; Aberdeen, G.W.; Lynch, T.J.; Nadler, J.L.; Kim, S.O.; Quon, M.J.; Pepe, G.J.; Albrecht, E.D. Estrogen deprivation in primate pregnancy leads to insulin resistance in offspring. J. Endocrinol. 2016, 230, 171–183. [Google Scholar] [CrossRef]
- Pepe, G.J.; Maniu, A.; Aberdeen, G.; Lynch, T.J.; Kim, S.O.; Nadler, J.; Albrecht, E.D. Insulin resistance elicited in postpubertal primate offspring deprived of estrogen in utero. Endocrine 2016, 54, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Aberdeen, G.; Lynch, T.J.; Albrecht, E.D.; Pepe, G.J. Adipose and Liver Function in Primate Offspring with Insulin resistance Induced by Estrogen Deprivation in utero. Endocrinol. Diabetes Metab J. 2017, 1, 1–7. Available online: http://researchopenworld.com/wp-content/uploads/2017/10/EDMJ-2017-109-Gerald-J.-Pepe-USA.pdf (accessed on 12 June 2023).
- Kim, S.O.; Albrecht, E.D.; Pepe, G.J. Estrogen promotes fetal skeletal muscle myofiber development important for insulin sensitivity in offspring. Endocrine 2022, 78, 32–41. [Google Scholar] [CrossRef]
- Hofman, P.L.; Regan, F.; Jackson, W.E.; Jefferies, C.; Knight, D.B.; Robinson, E.M.; Cutfield, W.S. Premature birth and later insulin resistance. N. Engl. J. Med. 2004, 351, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Bonamy, A.-K.E.; Martin, H.; Jörneskog, G.; Norman, M. Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J. Intern. Med. 2007, 262, 635–642. [Google Scholar] [CrossRef]
- Darendeliler, F.; Bas, F.; Bundak, R.; Coban, A.; Sancakli, O.; Eryilmaz, S.K.; Kucukemre, B.; Disci, R.; Gokcay, G.; Aki, S.; et al. Insulin resistance and body composition in preterm born children during prepubertal ages. Clin. Endocrinol. 2008, 68, 773–779. [Google Scholar] [CrossRef]
- Paz Levy, D.; Sheiner, E.; Wainstock, T.; Sergienko, R.; Landau, D.; Walfisch, A. Evidence that children born at early term (37-38 6/7 weeks) are at increased risk for diabetes and obesity-related disorders. Am. J. Obstet. Gynecol. 2017, 21, 588.E1–588.E11. [Google Scholar] [CrossRef]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010, 118, 1242–1250. [Google Scholar] [CrossRef]
- Chevalier, N.; Fénichel, P. Endocrine disruptors; new players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Koike, H.; Manabe, I.; Oishi, Y. Mechanisms of cooperative cell-cell interactions in skeletal muscle regeneration. Inflamm. Regen. 2022, 42, 48. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; Rando, T.A. A muscle stem cell support group: Coordinated cellular responses in muscle regeneration. Dev. Cell. 2018, 46, 135–143. [Google Scholar] [CrossRef]
- Fernando, C.A.; Pangan, A.M.; Cornelison, D.; Segal, S.S. Recovery of blood flow regulation in microvascular resistance networks during regeneration of mouse gluteus maximus muscle. J. Physiol. 2019, 597, 1401–1417. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Norton, C.E.; Jacobsen, N.L.; Fernando, C.A.; Cornelison, D.D.W.; Segal, S.S. Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels. Skelet. Muscle 2019, 9, 27. [Google Scholar] [CrossRef]
- Jacobsen, N.L.; Norton, C.E.; Shaw, R.L.; Cornelison, D.D.W.; Segal, S.S. Myofibre injury induces capillary disruption and regeneration of disorganized microvascular networks. J. Physiol. 2022, 600, 41–60. [Google Scholar] [CrossRef]
- Uccelli, A.; Wolff, T.; Valente, P.; Di, M.N.; Pellegrino, M.; Gurke, L.; Banfi, A.; Gianni-Barrera, R. Vascular endothelial growth factor biology for regenerative angiogenesis. Swiss. Med. Wkly. 2019, 149, w20011. [Google Scholar] [CrossRef]
- Ackermann, M.; Houdek, J.P.; Gibney, B.C.; Ysasi, A.; Wagner, W.; Belle, J.; Schittny, J.C.; Enzmann, F.; Tsuda, A.; Mentzer, S.J.; et al. Sprouting and intussusceptive angiogenesis in postpneumonectomy lung growth: Mechanisms of alveolar neovascularization. Angiogenesis 2014, 17, 541–551. [Google Scholar] [CrossRef]
- Egginton, S.; Zhou, A.L.; Brown, M.D.; Hudlicka, O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc. Res. 2001, 49, 634–646. [Google Scholar] [CrossRef]
- Latroche, C.; Weiss-Gayet, M.; Muller, L.; Gitiaux, C.; Leblanc, P.; Liot, S.; Ben-Larbi, S.; Abou-Khalil, R.; Verger, N.; Bardot, P.; et al. Coupling between myogenesis and angiogenesis during skeletal muscle regeneration is stimulated by restorative macrophages. Stem Cell Rep. 2017, 9, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Elhadidy, S.; Asakura, A. Vascular therapy for Duchenne muscular dystrophy (DMD). Fac. Rev. 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.C.; Ramos, S.V.; Turnbull, P.C.; Rebalka, I.A.; Cao, A.; Monaco, C.M.F.; Varah, N.E.; Edgett, B.A.; Huber, J.S.; Tadi, P.; et al. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H2O2 emission during impaired oxidative phosphorylation. J. Cachexia Sarcopenia Muscle 2019, 10, 643–661. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Mikheeva, I.B.; Sharapov, M.G.; Belosludtsev, K.N. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium-induced permeability transition. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165674. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, P.W.; Engel, W.K.; Zellweger, H. Experimental myopathy after microarterial embolization; comparison with childhood x-linked pseudohypertrophic muscular dystrophy. Arch. Neurol. 1970, 22, 365–378. [Google Scholar] [CrossRef]
- Nguyen, F.; Cherel, Y.; Guigand, L.; Goubault-Leroux, I.; Wyers, M. Muscle lesions associated with dystrophin deficiency in neonatal golden retriever puppies. J. Comp. Pathol. 2002, 126, 100–108. [Google Scholar] [CrossRef]
- Nguyen, F.; Guigand, L.; Goubault-Leroux, I.; Wyers, M.; Cherel, Y. Microvessel density in muscles of dogs with golden retriever muscular dystrophy. Neuromuscul. Disord. 2005, 15, 154–163. [Google Scholar] [CrossRef]
- Thomas, G.D. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front. Physiol. 2013, 4, 381. [Google Scholar] [CrossRef]
- Palladino, M.; Gatto, I.; Neri, V.; Straino, S.; Smith, R.C.; Silver, M.; Gaetani, E.; Marcantoni, M.; Giarretta, I.; Stigliano, E.; et al. Angiogenic impairment of the vascular endothelium: A novel mechanism and potential therapeutic target in muscular dystrophy. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2867–2876. [Google Scholar] [CrossRef]
- Deconinck, N.; Dan, B. Pathophysiology of duchenne muscular dystrophy: Current hypotheses. Pediatr. Neurol. 2007, 36, 1–7. [Google Scholar] [CrossRef]
- Deasy, B.M.; Feduska, J.M.; Payne, T.R.; Li, Y.; Ambrosio, F.; Huard, J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol. Ther. 2009, 17, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Mazzeo, A.; Bitto, A.; Aguennouz, M.; Migliorato, A.; De Pasquale, M.G.; Minutoli, L.; Altavilla, D.; Zentilin, L.; Giacca, M.; et al. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007, 21, 3737–3746. [Google Scholar] [CrossRef] [PubMed]
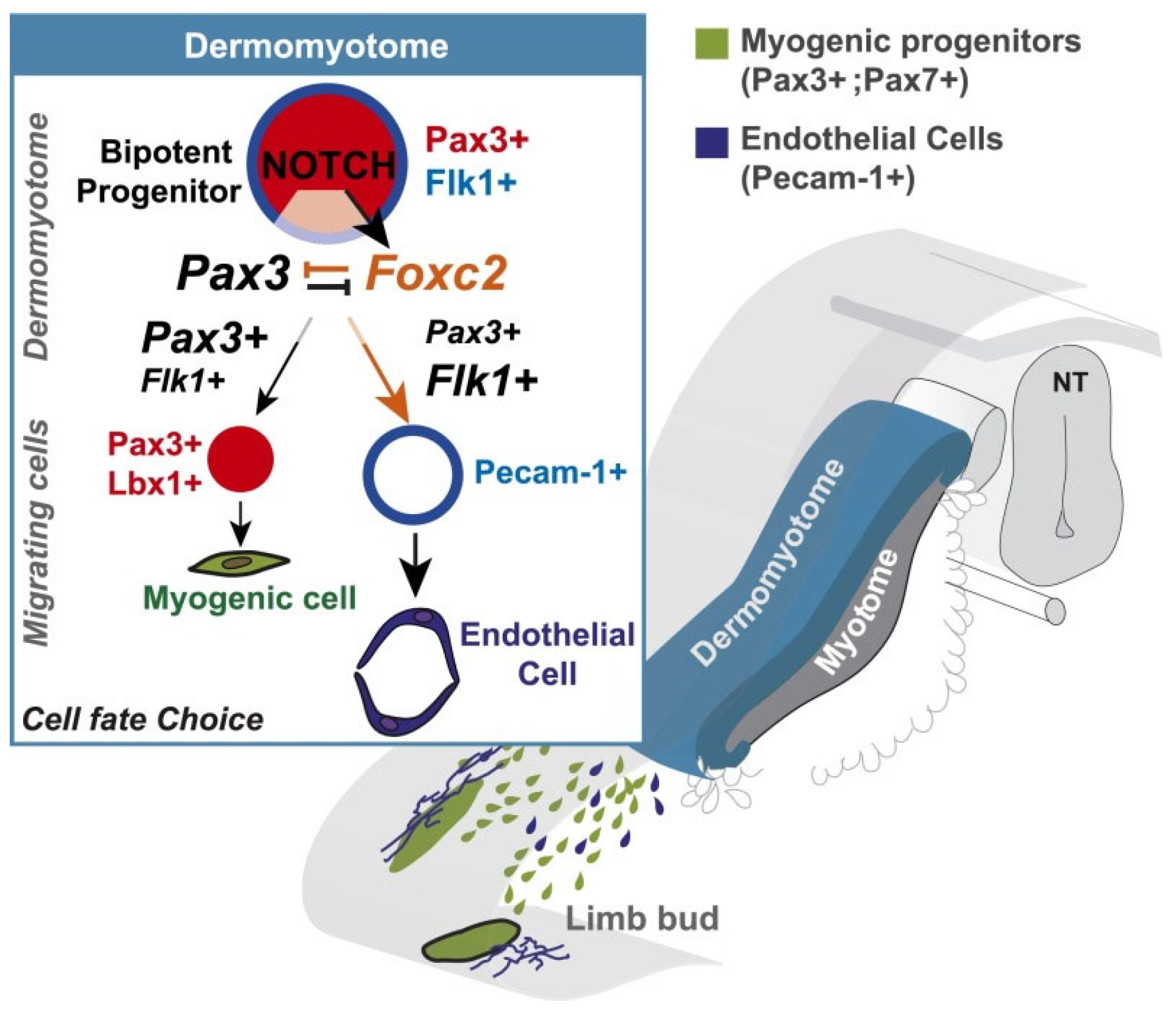



| Description | Reference |
|---|---|
| Myogenic and vascular endothelial cells derived from myogenic progenitor cells (MPC) in the embryonic dermomyotome | Latroche et al., 2015 [9]; Kassar-Duchossoy et al., 2005 [13]; Mayeuf-Louchart et al., 2014 [17]; Ben-Yair et al., 2008 [20] |
| MPCs express paired box transcription factors (pax) 3 and/or 7, the forkhead box protein C2 transcription factor (foxc2) and thenotch trans-membrane receptor and downstream signaling | Kassar-Duchossoy et al., 2005 [13]; Lagha et al., 2008 [14]; Lagha et al., 2009 [15]; Buckingham and Rigby, 2014 [16] |
| Notch signaling controls pax 3/7: foxc2 gene equilibrium and thus vascular (low pax 3/7) myogenic (high pax3/7) lineage of MPCs | Mayeuf-Louchart et al., 2014 [17] |
| Myogenic cells undergo coordinated expression of myogenic regulatory factors (Mrf), 5, 4, D and G that underpin muscle formation | Latroche et al., 2015 [9]; Kassar-Duchossoy et al., 2005 [13]; Lagha et al., 2008 [14]; Ben-Yair and Kalcheim, 2008 [20]; Bentzinger et al., 2012 [29] |
| Endothelial cells (EC) express vascular endothelial growth factor (VEGF) that controls vascular development and vascularization/capillarization of developing muscle fibers | Ferrara et al., 1996 [22]; Ferrara, 1999 [23] |
| Myogenic-stem-cell pool comprised of pax 3/7 Mrf5/NOTCH satellite cells (SC) established | Lagha et al., 2008 [14]; Lagha et al., 2009 [15]; Ramirez de et al., 2022 [18] |
| VEGF induced activation of Delta-like 4 (DII4), a ligand for notch, establishes SC quiescence | Verma et al., 2018 [32] |
| VEGF signaling brings ECs close to SCs, forming an EC–SC niche important for muscle regeneration in adulthood | Christov et al., 2007 [7]; Latroche et al., 2015 [9] |
| Placental estradiol (E2) secreted into the fetus important for muscle capillarization during second half of pregnancy | Albrecht and Pepe, 1990 [39]; Albrecht et al., 2022 [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, G.J.; Albrecht, E.D. Microvascular Skeletal-Muscle Crosstalk in Health and Disease. Int. J. Mol. Sci. 2023, 24, 10425. https://doi.org/10.3390/ijms241310425
Pepe GJ, Albrecht ED. Microvascular Skeletal-Muscle Crosstalk in Health and Disease. International Journal of Molecular Sciences. 2023; 24(13):10425. https://doi.org/10.3390/ijms241310425
Chicago/Turabian StylePepe, Gerald J., and Eugene D. Albrecht. 2023. "Microvascular Skeletal-Muscle Crosstalk in Health and Disease" International Journal of Molecular Sciences 24, no. 13: 10425. https://doi.org/10.3390/ijms241310425
APA StylePepe, G. J., & Albrecht, E. D. (2023). Microvascular Skeletal-Muscle Crosstalk in Health and Disease. International Journal of Molecular Sciences, 24(13), 10425. https://doi.org/10.3390/ijms241310425






