In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering
Abstract
1. Introduction
2. Results
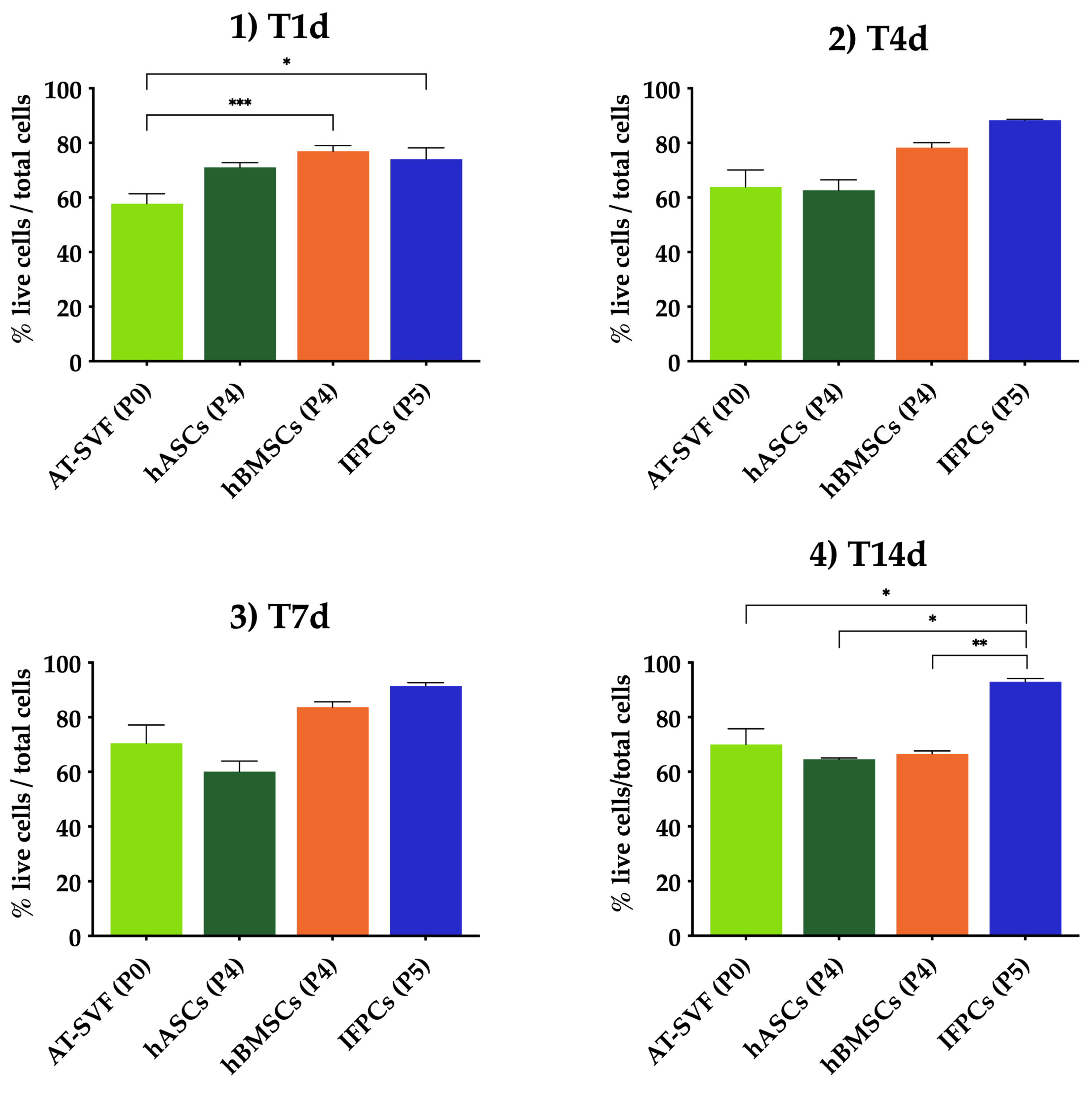
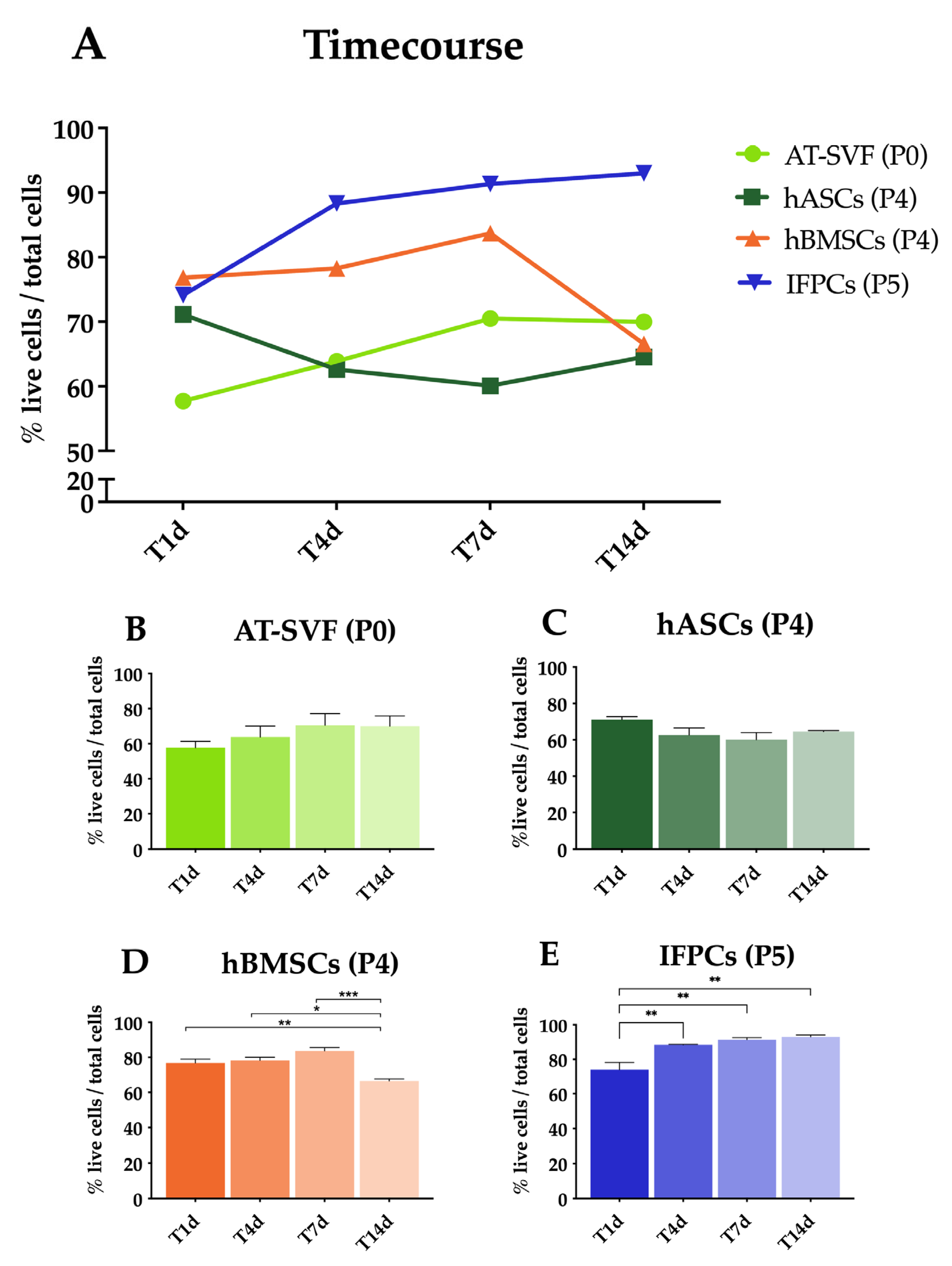
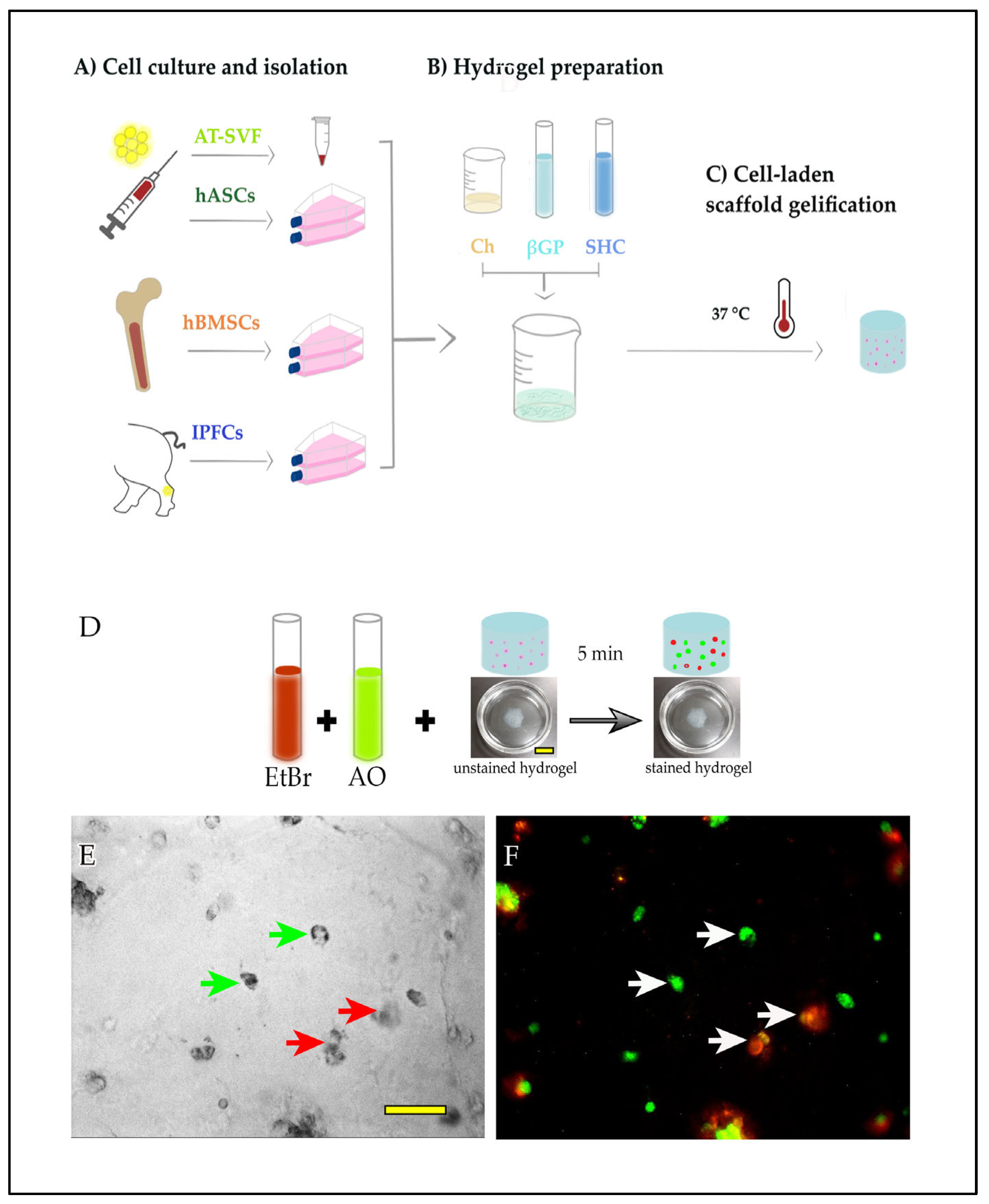
2.1. Cell Encapsulation in Hydrogel In Vitro
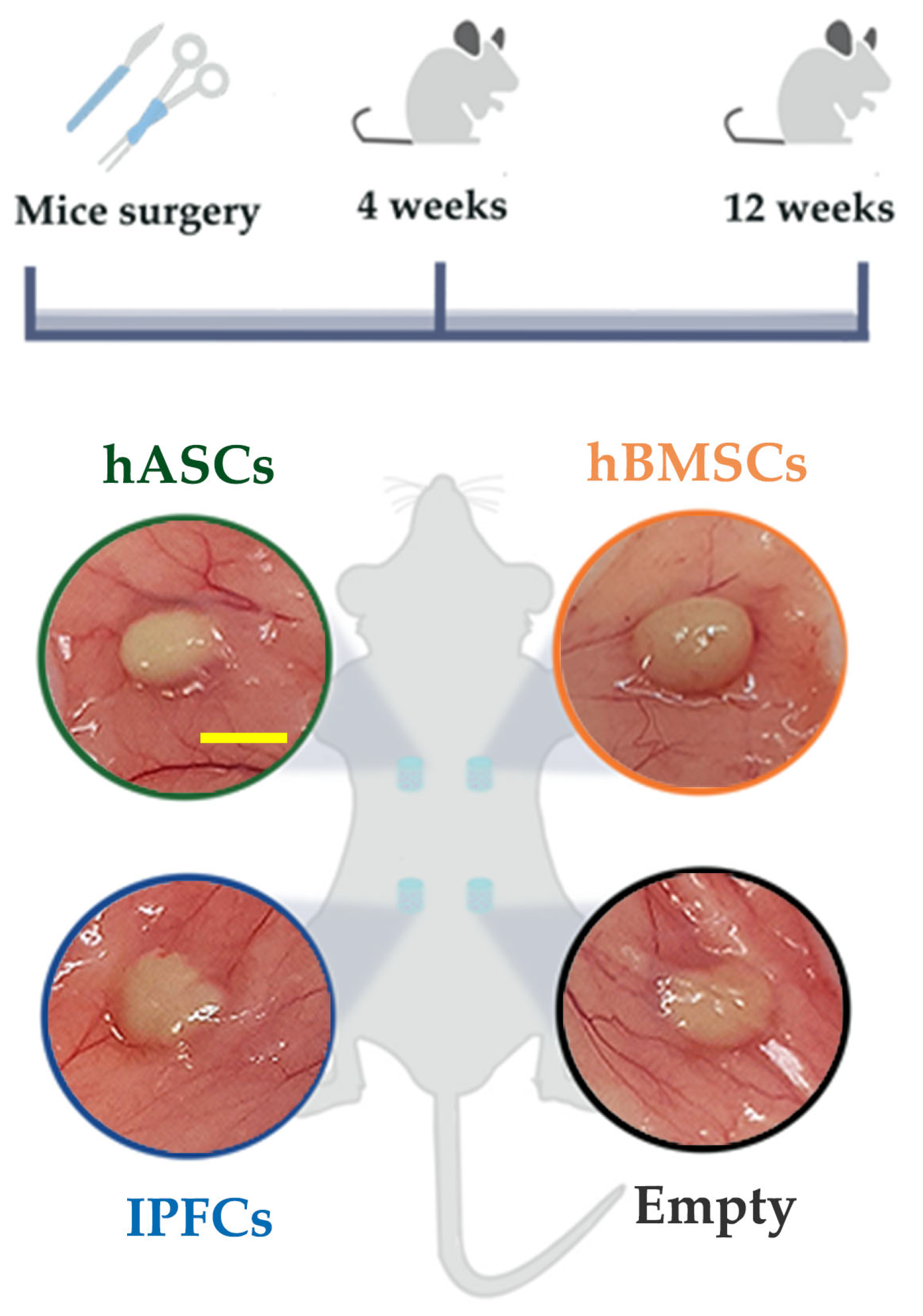
2.2. Hydrogel Ectopic Implant in Mice
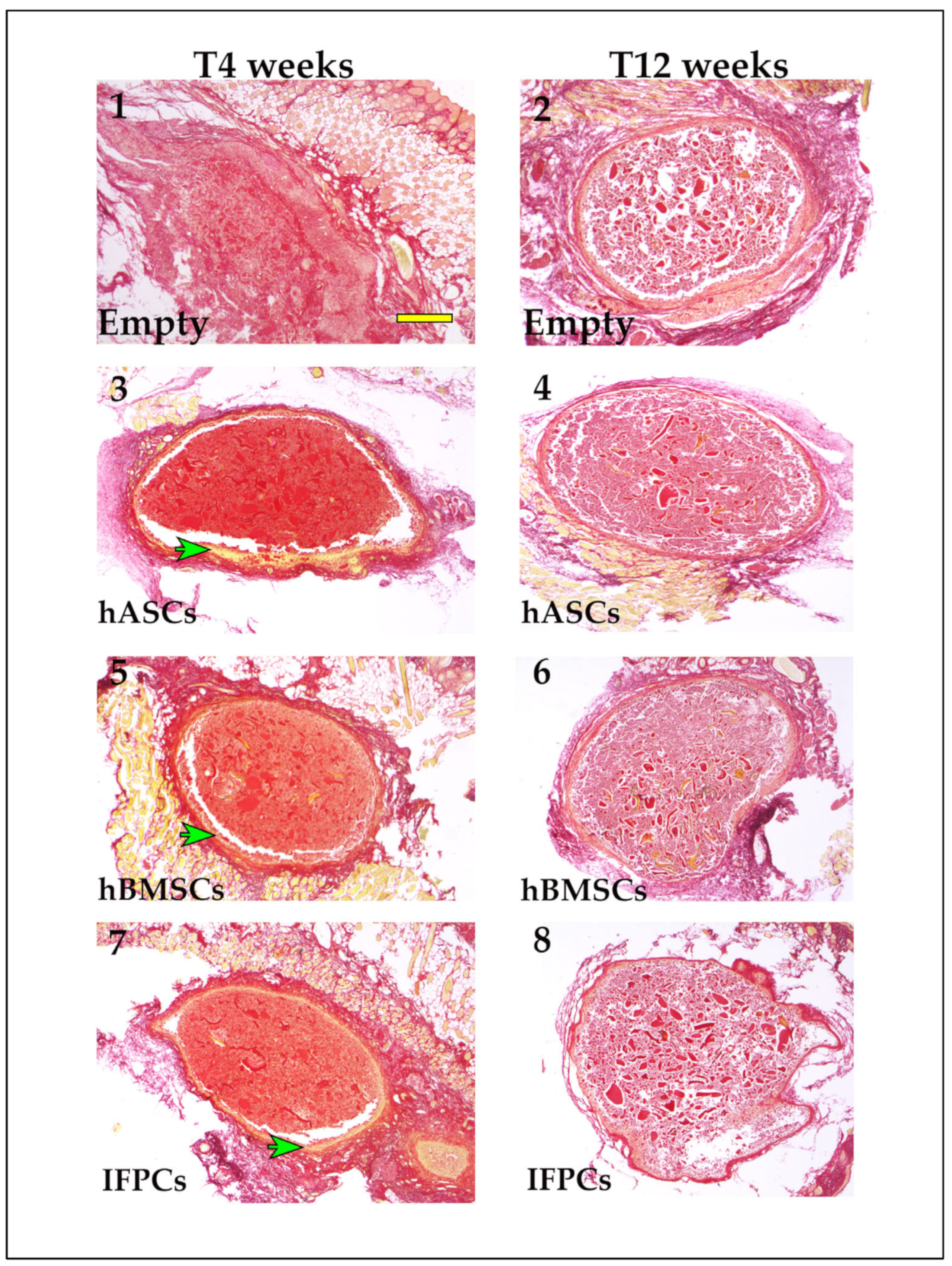
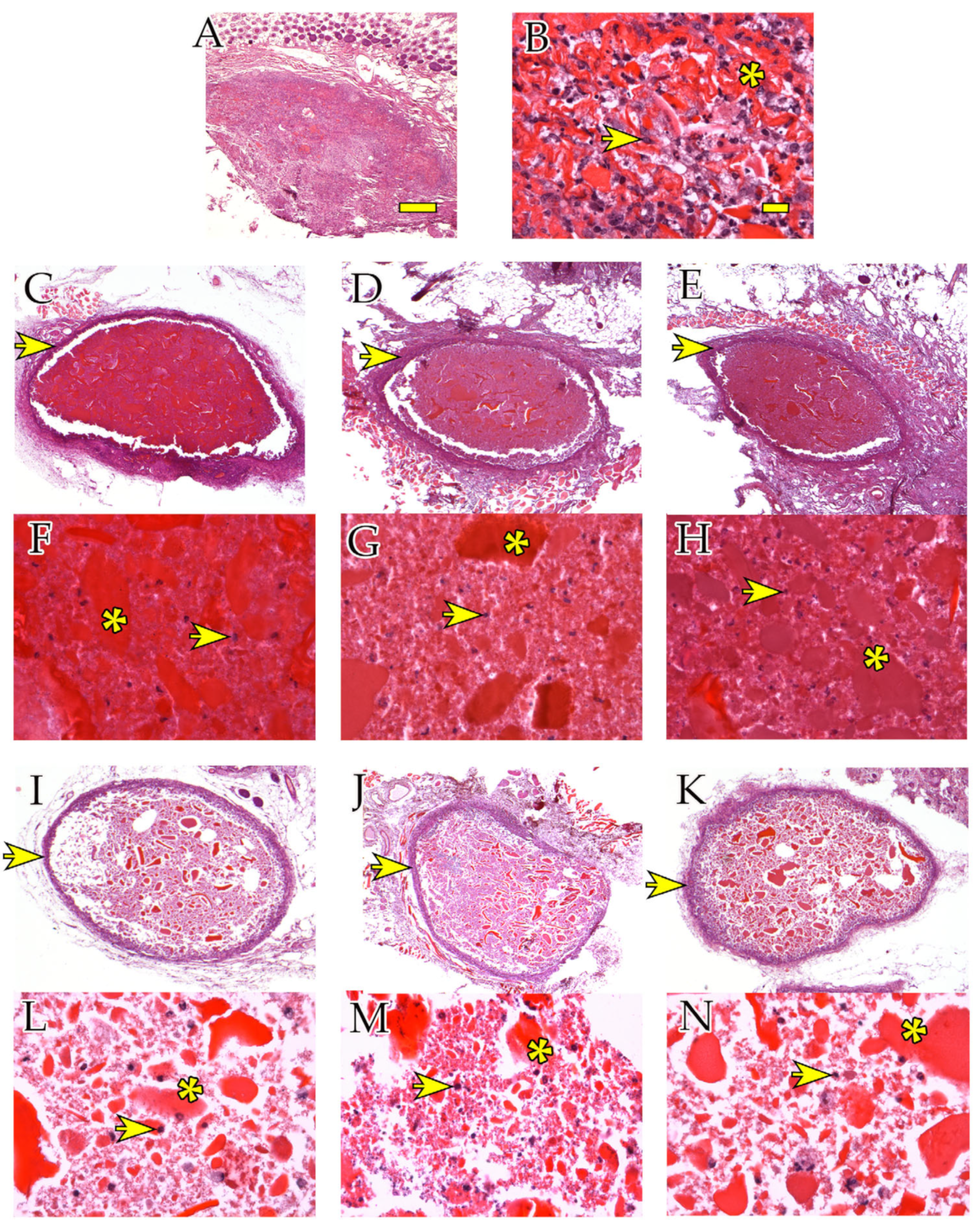
2.2.1. Histological Analysis
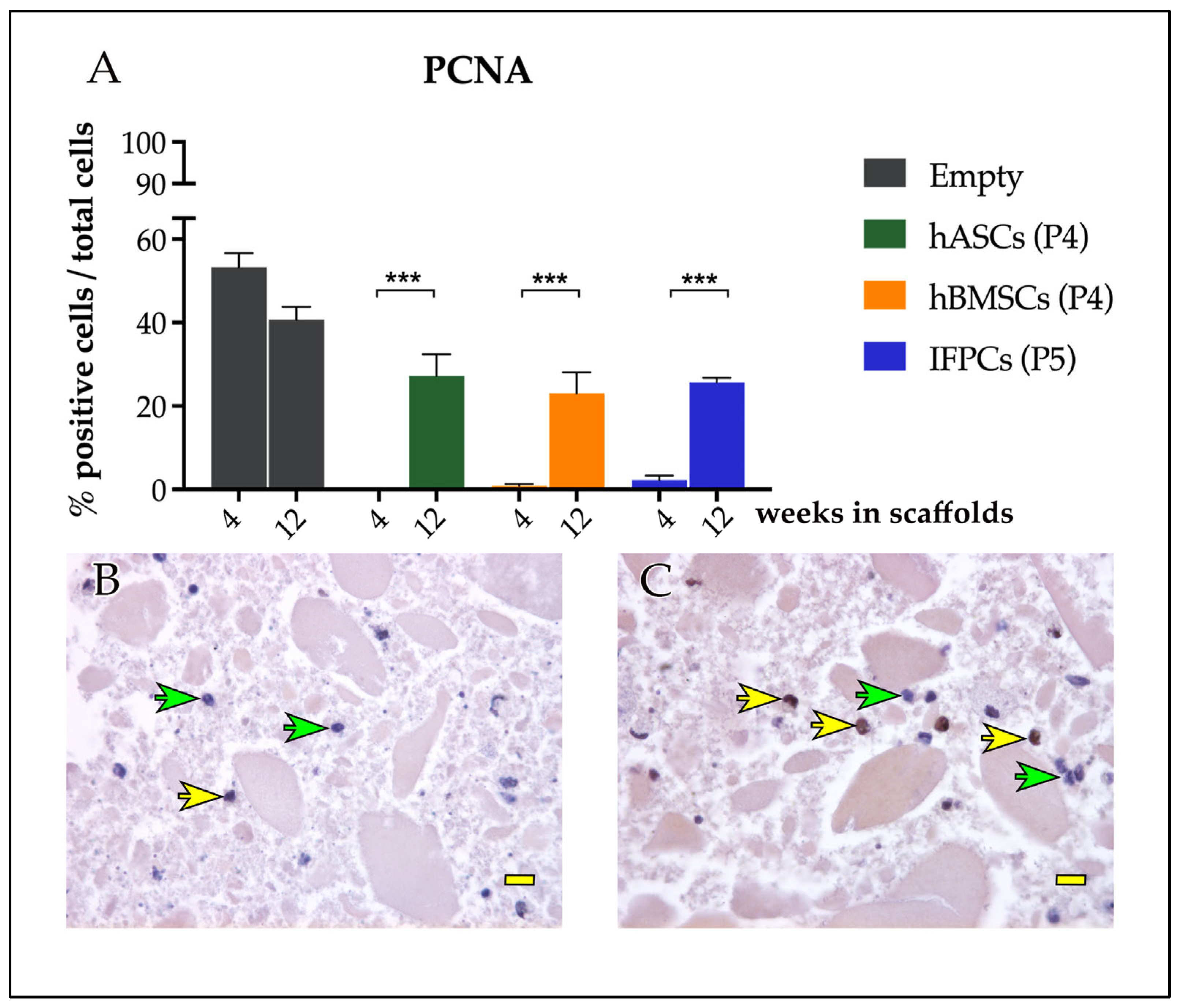
2.2.2. Immunohistochemical Analyses
3. Discussion
4. Materials and Methods
4.1. Chitosan-Based Hydrogel Preparation
4.2. In Vitro Study
4.2.1. Isolation and Culture of Bone Marrow Stem Cells
4.2.2. Isolation and Culture of Human Adipose Stem Cells
4.2.3. Isolation and Culture of Neonatal Porcine Hoffa’s Fat Pad Cells
4.3. Cell Encapsulation in Hydrogel
4.4. Live/Dead Cells Assay
4.5. In Vivo Study
4.5.1. Histological and Immunohistochemical Analyses
4.5.2. Immunohistochemical Evaluation: PCNA Expression
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Kamper, S.J.; Wiggers, J.H.; O’Brien, K.M.; Lee, H.; Wolfenden, L.; Yoong, S.L.; Robson, E.; McAuley, J.H.; Hartvigsen, J.; et al. Musculoskeletal Conditions May Increase the Risk of Chronic Disease: A Systematic Review and Meta-Analysis of Cohort Studies. BMC Med. 2018, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Cavendish, P.A.; Everhart, J.S.; Peters, N.J.; Sommerfeldt, M.F.; Flanigan, D.C. Osteochondral Allograft Transplantation for Knee Cartilage and Osteochondral Defects: A Review of Indications, Technique, Rehabilitation, and Outcomes. JBJS Rev. 2019, 7, e7. [Google Scholar] [CrossRef]
- Condron, N.B.; Kester, B.S.; Tokish, J.M.; Zumstein, M.A.; Gobezie, R.; Scheibel, M.; Cole, B.J. Nonoperative and Operative Soft-Tissue, Cartilage, and Bony Regeneration and Orthopaedic Biologics of the Shoulder: An Orthoregeneration Network (ON) Foundation Review. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2021, 37, 3200–3218. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, S.; Li, E.N.; Fritch, M.R.; Alexander, P.G.; Lin, H.; Tuan, R.S. Tissue Engineering for Musculoskeletal Regeneration and Disease Modeling. Handb. Exp. Pharmacol. 2021, 265, 235–268. [Google Scholar] [CrossRef]
- Colombini, A.; Lopa, S.; Ceriani, C.; Lovati, A.B.; Croiset, S.J.; Di Giancamillo, A.; Lombardi, G.; Banfi, G.; Moretti, M. In Vitro Characterization and In Vivo Behavior of Human Nucleus Pulposus and Annulus Fibrosus Cells in Clinical-Grade Fibrin and Collagen-Enriched Fibrin Gels. Tissue Eng. Part A 2015, 21, 793–802. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Behfar, A.; Terzic, A. Mesenchymal Stem Cells: Engineering Regeneration. Clin. Transl. Sci. 2008, 1, 34–35. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Fisher, J.N.; Tessaro, I.; Bertocco, T.; Peretti, G.M.; Mangiavini, L. The Application of Stem Cells from Different Tissues to Cartilage Repair. Stem Cells Int. 2017, 2017, 2761678. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural Hydrogels for Cartilage Regeneration: Modification, Preparation and Application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef]
- Molinaro, G.; Leroux, J.-C.; Damas, J.; Adam, A. Biocompatibility of Thermosensitive Chitosan-Based Hydrogels: An in Vivo Experimental Approach to Injectable Biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable Thermosensitive Chitosan Hydrogels with Controlled Gelation Kinetics and Enhanced Mechanical Resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef]
- Chenite, A. Rheological Characterisation of Thermogelling Chitosan/Glycerol-Phosphate Solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Stanzione, A.; Polini, A.; La Pesa, V.; Romano, A.; Quattrini, A.; Gigli, G.; Moroni, L.; Gervaso, F. Development of Injectable Thermosensitive Chitosan-Based Hydrogels for Cell Encapsulation. Appl. Sci. 2020, 10, 6550. [Google Scholar] [CrossRef]
- Morello, G.; De Iaco, G.; Gigli, G.; Polini, A.; Gervaso, F. Chitosan and Pectin Hydrogels for Tissue Engineering and In Vitro Modeling. Gels 2023, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, A.; Polini, A.; La Pesa, V.; Quattrini, A.; Romano, A.; Gigli, G.; Moroni, L.; Gervaso, F. Thermosensitive Chitosan-Based Hydrogels Supporting Motor Neuron-like NSC-34 Cell Differentiation. Biomater. Sci. 2021, 9, 7492–7503. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-Based Chitosan Thermosensitive Hydrogels and Their Biomedical Applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Lombardo, M.D.M.; Mangiavini, L.; Peretti, G.M. Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective. Pharmaceutics 2021, 13, 1886. [Google Scholar] [CrossRef]
- Wieringa, P.A.; Gonçalves de Pinho, A.R.; Micera, S.; van Wezel, R.J.A.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv. Healthc. Mater. 2018, 7, e1701164. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as Stem Cell Regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.H.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar] [CrossRef]
- Roncada, T.; Bonithon, R.; Blunn, G.; Roldo, M. Soft Substrates Direct Stem Cell Differentiation into the Chondrogenic Lineage without the Use of Growth Factors. J. Tissue Eng. 2022, 13, 20417314221122120. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers 2021, 13, 2950. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Tessaro, I.; Marmotti, A.; Sirtori, C.; Peretti, G.M.; Mangiavini, L. Does the Harvesting Site Influence the Osteogenic Potential of Mesenchymal Stem Cells? Stem Cells Int. 2019, 2019, 9178436. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Pierini, M.; Dozza, B.; Lucarelli, E.; Tazzari, P.L.; Ricci, F.; Remondini, D.; di Bella, C.; Giannini, S.; Donati, D. Efficient Isolation and Enrichment of Mesenchymal Stem Cells from Bone Marrow. Cytotherapy 2012, 14, 686–693. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and Characterization of Primary Bone Marrow Mesenchymal Stromal Cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, M.; Kim, H.-S.; Kim, Y.-M.; Choi, E.-H. Adipose-Derived Stem Cells as a New Therapeutic Modality for Ageing Skin. Exp. Dermatol. 2011, 20, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Ozpur, M.A.; Guneren, E.; Canter, H.I.; Karaaltin, M.V.; Ovali, E.; Yogun, F.N.; Baygol, E.G.; Kaplan, S. Generation of Skin Tissue Using Adipose Tissue-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells–a Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.M.; Ulivi, M.; De Girolamo, L.; Meroni, V.; Lombardo, M.D.; Mangiavini, L. Evaluation of the Use of Autologous Micro-Fragmented Adipose Tissue in the Treatment of Knee Osteoarthritis: Preliminary Results of a Randomized Controlled Trial. J. Biol. Regul. Homeost. Agents 2018, 32, 193–199. [Google Scholar] [PubMed]
- Hoemann, C.D.; Sun, J.; Légaré, A.; McKee, M.D.; Buschmann, M.D. Tissue Engineering of Cartilage Using an Injectable and Adhesive Chitosan-Based Cell-Delivery Vehicle. Osteoarthr. Cartil. 2005, 13, 318–329. [Google Scholar] [CrossRef]
- Logerstedt, D.S.; Ebert, J.R.; MacLeod, T.D.; Heiderscheit, B.C.; Gabbett, T.J.; Eckenrode, B.J. Effects of and Response to Mechanical Loading on the Knee. Sports Med. 2022, 52, 201–235. [Google Scholar] [CrossRef]
- Herrera Millar, V.R.; Mangiavini, L.; Polito, U.; Canciani, B.; Nguyen, V.T.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Di Giancamillo, A. Hypoxia as a Stimulus for the Maturation of Meniscal Cells: Highway to Novel Tissue Engineering Strategies? Int. J. Mol. Sci. 2021, 22, 6905. [Google Scholar] [CrossRef]
- Szojka, A.R.A.; Li, D.X.; Sopcak, M.E.J.; Ma, Z.; Kunze, M.; Mulet-Sierra, A.; Adeeb, S.M.; Westover, L.; Jomha, N.M.; Adesida, A.B. Mechano-Hypoxia Conditioning of Engineered Human Meniscus. Front. Bioeng. Biotechnol. 2021, 9, 739438. [Google Scholar] [CrossRef]
- Herrera Millar, V.R.; Canciani, B.; Mangiavini, L.; Filipe, J.F.S.; Aidos, L.; Pallaoro, M.; Peretti, G.M.; Pocar, P.; Modina, S.C.; Di Giancamillo, A. Endostatin in 3D Fibrin Hydrogel Scaffolds Promotes Chondrogenic Differentiation in Swine Neonatal Meniscal Cells. Biomedicines 2022, 10, 2415. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Canciani, B.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Mangiavini, L. Effect of Chemically Induced Hypoxia on Osteogenic and Angiogenic Differentiation of Bone Marrow Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in Direct Coculture. Cells 2020, 9, 757. [Google Scholar] [CrossRef]
- Canciani, B.; Herrera Millar, V.R.; Pallaoro, M.; Aidos, L.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Mangiavini, L.; Di Giancamillo, A. Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus. Int. J. Mol. Sci. 2021, 22, 12465. [Google Scholar] [CrossRef]
- Dubois, S.G.; Floyd, E.Z.; Zvonic, S.; Kilroy, G.; Wu, X.; Carling, S.; Halvorsen, Y.D.C.; Ravussin, E.; Gimble, J.M. Isolation of Human Adipose-Derived Stem Cells from Biopsies and Liposuction Specimens. In Mesenchymal Stem Cells; Prockop, D.J., Bunnell, B.A., Phinney, D.G., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 69–79. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canciani, B.; Semeraro, F.; Herrera Millar, V.R.; Gervaso, F.; Polini, A.; Stanzione, A.; Peretti, G.M.; Di Giancamillo, A.; Mangiavini, L. In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10446. https://doi.org/10.3390/ijms241310446
Canciani B, Semeraro F, Herrera Millar VR, Gervaso F, Polini A, Stanzione A, Peretti GM, Di Giancamillo A, Mangiavini L. In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(13):10446. https://doi.org/10.3390/ijms241310446
Chicago/Turabian StyleCanciani, Barbara, Francesca Semeraro, Valentina Rafaela Herrera Millar, Francesca Gervaso, Alessandro Polini, Antonella Stanzione, Giuseppe Michele Peretti, Alessia Di Giancamillo, and Laura Mangiavini. 2023. "In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering" International Journal of Molecular Sciences 24, no. 13: 10446. https://doi.org/10.3390/ijms241310446
APA StyleCanciani, B., Semeraro, F., Herrera Millar, V. R., Gervaso, F., Polini, A., Stanzione, A., Peretti, G. M., Di Giancamillo, A., & Mangiavini, L. (2023). In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering. International Journal of Molecular Sciences, 24(13), 10446. https://doi.org/10.3390/ijms241310446






