Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development
Abstract
1. Introduction
2. Results
2.1. SNV and Indel Analysis
2.2. Recurrent Coding Non-Synonymous Variants
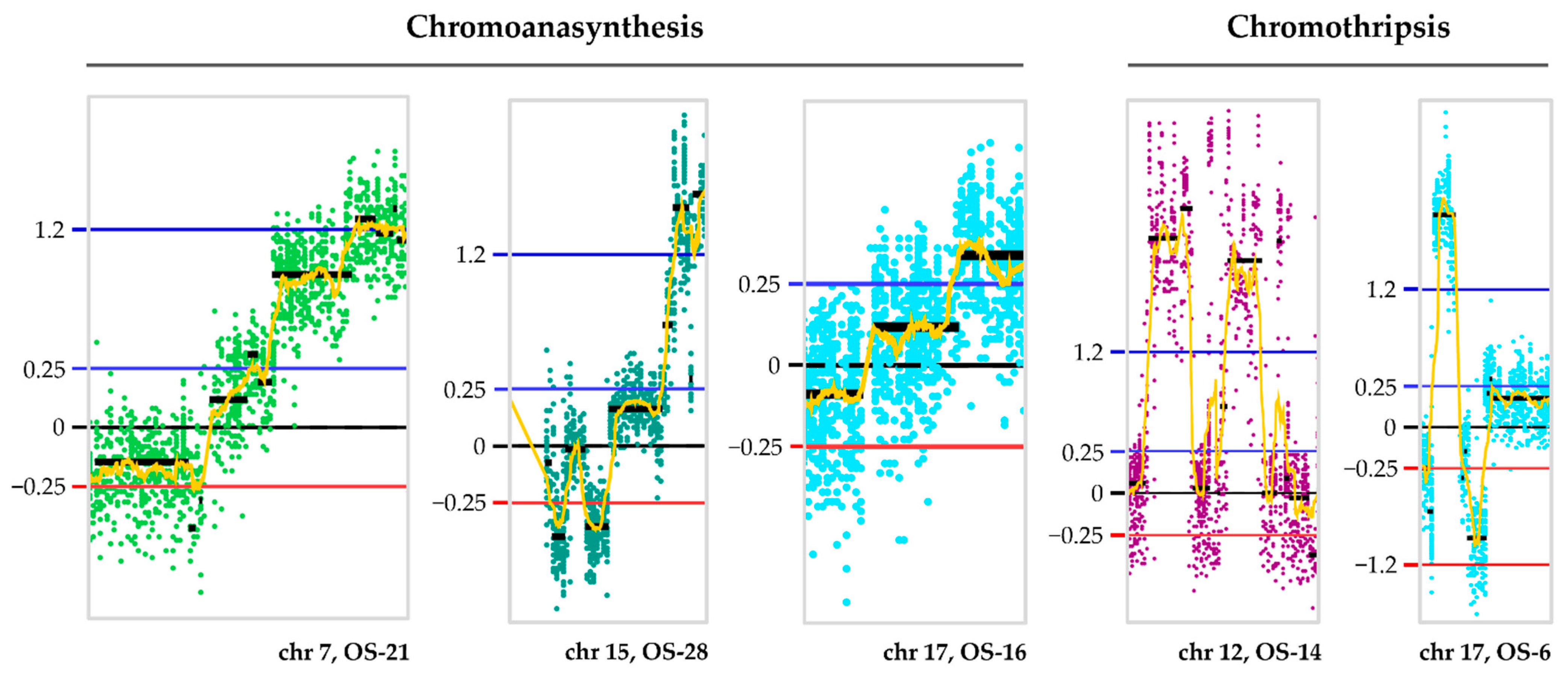
2.3. CNA
2.4. Recurrent CNA Events
2.5. Candidate Germline Variants
2.6. Protein–Protein Interaction Network and Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Characterization of the Patients
4.2. Clinical Exome Sequencing and Analysis
4.3. CNA Analysis
4.4. Protein–Protein Interaction (PPI) Network and Functional Enrichment Analysis of OS-Altered Genes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma Incidence and Survival Rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Balmant, N.V.; Reis, R.D.S.; Santos, M.D.O.; Maschietto, M.; de Camargo, B. Incidence and Mortality of Bone Cancer among Children, Adolescents and Young Adults of Brazil. Clinics 2019, 74, e858. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A Comprehensive Review. SICOT J. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.J.; Taran, R.; Malipatil, N.B. Pediatric Osteosarcoma: An Updated Review. Indian J. Med. Paediatr. Oncol. 2017, 38, 33–43. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and Somatic Genetics of Osteosarcoma—Connecting Aetiology, Biology and Therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Franceschini, N.; Lam, S.W.; Cleton-Jansen, A.M.; Bovée, J.V.M.G. What’s New in Bone Forming Tumours of the Skeleton? Virchows Arch. 2020, 476, 147–157. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- INCA. Incidência, Mortalidade e Morbidade Hospitalar Por Câncer Em Crianças, Adolescentes e Adultos Jovens No Brasil: Informações Dos Registros de Câncer e Do Sistema de Mortalidade; Ministério da Saúde: Rio de Janeiro, Brazil, 2016.
- Petrilli, A.S.; Brunetto, A.L.; Cypriano, M.D.S.; Ferraro, A.A.; Macedo, C.R.P.D.; Senerchia, A.A.; Almeida, M.T.; da Costa, C.M.; Lustosa, D.; Borsato, M.L.; et al. Fifteen Years’ Experience of the Brazilian Osteosarcoma Treatment Group (BOTG): A Contribution from an Emerging Country. J. Adolesc. Young Adult Oncol. 2013, 2, 145–152. [Google Scholar] [CrossRef]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer. Increasing Access, Advancing Quality, Saving Lives; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Savage, S.A.; Mirabello, L. Using Epidemiology and Genomics to Understand Osteosarcoma Etiology. Sarcoma 2011, 2011, 548151. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araújo, J.M.G.; Fernandes, J.V. Biology and Pathogenesis of Human Osteosarcoma (Review). Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef]
- Chen, X.; Pappo, A.; Dyer, M.A. Pediatric Solid Tumor Genomics and Developmental Pliancy. Oncogene 2015, 34, 5207–5215. [Google Scholar] [CrossRef] [PubMed]
- Tirtei, E.; Cereda, M.; De Luna, E.; Quarello, P.; Asaftei, S.D.; Fagioli, F. Omic Approaches to Pediatric Bone Sarcomas. Pediatr. Blood Cancer 2020, 67, e28072. [Google Scholar] [CrossRef] [PubMed]
- Poos, K.; Smida, J.; Maugg, D.; Eckstein, G.; Baumhoer, D.; Nathrath, M.; Korsching, E. Genomic Heterogeneity of Osteosarcoma—Shift from Single Candidates to Functional Modules. PLoS ONE 2015, 10, e0123082. [Google Scholar] [CrossRef]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients with Osteosarcoma. JAMA Oncol. 2020, 6, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.F.; de Barros, J.S.; da Costa, S.S.; de Oliveira Scliar, M.; Van Helvoort Lengert, A.; Boldrini, É.; da Silva, S.R.M.; Tasic, L.; Vidal, D.O.; Krepischi, A.C.V.; et al. DNA Methylation Patterns Suggest the Involvement of DNMT3B and TET1 in Osteosarcoma Development. Mol. Genet. Genom. 2023, 298, 721–733. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Defining Driver DNA Methylation Changes in Human Cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. Insights from Large-Scale Cancer Genome Sequencing. Annu. Rev. Cancer Biol. 2018, 2, 429–444. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; John, E.; et al. Pan-Cancer Genome and Transcriptome Analyses of 1699 Paediatric Leukaemias and Solid Tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Rickel, K.; Fang, F.; Tao, J. Molecular Genetics of Osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Bousquet, M.; Noirot, C.; Accadbled, F.; Sales de Gauzy, J.; Castex, M.P.; Brousset, P.; Gomez-Brouchet, A. Whole-Exome Sequencing in Osteosarcoma Reveals Important Heterogeneity of Genetic Alterations. Ann. Oncol. 2016, 27, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.J.; Bayles, I.; Funnell, A.P.W.; Miller, T.E.; Saiakhova, A.; Lizardo, M.M.; Bartels, C.F.; Kapteijn, M.Y.; Hung, S.; Mendoza, A.; et al. Positively Selected Enhancer Elements Endow Osteosarcoma Cells with Metastatic Competence. Nat. Med. 2018, 24, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef]
- Kovac, M.; Blattmann, C.; Ribi, S.; Smida, J.; Mueller, N.S.; Engert, F.; Castro-Giner, F.; Weischenfeldt, J.; Kovacova, M.; Krieg, A.; et al. Exome Sequencing of Osteosarcoma Reveals Mutation Signatures Reminiscent of BRCA Deficiency. Nat. Commun. 2015, 6, 8940. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The Landscape of Genomic Alterations across Childhood Cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Rahman, N. Realizing the Promise of Cancer Predisposition Genes. Nature 2014, 505, 302–308. [Google Scholar] [CrossRef]
- McGee, R.B.; Nichols, K.E. Introduction to Cancer Genetic Susceptibility Syndromes. Hematology 2016, 2016, 293–301. [Google Scholar] [CrossRef]
- Kesserwan, C.; Friedman Ross, L.; Bradbury, A.R.; Nichols, K.E. The Advantages and Challenges of Testing Children for Heritable Predisposition to Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 251–269. [Google Scholar] [CrossRef]
- Simpson, E.; Brown, H.L. Understanding Osteosarcomas. J. Am. Acad. Physician Assist. 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary Genomic Approaches Highlight the PI3K/MTOR Pathway as a Common Vulnerability in Osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Smida, J.; Xu, H.; Zhang, Y.; Baumhoer, D.; Ribi, S.; Kovac, M.; von Luettichau, I.; Bielack, S.; O’Leary, V.B.; Leib-Mösch, C.; et al. Genome-Wide Analysis of Somatic Copy Number Alterations and Chromosomal Breakages in Osteosarcoma. Int. J. Cancer 2017, 141, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Z.; Fan, S.; Yao, D.; Zhang, Z.; Guan, C.; Deng, W.; Ying, Y. Protein Tyrosine Phosphatase Receptor-Type Q: Structure, Activity, and Implications in Human Disease. Protein Pept. Lett. 2022, 29, 567–573. [Google Scholar] [CrossRef]
- Du, Y.; Grandis, J.R. Receptor-Type Protein Tyrosine Phosphatases in Cancer. Chin. J. Cancer 2015, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Nakano, T.; Hosonaga, M.; Sampetrean, O.; Harigai, R.; Sasaki, T.; Koya, I.; Okano, H.; Kudoh, J.; Saya, H.; et al. RNA Sequencing Analysis Reveals Interactions between Breast Cancer or Melanoma Cells and the Tissue Microenvironment during Brain Metastasis. BioMed Res. Int. 2017, 2017, 8032910. [Google Scholar] [CrossRef]
- Poturnajova, M.; Furielova, T.; Balintova, S.; Schmidtova, S.; Kucerova, L.; Matuskova, M. Molecular Features and Gene Expression Signature of Metastatic Colorectal Cancer (Review). Oncol. Rep. 2021, 45, 10. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Liao, Y.; Liu, J.; Huang, J.; Xia, M.; Chen, M.; Tan, H.; He, W.; Xu, M.; et al. High Expression of PTPRM Predicts Poor Prognosis and Promotes Tumor Growth and Lymph Node Metastasis in Cervical Cancer. Cell Death Dis. 2020, 11, 687. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Liao, P.-J.; Liu, Y.; Zhong, M.; Sun, A.; Jiang, X.; Wang, X.-P.; Zhang, M. Protein Tyrosine Phosphatase, Receptor Type B Is a Potential Biomarker and Facilitates Cervical Cancer Metastasis via Epithelial-Mesenchymal Transition. Bioengineered 2021, 12, 5739–5748. [Google Scholar] [CrossRef]
- Caldas, G.V.; DeLuca, J.G. KNL1: Bringing Order to the Kinetochore. Chromosoma 2014, 123, 169–181. [Google Scholar] [CrossRef]
- McLeod, C.; Gout, A.M.; Zhou, X.; Thrasher, A.; Rahbarinia, D.; Brady, S.W.; Macias, M.; Birch, K.; Finkelstein, D.; Sunny, J.; et al. Jude Cloud: A Pediatric Cancer Genomic Data-Sharing Ecosystem. Cancer Discov. 2021, 11, 1082–1099. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhao, Y.; Liu, Y.; Cai, B.; Dong, N.; Li, B. Effect of KNL1 on the Proliferation and Apoptosis of Colorectal Cancer Cells. Technol. Cancer Res. Treat. 2019, 18, 153303381985866. [Google Scholar] [CrossRef]
- Nakamura, E.; Hata, K.; Takahata, Y.; Kurosaka, H.; Abe, M.; Abe, T.; Kihara, M.; Komori, T.; Kobayashi, S.; Murakami, T.; et al. Zfhx4 Regulates Endochondral Ossification as the Transcriptional Platform of Osterix in Mice. Commun. Biol. 2021, 4, 1258. [Google Scholar] [CrossRef]
- Zong, S.; Xu, P.; Xu, Y.H.; Guo, Y. A Bioinformatics Analysis: ZFHX4 Is Associated with Metastasis and Poor Survival in Ovarian Cancer. J. Ovarian Res. 2022, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Millstein, J.; Budden, T.; Goode, E.L.; Anglesio, M.S.; Talhouk, A.; Intermaggio, M.P.; Leong, H.S.; Chen, S.; Elatre, W.; Gilks, B.; et al. Prognostic Gene Expression Signature for High-Grade Serous Ovarian Cancer. Ann. Oncol. 2020, 31, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, J.; Park, S.M.; Hong, C.M.; Han, M.E.; Song, P.; Kang, C.D.; Lee, D.; Kim, Y.H.; Hur, J.; et al. Prognostic Role of Zinc Finger Homeobox 4 in Ovarian Serous Cystadenocarcinoma. Genet. Test. Mol. Biomark. 2020, 24, 145–149. [Google Scholar] [CrossRef]
- Qing, T.; Zhu, S.; Suo, C.; Zhang, L.; Zheng, Y.; Shi, L. Somatic Mutations in ZFHX4 Gene Are Associated with Poor Overall Survival of Chinese Esophageal Squamous Cell Carcinoma Patients. Sci. Rep. 2017, 7, 4951. [Google Scholar] [CrossRef]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The Genetics of Osteosarcoma. Sarcoma 2012, 2012, 627254. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef]
- Maire, G.; Yoshimoto, M.; Chilton-MacNeill, S.; Thorner, P.S.; Zielenska, M.; Squire, J.A. Recurrent RECQL4 Imbalance and Increased Gene Expression Levels Are Associated with Structural Chromosomal Instability in Sporadic Osteosarcoma. Neoplasia 2009, 11, 260–268, IN4–IN6. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent Mutation of IGF Signalling Genes and Distinct Patterns of Genomic Rearrangement in Osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Brunetti, G.; Marzano, F.; Colucci, S.; Ventura, A.; Cavallo, L.; Grano, M.; Faienza, M.F. Genotype–Phenotype Correlation in Juvenile Paget Disease: Role of Molecular Alterations of the TNFRSF11B Gene. Endocrine 2012, 42, 266–271. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The Roles of Osteoprotegerin in Cancer, Far beyond a Bone Player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef]
- Marley, K.; Bracha, S.; Seguin, B. Osteoprotegerin Activates Osteosarcoma Cells That Co-Express RANK and RANKL. Exp. Cell Res. 2015, 338, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A Copy Number Variation Map of the Human Genome. Nat. Rev. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, W.; Hurles, M.E.; Lupski, J.R. Copy Number Variation in Human Health, Disease, and Evolution. Annu. Rev. Genomics Hum. Genet. 2009, 10, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.L.; Sivaprakasam, K.; Briones, N.; Zismann, V.; Perdigones, N.; Drenner, K.; Facista, S.; Richholt, R.; Liang, W.; Aldrich, J.; et al. Canine Osteosarcoma Genome Sequencing Identifies Recurrent Mutations in DMD and the Histone Methyltransferase Gene SETD2. Commun. Biol. 2019, 2, 266. [Google Scholar] [CrossRef]
- Wang, Y.; Marino-Enriquez, A.; Bennett, R.R.; Zhu, M.; Shen, Y.; Eilers, G.; Lee, J.C.; Henze, J.; Fletcher, B.S.; Gu, Z.; et al. Dystrophin Is a Tumor Suppressor in Human Cancers with Myogenic Programs. Nat. Genet. 2014, 46, 601–606. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, X.; Ren, T.; Huang, Y.; Zhang, H.; Yu, Y.; Chen, C.; Wang, W.; Niu, J.; Lou, J.; et al. The Role of Tumor-Associated Macrophages in Osteosarcoma Progression—Therapeutic Implications. Cell. Oncol. 2021, 44, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, D.; Mulcrone, P.L.; Owens, P.; Sterling, J.A. The Bone Microenvironment: A Fertile Soil for Tumor Growth. Curr. Osteoporos. Rep. 2016, 14, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.-H.; Yan, Y.-G.; Wang, C.; Wang, W.-J. PI3K/Akt Signaling in Osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Y.; Huang, Q.; Wang, B.; Wang, W.; Niu, J.; Lou, J.; Xu, J.; Ren, T.; Huang, Y.; et al. PI3K Inhibitor Impairs Tumor Progression and Enhances Sensitivity to Anlotinib in Anlotinib-Resistant Osteosarcoma. Cancer Lett. 2022, 536, 215660. [Google Scholar] [CrossRef]
- Lézot, F.; Corre, I.; Morice, S.; Rédini, F.; Verrecchia, F. SHH Signaling Pathway Drives Pediatric Bone Sarcoma Progression. Cells 2020, 9, 536. [Google Scholar] [CrossRef]
- Du, X.; Yang, J.; Yang, D.; Tian, W.; Zhu, Z. The Genetic Basis for Inactivation of Wnt Pathway in Human Osteosarcoma. BMC Cancer 2014, 14, 450. [Google Scholar] [CrossRef]
- Lamora, A.; Talbot, J.; Mullard, M.; Brounais-Le Royer, B.; Redini, F.; Verrecchia, F. TGF-β Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, 96. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Xie, X.; Wang, Z.; Lei, Q. Identification of Potential Crucial Genes and Key Pathways in Osteosarcoma. Hereditas 2020, 157, 29. [Google Scholar] [CrossRef]
- Mandelker, D.; Donoghue, M.; Talukdar, S.; Bandlamudi, C.; Srinivasan, P.; Vivek, M.; Jezdic, S.; Hanson, H.; Snape, K.; Kulkarni, A.; et al. Germline-Focussed Analysis of Tumour-Only Sequencing: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2019, 30, 1221–1231. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 11, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- The 1000 Genomes Project Consortium A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74.
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, M.S.; Yamamoto, G.L.; de Almeida, T.F.; Ezquina, S.A.M.; Sunaga, D.Y.; Pho, N.; Bozoklian, D.; Sandberg, T.O.M.; Brito, L.A.; Lazar, M.; et al. Exomic Variants of an Elderly Cohort of Brazilians in the ABraOM Database. Hum. Mutat. 2017, 38, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.-H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. DbSNP: The NCBI Database of Genetic Variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- The UK10K Consortium The UK10K Project Identifies Rare Variants in Health and Disease. Nature 2015, 526, 82–90. [CrossRef]
- Griffith, M.; Spies, N.C.; Krysiak, K.; McMichael, J.F.; Coffman, A.C.; Danos, A.M.; Ainscough, B.J.; Ramirez, C.A.; Rieke, D.T.; Kujan, L.; et al. CIViC Is a Community Knowledgebase for Expert Crowdsourcing the Clinical Interpretation of Variants in Cancer. Nat. Genet. 2017, 49, 170–174. [Google Scholar] [CrossRef]
- Zhang, J.; Bajari, R.; Andric, D.; Gerthoffert, F.; Lepsa, A.; Nahal-Bose, H.; Stein, L.D.; Ferretti, V. The International Cancer Genome Consortium Data Portal. Nat. Biotechnol. 2019, 37, 367–369. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Fuentes Fajardo, K.V.; Adams, D.; Mason, C.E.; Sincan, M.; Tifft, C.; Toro, C.; Boerkoel, C.F.; Gahl, W.; Markello, T. Detecting False-Positive Signals in Exome Sequencing. Hum. Mutat. 2012, 33, 609–613. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Li, C.; Boerwinkle, E. DbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum. Mutat. 2016, 37, 235–241. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.R.; Ziman, R.; Yuen, R.K.C.; Feuk, L.; Scherer, S.W. The Database of Genomic Variants: A Curated Collection of Structural Variation in the Human Genome. Nucleic Acids Res. 2014, 42, D986–D992. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
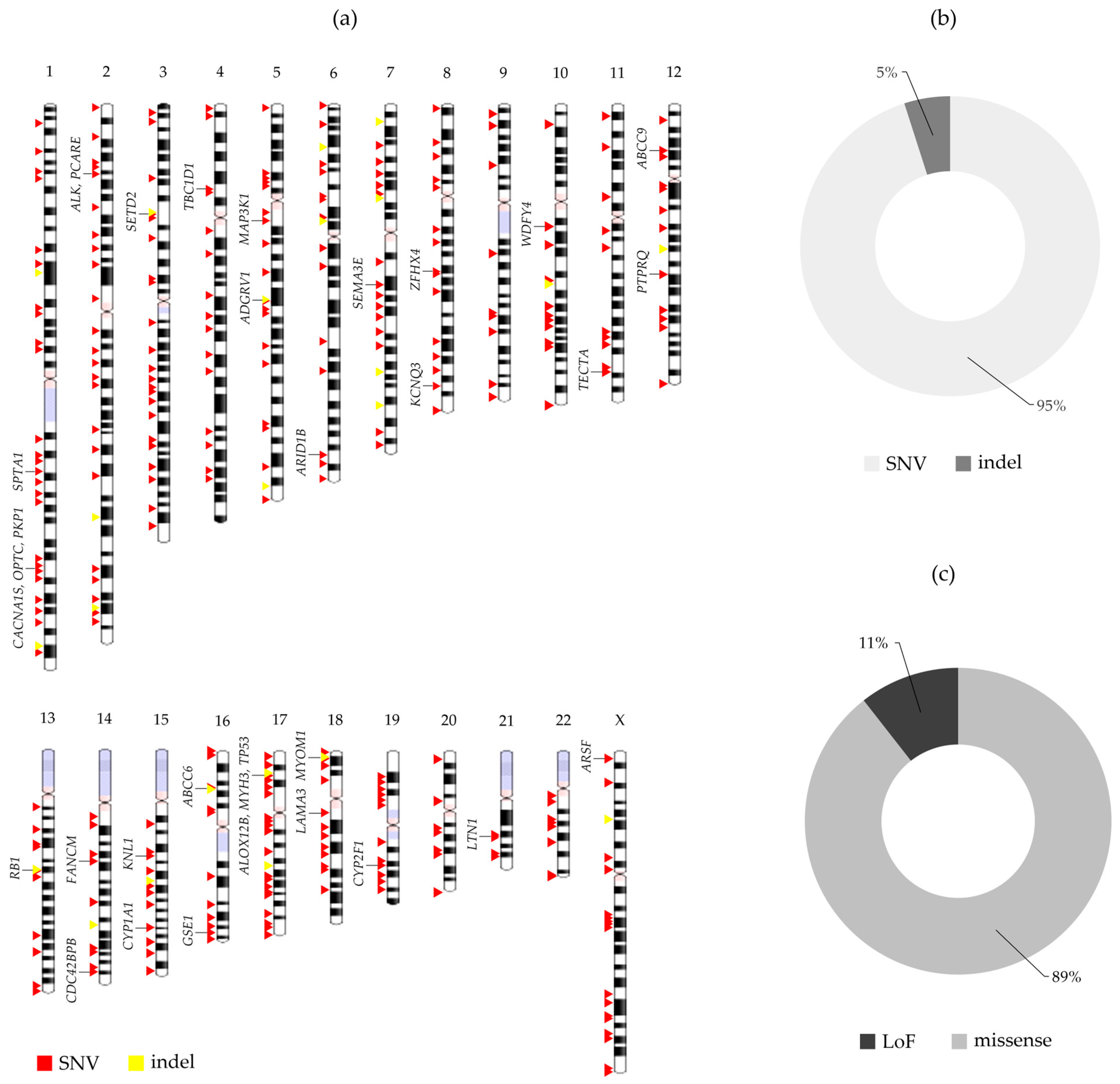
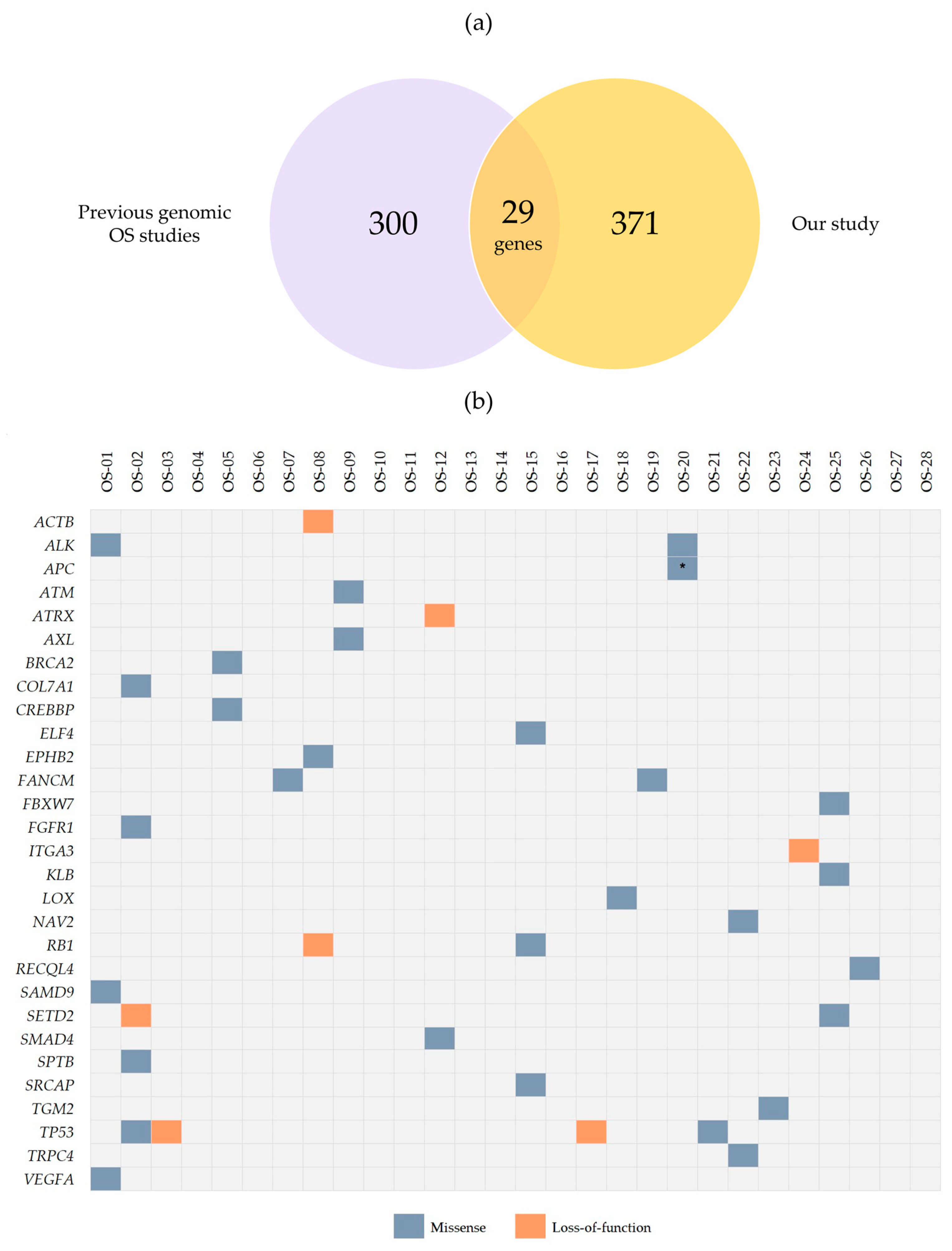
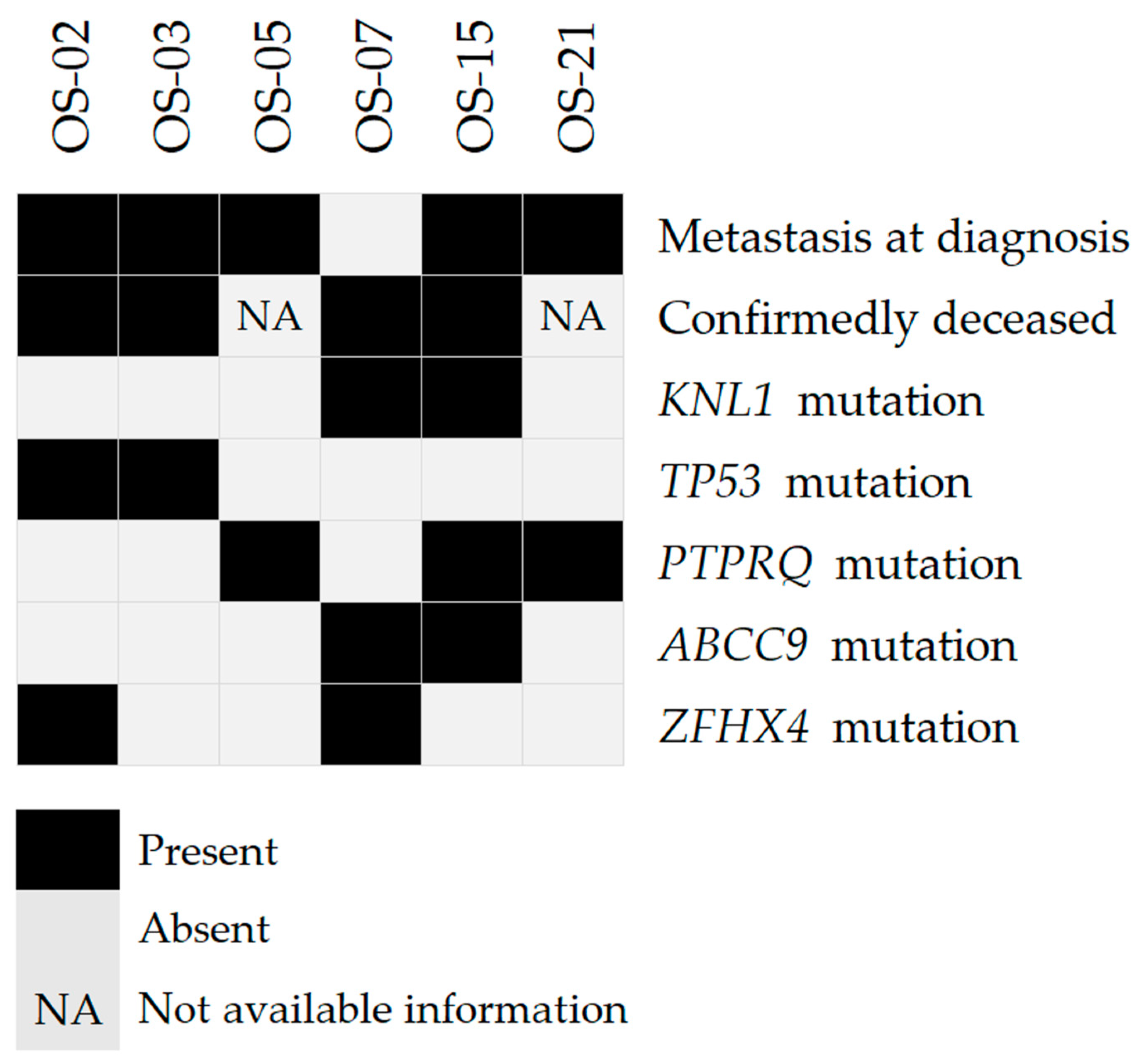
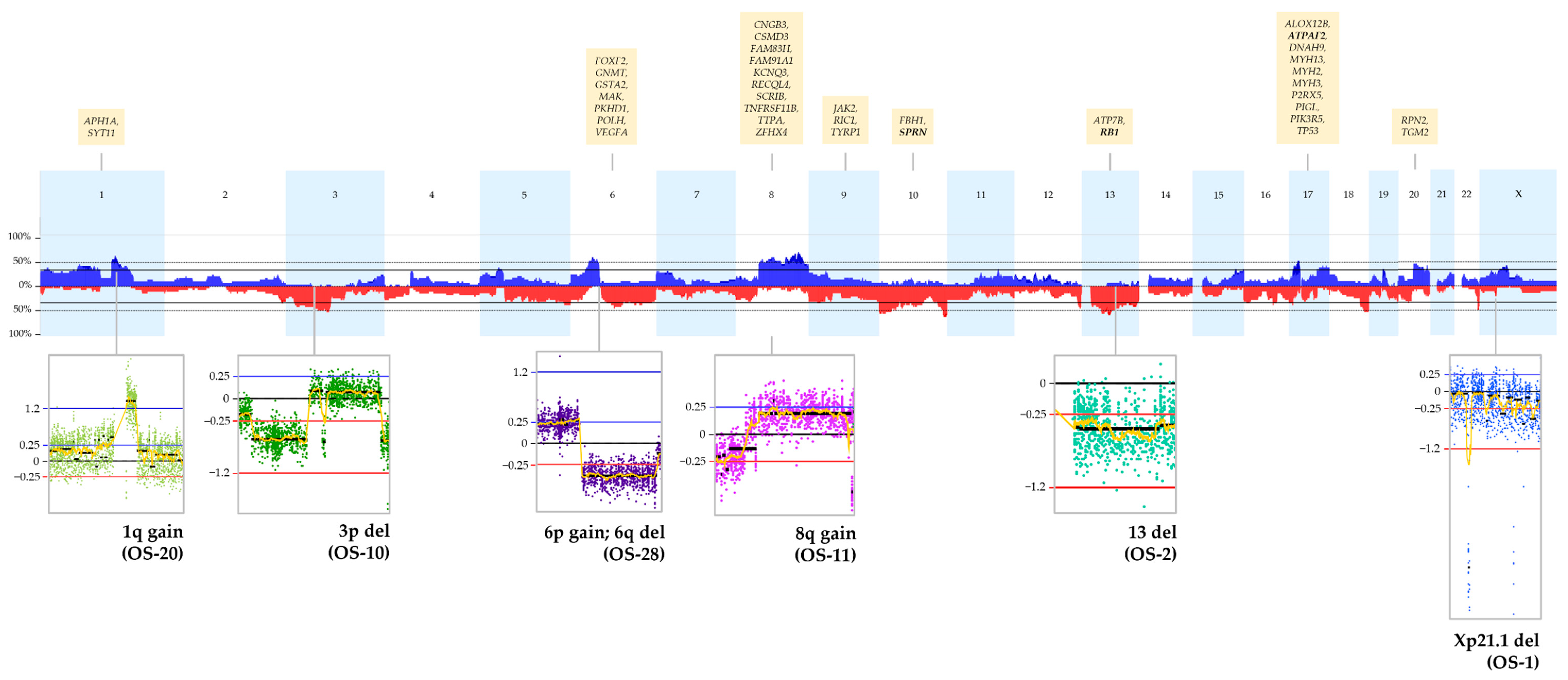
| Classification * | Genes |
|---|---|
| Tumor suppressors | ADAMTS18, APC, ATM, AXIN2, BAX, BRCA2, CDH13, CIC, CREBBP, CSMD1, DCDC2, DNMT1, EPHB2, FBXW7, GNMT, HRG, IRF8, ITGA7, LIFR, LOX, LZTS1, MAX, MST1R, MYO18B, PAWR, PIN1, PKD1, PPP2R1B, PRDM5, PTPN13, RB1, ROBO1, SCRIB, SERPINB5, SETD2, SMAD4, SYNM, TGFBR3, THBS1, TP53, VEGFA, ZBTB16, ZFHX3 |
| Oncogenes | ALK, AXL, BAX, BCL11A, CSF1, ELF4, EVI5, FGFR1, GLI2, ITGA3, JAK2, KIT, MBD1, MEIS1, MST1R, NCOA3, NTRK1, PPM1D, RALGDS, RHO, TBC1D1, TYRP1, ZBTB16 |
| Variant Info # | RefSeq Genes 105 Interim v3.1, NCBI | OS Genes b | Cancer Databases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genomic Coordinates (Chr:Start-Stop) a | Ref/Alt Alleles | VAF | Gene Name | Effect | Match | COSMIC | ICGC | CIViC | TCGA |
| chr1:197316605-197316605 | G/A | 0.56 | CRB1 | LoF | |||||
| chr1:76215170-76215174 | AAAGA/- | 0.52 | ACADM | LoF | |||||
| chr2:169761127-169761127 | G/A | 0.33 | G6PC2 | LoF | |||||
| chr2:188331670-188331669 | -/T | 0.75 | TFPI | LoF | |||||
| chr2:227661111-227661111 | C/A | 0.15 | IRS1 | LoF | |||||
| chr2:227896863-227896871 | CCTGGGGGT/- | 0.53 | COL4A4 | LoF | |||||
| chr2:234201046-234201046 | A/T | 0.16 | ATG16L1 | LoF | |||||
| chr2:242149051-242149051 | G/T | 0.55 | ANO7 | LoF | |||||
| chr3:38798294-38798294 | G/T | 0.26 | SCN10A | LoF | |||||
| chr3:47061249-47061249 | C/A | 0.52 | SETD2 TS | LoF | Y | ||||
| chr3:49929189-49929189 | A/G | 0.38 | MST1R TS/OG | LoF | |||||
| chr4:100350727-100350727 | C/A | 0.5 | ADH7 | LoF | |||||
| chr5:176830344-176830343 | -/T | 0.38 | F12 | LoF | |||||
| chr5:89979528-89979527 | -/T | 0.46 | ADGRV1 | LoF | |||||
| chr6:42931340-42931340 | G/T | 0.75 | CNPY3-GNMT, GNMT | LoF | |||||
| chr6:52617789-52617789 | C/- | 0.48 | GSTA2 | LoF | |||||
| chr7:107198514-107198514 | A/C | 0.6 | COG5 | LoF | |||||
| chr7:121726081-121726081 | C/- | 0.5 | AASS | LoF | |||||
| chr7:138406691-138406691 | T/- | 0.73 | ATP6V0A4 | LoF | |||||
| chr7:40174717-40174717 | C/G | 0.45 | SUGCT | LoF | |||||
| chr7:44561787-44561787 | C/- | 0.67 | NPC1L1 | LoF | |||||
| chr7:5569193-5569193 | G/- | 0.45 | ACTB | LoF | Y | ||||
| chr8:77761365-77761365 | G/T | 0.65 | ZFHX4 | LoF | |||||
| chr9:130885414-130885414 | C/A | 0.22 | PTGES2 | LoF | |||||
| chr10:79795134-79795135 | AG/- | 0.32 | RPS24 | LoF | |||||
| chr10:79795137-79795137 | A/T | 0.31 | RPS24 | LoF | |||||
| chr11:114057673-114057673 | G/A | 0.93 (*) | ZBTB16 TS/OG | LoF | |||||
| chr11:93545017-93545017 | A/C | 0.37 | MED17 | LoF | |||||
| chr12:40645036-40645036 | G/T | 0.4 | LRRK2 | LoF | |||||
| chr12:66788074-66788073 | -/C | 0.51 | GRIP1 | LoF | Y | ||||
| chr13:48955486-48955486 | T/- | 0.45 | RB1TS | LoF | Y | Y | |||
| chr15:50904973-50904972 | -/TA | 0.26 | TRPM7 | LoF | |||||
| chr15:53889390-53889390 | G/A | 0.18 | WDR72 | LoF | |||||
| chr15:84566757-84566757 | G/T | 0.31 | ADAMTSL3 | LoF | |||||
| chr16:15850335-15850335 | C/A | 0.22 | MYH11 | LoF | Y | Y | |||
| chr16:16315529-16315528 | -/A | 0.28 | ABCC6 | LoF | |||||
| chr17:48149353-48149353 | G/A | 0.43 | ITGA3 OG | LoF | Y | ||||
| chr17:48268206-48268206 | G/- | 0.36 | COL1A1 | LoF | |||||
| chr17:7574003-7574003 | G/A | 0.7 | TP53TS | LoF | Y | Y | Y | Y | Y |
| chr17:7578190-7578189 | -/T | 0.81 | TP53TS | LoF | Y | Y | |||
| chr17:7578370-7578370 | C/T | 0.43 | TP53TS | LoF | Y | Y | Y | Y | Y |
| chr18:3193953-3193959 | AAGTCTG/- | 0.57 | MYOM1 | LoF | |||||
| chr18:39637927-39637927 | G/T | 0.18 | PIK3C3 | LoF | |||||
| chr18:47806245-47806245 | C/T | 0.47 | MBD1OG | LoF | |||||
| chrX:31089722-31089722 | C/- | 0.96 (**) | FTHL17 | LoF | |||||
| chrX:57318934-57318934 | A/T | 0.34 | FAAH2 | LoF | |||||
| chrX:76939760-76939760 | T/A | 0.28 | ATRX | LoF | Y | Y | |||
| chr1:155851183-155851183 | G/T | 0.23 | SYT11 | Missense | Y | ||||
| chr1:225594417-225594417 | G/A | 0.55 | LBR | Missense | |||||
| chr1:23234503-23234503 | G/C | 0.14 | EPHB2 TS | Missense | Y | Y | |||
| chr1:237802396-237802396 | G/C | 0.2 | RYR2 | Missense | |||||
| chr2:116447461-116447461 | G/T | 0.22 | DPP10 | Missense | Y | ||||
| chr2:169781238-169781238 | C/T | 0.55 | ABCB11 | Missense | Y | ||||
| chr2:228154801-228154801 | C/A | 0.2 | COL4A3 | Missense | |||||
| chr2:234343485-234343485 | C/G | 0.44 | DGKD | Missense | |||||
| chr2:26501668-26501668 | A/G | 0.48 | HADHB | Missense | |||||
| chr3:164764634-164764634 | G/T | 0.17 | SI | Missense | |||||
| chr3:4558263-4558263 | T/G | 0.35 | ITPR1 | Missense | |||||
| chr5:78181435-78181435 | C/T | 0.58 | ARSB | Missense | |||||
| chr6:157502299-157502299 | G/A | 0.12 | ARID1B | Missense | |||||
| chr6:161139813-161139813 | T/A | 0.23 | PLG | Missense | |||||
| chr6:33143809-33143809 | C/A | 0.17 | COL11A2 | Missense | |||||
| chr7:27169095-27169095 | G/C | 0.57 | HOXA4 | Missense | |||||
| chr8:38272404-38272404 | G/T | 0.25 | FGFR1OG | Missense | Y | Y | Y | ||
| chr9:33447464-33447464 | A/G | 0.41 | AQP3 | Missense | |||||
| chr10:105792709-105792709 | C/T | 0.85 | COL17A1 | Missense | Y | ||||
| chr11:119213431-119213431 | C/A | 0.54 | C1QTNF5, MFRP | Missense | |||||
| chr12:21964993-21964993 | C/A | 0.23 | ABCC9 | Missense | |||||
| chr13:113793695-113793695 | C/T | 0.22 | F10 | Missense | |||||
| chr13:52544680-52544680 | C/T | 0.33 | ATP7B | Missense | Y | Y | Y | ||
| chr14:103371559-103371559 | A/C | 0.57 | TRAF3 | Missense | |||||
| chr14:103418914-103418914 | C/A | 0.31 | CDC42BPB | Missense | |||||
| chr16:3781285-3781285 | C/T | 0.27 | CREBBPTS | Missense | Y | Y | |||
| chr17:61958178-61958178 | T/C | 0.52 | GH2 | Missense | |||||
| chr17:7577124-7577124 | C/G | 0.74 | TP53 TS | Missense | Y | Y | Y | Y | |
| chr17:76989711-76989711 | G/C | 0.43 | CANT1 | Missense | |||||
| chr19:14208479-14208479 | T/C | 0.31 | PRKACA | Missense | Y | ||||
| chr19:33716477-33716477 | C/A | 0.62 | SLC7A10 | Missense | |||||
| chr19:41759546-41759546 | G/A | 0.18 | AXL OG | Missense | Y | Y | Y | Y | |
| chrX:138623259-138623259 | C/T | 0.45 | F9 | Missense | Y | ||||
| Region * | Length (Mb) | Cytoband Location | Event | Genes | miRNAs | Cancer Gene Census # |
|---|---|---|---|---|---|---|
| chr1:149683910-153535697 | 3.85 | q21.2–q21.3 | Gain | 160 | 2 | ARNT |
| chr6:42848233-45479933 | 2.64 | p21.1 | Gain | 59 | 1 | |
| chr8:71415111-133492411 | 28.43 | q13.3–q24.22 | Gain | 169 | 12 | EXT1, MYC |
| chr10:128038823-135454121 | 7.41 | q26.2–q26.3 | Loss | 62 | 3 | |
| chr13:53415739-57786339 | 4.37 | q14.3–q21.1 | Loss | 32 | 1 |
| Gene | Variant (GRCh37/hg19) | ID | Read Depth | VAF (%) | ClinVar ID | ClinVar Classification | Franklin Classification | ACMG Criteria * |
|---|---|---|---|---|---|---|---|---|
| RB1 | chr13:48937089-A/G | OS-15 | 53 | 55 | 428682 | P/LP | LP | PM2, PP3, PP5 |
| chr13:48955486:T/- | OS-8 | 63 | 45 | - | - | LP | PVS1, PM2 | |
| TP53 | chr17:7578370-C/T | OS-3 | 23 | 43 | 428908 | P/LP | P | PVS1, PM2, PP5 |
| chr17:7574003-G/A | OS-17 | 40 | 70 | 182970 | P | P | PVS1, PM2, PM1, PP5 | |
| chr17:7578190:-/T | OS-2 | 32 | 81 | - | - | LP | PVS1, PM2 |
| Type of Alteration | GO | KEGG |
|---|---|---|
| Coding SNVs and indels 400 inputted genes 405 nodes 218 edges | Multicellular organismal process Anatomical structure development Developmental process Multicellular organism development Regulation of biological quality System development Cellular component organization Homeostatic process Anatomical structure morphogenesis Animal organ development | Pathways in cancer PI3K–Akt signaling pathway ECM–receptor interaction Human papillomavirus infection Small cell lung cancer Arrhythmogenic right ventricular cardiomyopathy Focal adhesion Proteoglycans in cancer Dilated cardiomyopathy Hypertrophic cardiomyopathy |
| Copy number alterations 1677 inputted genes 1132 nodes 582 edges | Positive regulation of peptidyl-serine phosphorylation of stat protein Regulation of peptidyl-serine phosphorylation of stat protein Natural killer cell activation involved in immune response Response to exogenous dsiRNA B-cell proliferation Humoral immune response T-cell activation involved in immune response Lymphocyte proliferation Leukocyte proliferation Negative regulation of glucuronosyltransferase activity | Autoimmune thyroid disease Cytosolic DNA-sensing pathway RIG-I-like receptor signaling pathway JAK–STAT signaling pathway Hepatitis B Epstein–Barr virus infection Ascorbate and aldarate metabolism Toll-like receptor signaling pathway Human papillomavirus infection PI3K–Akt signaling pathway |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, S.F.; Barros, J.S.d.; Costa, S.S.d.; Carmo, G.B.d.; Scliar, M.d.O.; Lengert, A.v.H.; Boldrini, É.; Silva, S.R.M.d.; Vidal, D.O.; Maschietto, M.; et al. Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development. Int. J. Mol. Sci. 2023, 24, 10463. https://doi.org/10.3390/ijms241310463
Pires SF, Barros JSd, Costa SSd, Carmo GBd, Scliar MdO, Lengert AvH, Boldrini É, Silva SRMd, Vidal DO, Maschietto M, et al. Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development. International Journal of Molecular Sciences. 2023; 24(13):10463. https://doi.org/10.3390/ijms241310463
Chicago/Turabian StylePires, Sara Ferreira, Juliana Sobral de Barros, Silvia Souza da Costa, Gabriel Bandeira do Carmo, Marília de Oliveira Scliar, André van Helvoort Lengert, Érica Boldrini, Sandra Regini Morini da Silva, Daniel Onofre Vidal, Mariana Maschietto, and et al. 2023. "Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development" International Journal of Molecular Sciences 24, no. 13: 10463. https://doi.org/10.3390/ijms241310463
APA StylePires, S. F., Barros, J. S. d., Costa, S. S. d., Carmo, G. B. d., Scliar, M. d. O., Lengert, A. v. H., Boldrini, É., Silva, S. R. M. d., Vidal, D. O., Maschietto, M., & Krepischi, A. C. V. (2023). Analysis of the Mutational Landscape of Osteosarcomas Identifies Genes Related to Metastasis and Prognosis and Disrupted Biological Pathways of Immune Response and Bone Development. International Journal of Molecular Sciences, 24(13), 10463. https://doi.org/10.3390/ijms241310463








