Molecular BCR::ABL1 Quantification and ABL1 Mutation Detection as Essential Tools for the Clinical Management of Chronic Myeloid Leukemia Patients: Results from a Brazilian Single-Center Study
Abstract
:1. Introduction
2. Results
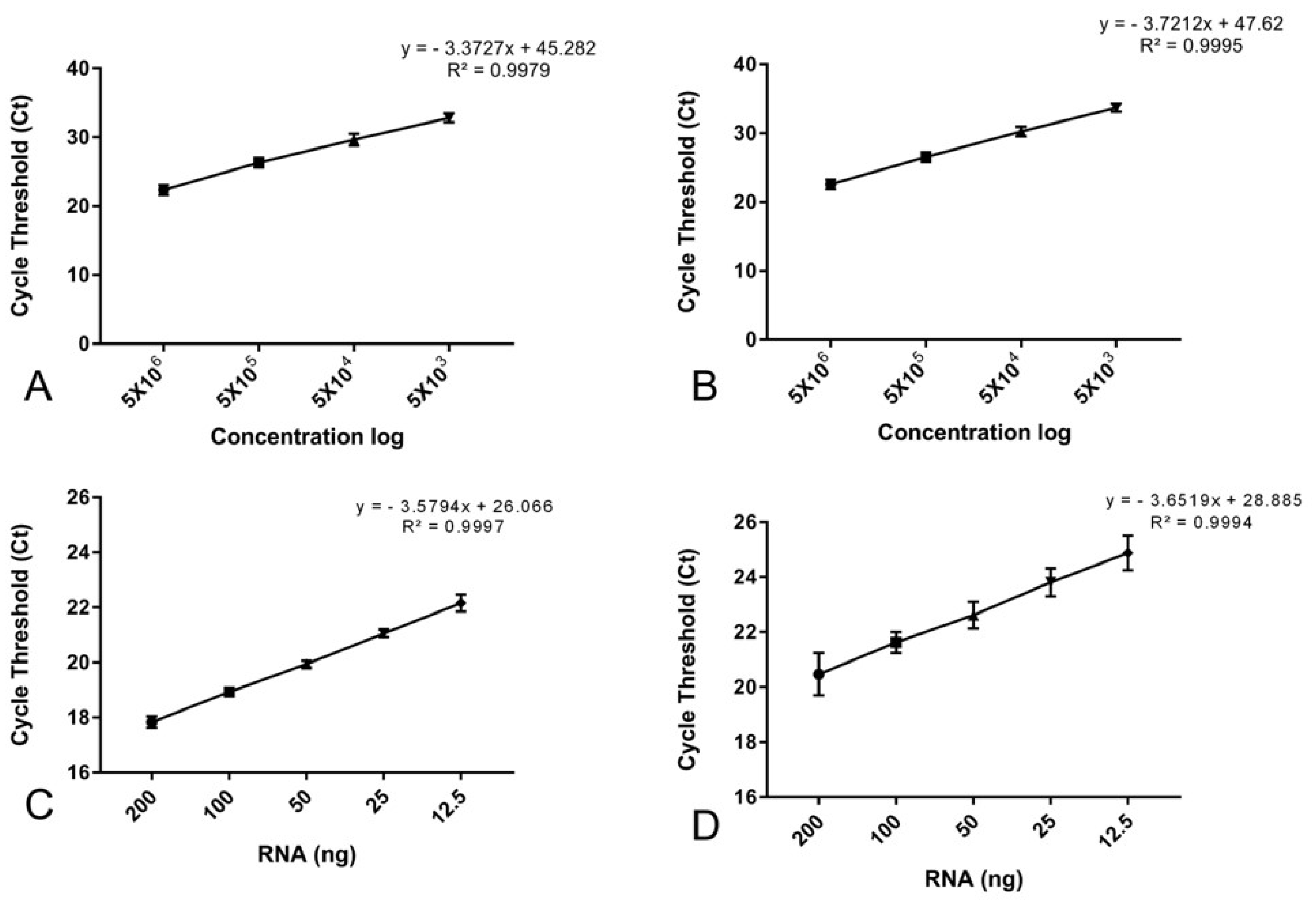
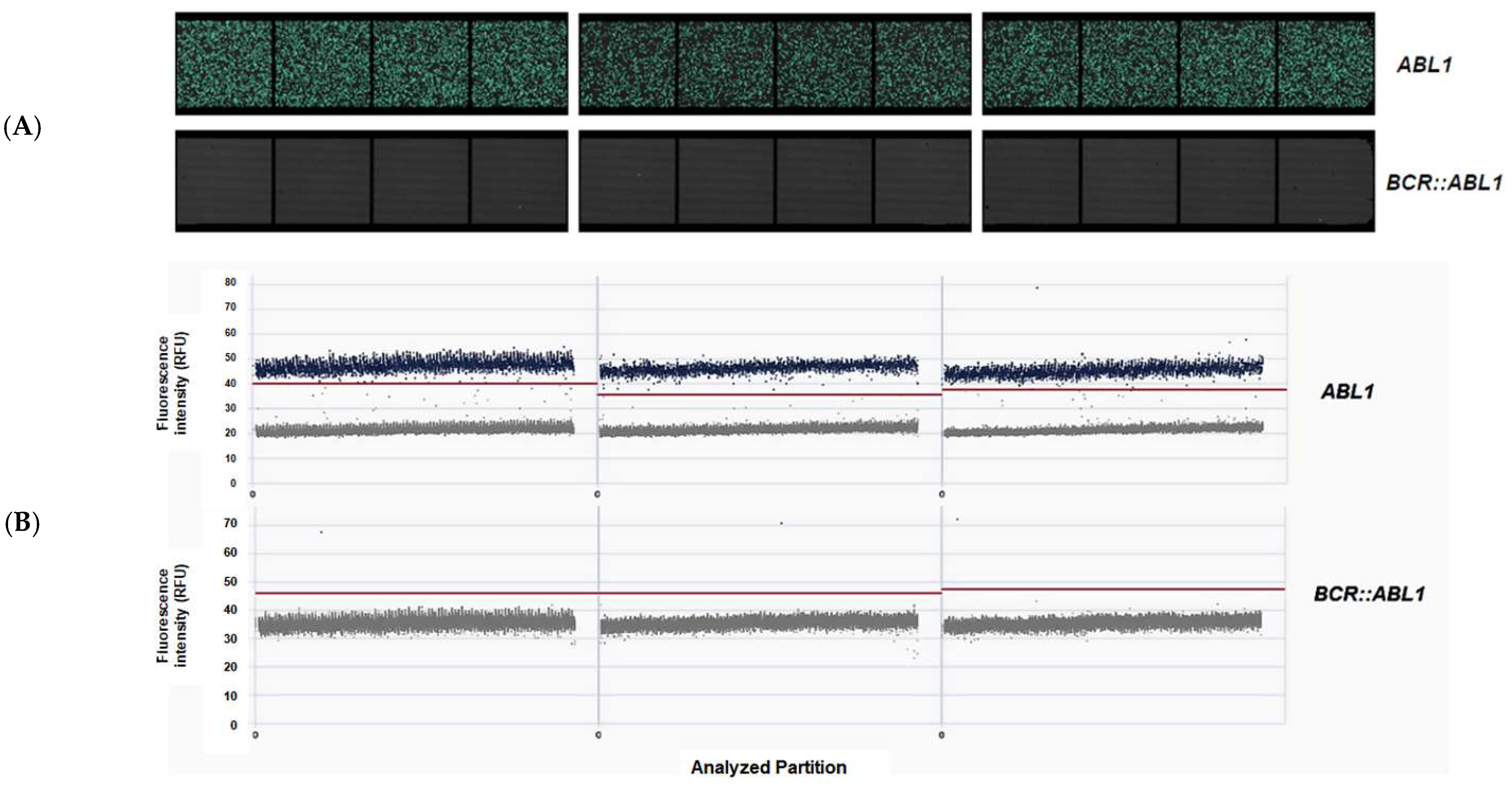
2.1. Characterization of Duplex One-Step RT-qPCR for BCR::ABL1 Quantification Assay
2.2. Amplification Efficiency and Repeatability
2.3. Cross-Validation, Specificity, and Sensitivity
2.4. Assay Validation and Certification
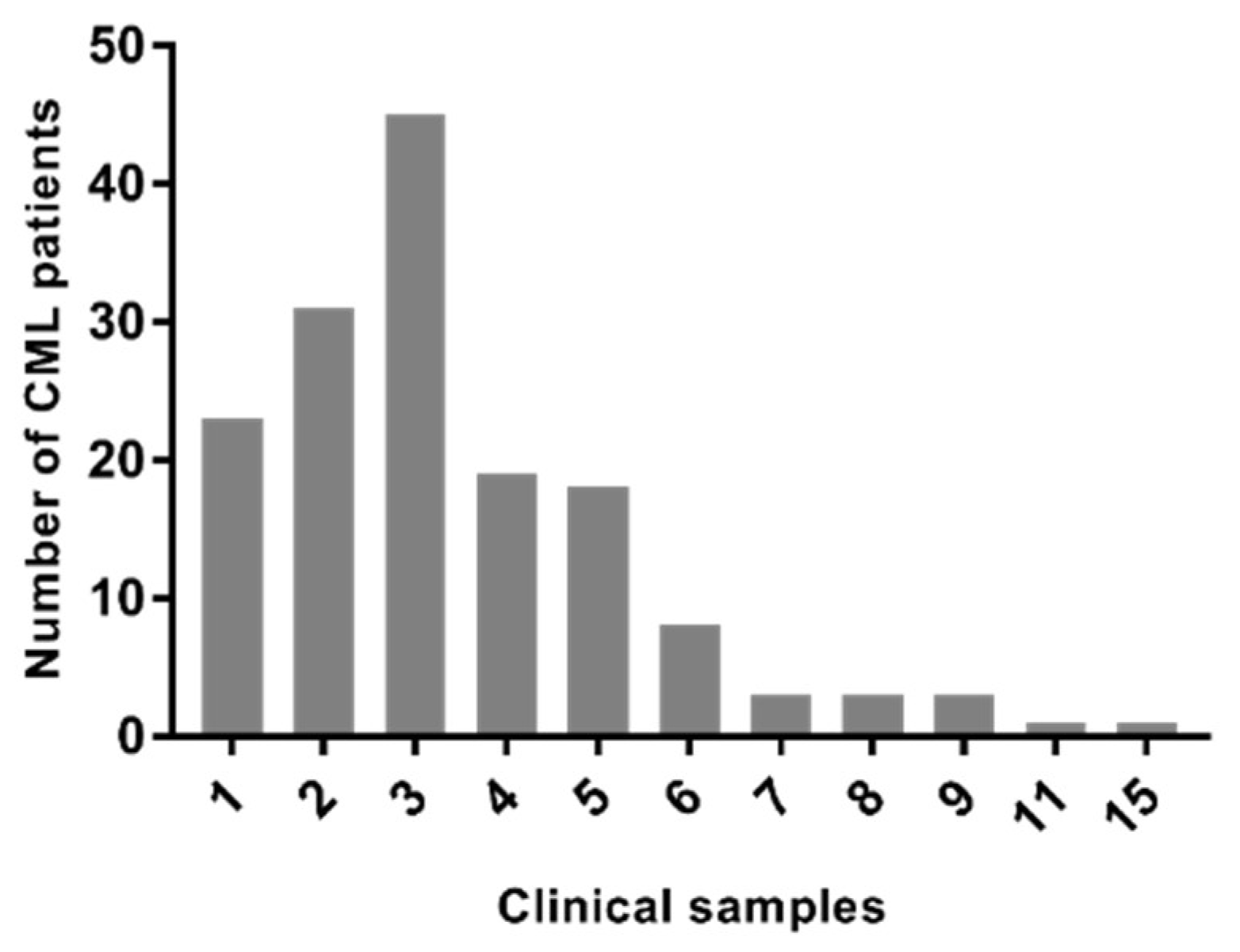
2.5. Epidemiological Data of CML Samples
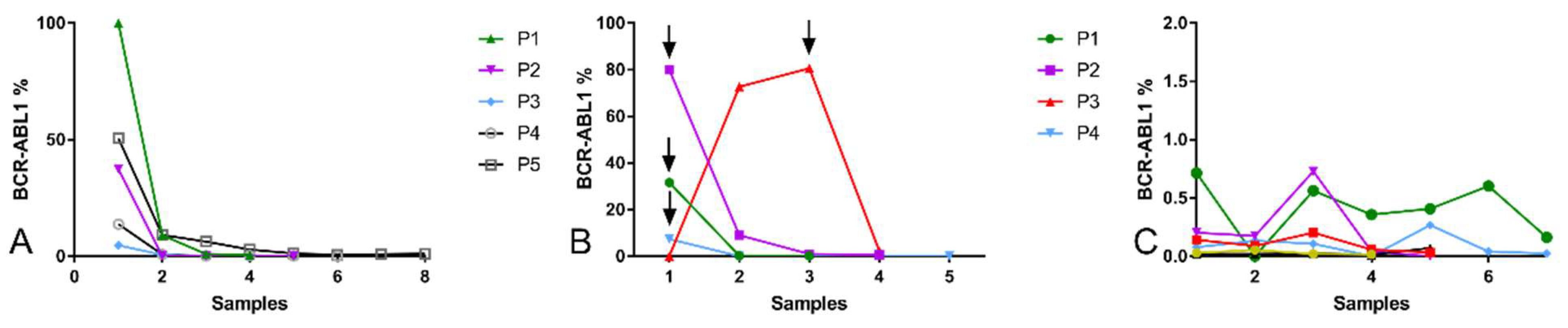
2.6. Response to Treatment Evaluated by Quantification of BCR::ABL1 Expression
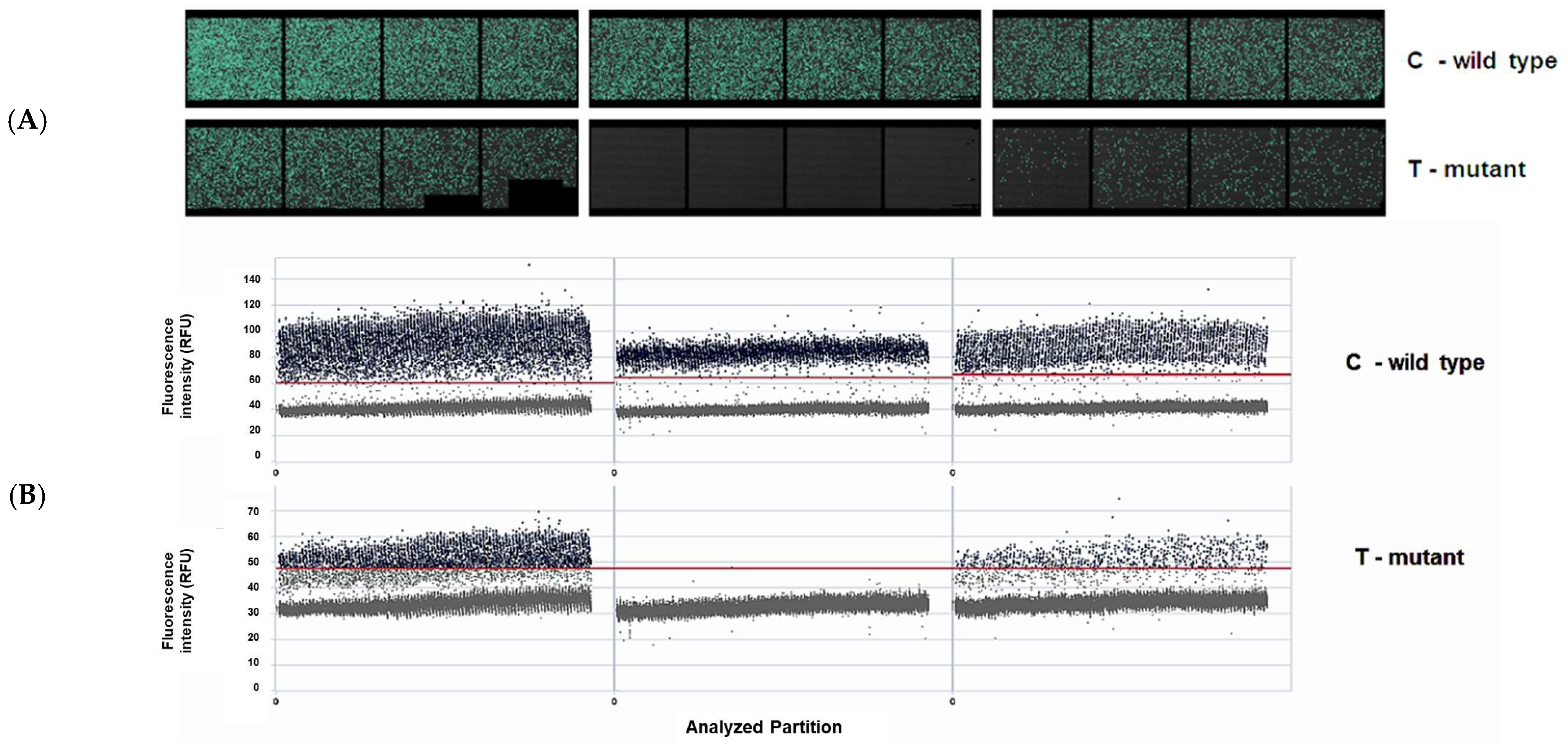
2.7. ABL1 Mutation
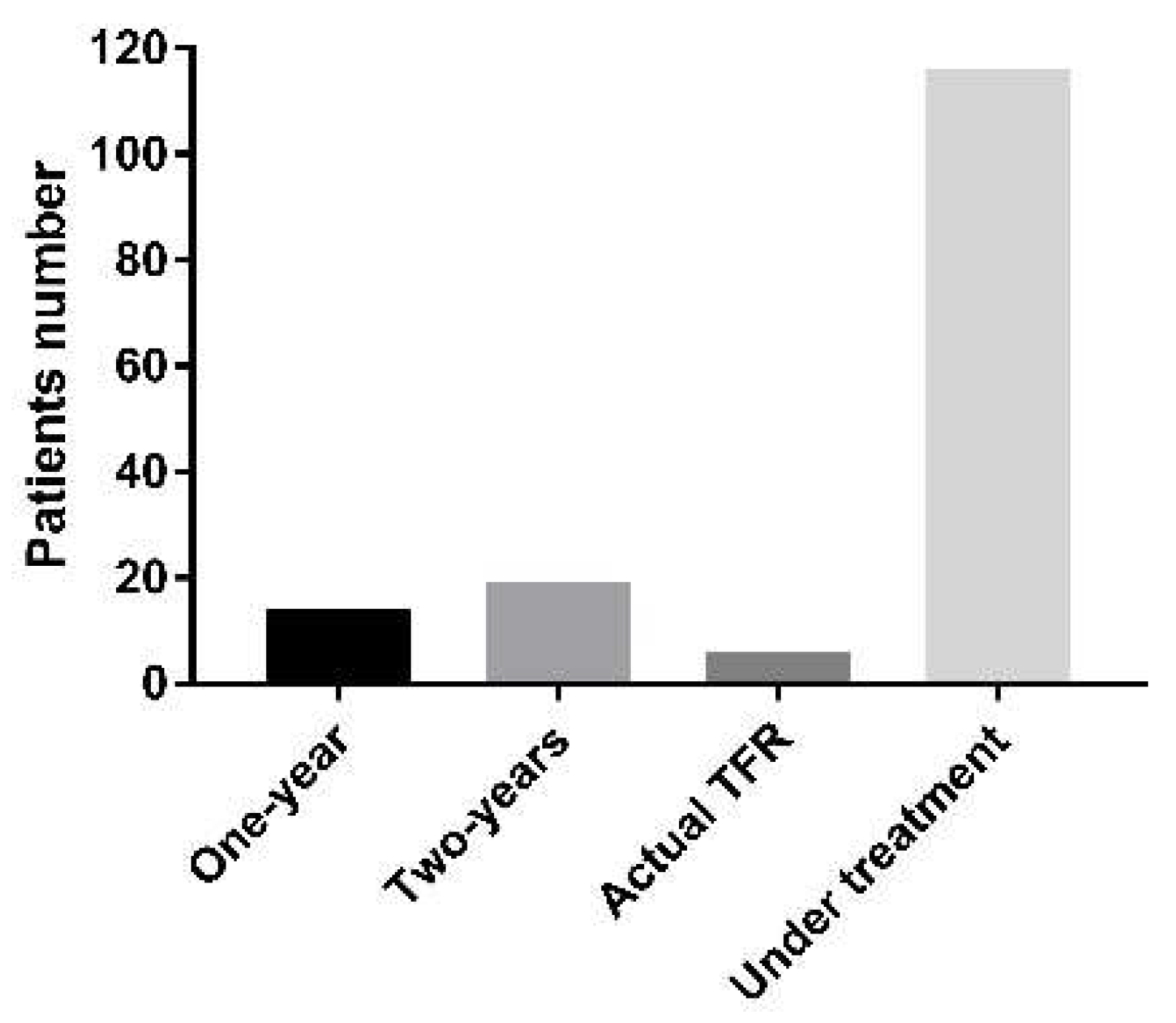
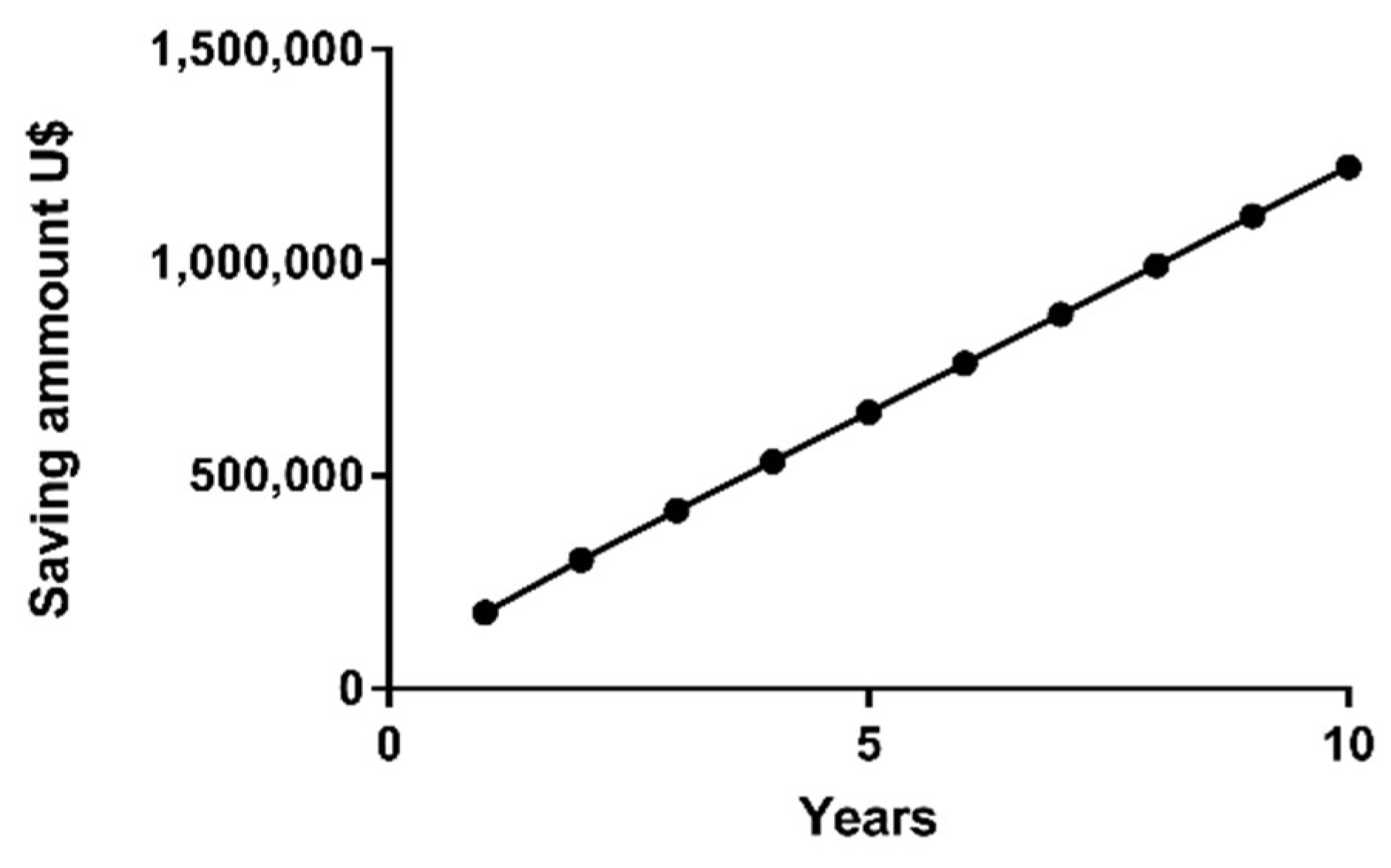
2.8. Cost-Effectiveness
3. Discussion
4. Methods
BCR::ABL Quantification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apperley, J.F. Chronic Myeloid Leukaemia. Lancet 2015, 385, 1447–1459. [Google Scholar] [CrossRef]
- Comissão Nacional de Incorporação de Tecnologias no SUS. Protocolo Clínico e Diretrizes Terapêuticas da Leucemia Mieloide Crônica do Adulto; Comissão Nacional de Incorporação de Tecnologias no SUS: Brasília, Brazil, 2020.
- Hu, Y.; Li, Q.; Hou, M.; Peng, J.; Yang, X.; Xu, S. Magnitude and Temporal Trend of the Chronic Myeloid Leukemia: On the Basis of the Global Burden of Disease Study 2019. JCO Glob. Oncol. 2021, 7, 1429–1441. [Google Scholar] [CrossRef]
- Bhamidipati, P.K.; Kantarjian, H.; Cortes, J.; Cornelison, A.M.; Jabbour, E. Management of Imatinib-Resistant Patients with Chronic Myeloid Leukemia. Adv. Hematol. 2013, 4, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Craddock, C.F. We Do Still Transplant CML, Don’t We? Hematology 2018, 2018, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbour, E.; Cortes, J.E.; Kantarjian, H.M. Imatinib Treatment for Chronic Myeloid Leukemia Suboptimal Response to or Failure of Imatinib Treatment for Chronic Myeloid Leukemia: What Is the Optimal Strategy? Mayo Clin. Proc. 2009, 84, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, I.; Winston, K. Chronic Myeloid Leukemia, from Pathophysiology to Treatment-Free Remission: A Narrative Literature Review. J. Blood Med. 2023, 14, 261–277. [Google Scholar] [CrossRef]
- Radich, J.P.; Dai, H.; Mao, M.; Oehler, V.; Schelter, J.; Druker, B.; Sawyers, C.; Shah, N.; Stock, W.; Willman, C.L.; et al. Gene Expression Changes Associated with Progression and Response in Chronic Myeloid Leukemia. Proc. Natl. Acad. Sci. USA 2006, 103, 2794–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deininger, M.W.; Shah, N.P.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; DeAngelo, D.J.; Gotlib, J.; Hobbs, G.; Maness, L.; et al. Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1385–1415. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Kantarjian, H.M.; Cortes, J.E. Diagnosis and Treatment of Chronic Myeloid Leukemia in 2015. Mayo Clin. Proc. 2015, 90, 1440–1454. [Google Scholar] [CrossRef] [Green Version]
- Branford, S.; Fletcher, L.; Cross, N.C.P.; Müller, M.C.; Hochhaus, A.; Kim, D.-W.; Radich, J.P.; Saglio, G.; Pane, F.; Kamel-Reid, S.; et al. Desirable Performance Characteristics for BCR-ABL Measurement on an International Reporting Scale to Allow Consistent Interpretation of Individual Patient Response and Comparison of Response Rates between Clinical Trials. Blood 2008, 112, 3330–3338. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.M.F.-C.S.; Romvari, R.M.F.-C.E. Interpreting Molecular Monitoring Results and International Standardization in Chronic Myeloid Leukemia. J. Adv. Pract. Oncol. 2012, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochhaus, A.; Larson, R.A.; Guilhot, F.; Radich, J.P.; Branford, S.; Hughes, T.P.; Baccarani, M.; Deininger, M.W.; Cervantes, F.; Fujihara, S.; et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 2017, 376, 917–927. [Google Scholar] [CrossRef]
- O’Brien, S.G.; Guilhot, F.; Larson, R.A.; Gathmann, I.; Baccarani, M.; Cervantes, F.; Cornelissen, J.J.; Fischer, T.; Hochhaus, A.; Hughes, T.; et al. Imatinib Compared with Interferon and Low-Dose Cytarabine for Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2003, 348, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Hehlmann, R.; Lauseker, M.; Saußele, S.; Pfirrmann, M.; Krause, S.; Kolb, H.J.; Neubauer, A.; Hossfeld, D.K.; Nerl, C.; Gratwohl, A.; et al. Assessment of Imatinib as First-Line Treatment of Chronic Myeloid Leukemia: 10-Year Survival Results of the Randomized CML Study IV and Impact of Non-CML Determinants. Leukemia 2017, 31, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, S.; Mologni, L.; Rostagno, R.; Piazza, R.; Magistroni, V.; Ceccon, M.; Viltadi, M.; Flynn, D.; Gambacorti-Passerini, C. Three Novel Patient-Derived BCR/ABL Mutants Show Different Sensitivity to Second and Third Generation Tyrosine Kinase Inhibitors. Am. J. Hematol. 2012, 87, E125–E128. [Google Scholar] [CrossRef]
- Breccia, M.; Alimena, G. Second-generation tyrosine kinase inhibitors (tki) as salvage therapy for resistant or intolerant patients to prior tkis. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014003. [Google Scholar] [CrossRef]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef] [Green Version]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-Term Benefits and Risks of Frontline Nilotinib vs Imatinib for Chronic Myeloid Leukemia in Chronic Phase: 5-Year Update of the Randomized ENESTnd Trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef] [Green Version]
- Cortes, J.E.; Gambacorti-Passerini, C.; Deininger, M.W.; Mauro, M.J.; Chuah, C.; Kim, D.-W.; Dyagil, I.; Glushko, N.; Milojkovic, D.; le Coutre, P.; et al. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results from the Randomized BFORE Trial. J. Clin. Oncol. 2018, 36, 231–237. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, D.L.; Pricl, S.; Kantarjian, H.; Cortes, J.; Quintás-Cardama, A. The Rise and Fall of Gatekeeper Mutations? The BCR-ABL1 T315I Paradigm. Cancer 2012, 118, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Brümmendorf, T.H.; Cortes, J.E.; Milojkovic, D.; Gambacorti-Passerini, C.; Clark, R.E.; le Coutre, P.; Garcia-Gutierrez, V.; Chuah, C.; Kota, V.; Lipton, J.H.; et al. Bosutinib versus Imatinib for Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia: Final Results from the BFORE Trial. Leukemia 2022, 36, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Shah, N.P.; Hochhaus, A.; Cortes, J.; Shah, S.; Ayala, M.; Moiraghi, B.; Shen, Z.; Mayer, J.; Pasquini, R.; et al. Dasatinib versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Hochhaus, A.; Saglio, G.; De Souza, C.; Flinn, I.W.; Stenke, L.; Goh, Y.-T.; Rosti, G.; Nakamae, H.; Gallagher, N.J.; et al. Nilotinib versus Imatinib for the Treatment of Patients with Newly Diagnosed Chronic Phase, Philadelphia Chromosome-Positive, Chronic Myeloid Leukaemia: 24-Month Minimum Follow-up of the Phase 3 Randomised ENESTnd Trial. Lancet Oncol. 2011, 12, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Shah, N.P.; Cortes, J.E.; Baccarani, M.; Agarwal, M.B.; Undurraga, M.S.; Wang, J.; Kassack Ipiña, J.J.; Kim, D.-W.; Ogura, M.; et al. Dasatinib or Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: 2-Year Follow-up from a Randomized Phase 3 Trial (DASISION). Blood 2012, 119, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef] [Green Version]
- Atallah, E.; Sweet, K. Treatment-Free Remission: The New Goal in CML Therapy. Curr. Hematol. Malig. Rep. 2021, 16, 433–439. [Google Scholar] [CrossRef]
- Pavlovsky, C.; Abello Polo, V.; Pagnano, K.; Varela, A.I.; Agudelo, C.; Bianchini, M.; Boquimpani, C.; Centrone, R.; Conchon, M.; Delgado, N.; et al. Treatment-Free Remission in Patients with Chronic Myeloid Leukemia: Recommendations of the LALNET Expert Panel. Blood Adv. 2021, 5, 4855–4863. [Google Scholar] [CrossRef]
- Sharf, G.; Marin, C.; Bradley, J.A.; Pemberton-Whiteley, Z.; Bombaci, F.; Christensen, R.I.O.; Gouimi, B.; Deekes, N.B.; Daban, M.; Geissler, J. Treatment-Free Remission in Chronic Myeloid Leukemia: The Patient Perspective and Areas of Unmet Needs. Leukemia 2020, 34, 2102–2112. [Google Scholar] [CrossRef]
- Kantarjian, H.; Rajkumar, S.V. Why Are Cancer Drugs So Expensive in the United States, and What Are the Solutions? Mayo Clin. Proc. 2015, 90, 500–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Experts in Chronic Myeloid Leukemia. The Price of Drugs for Chronic Myeloid Leukemia (CML) Is a Reflection of the Unsustainable Prices of Cancer Drugs: From the Perspective of a Large Group of CML Experts. Blood 2013, 121, 4439–4442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.M.; Hughes, T.P.; Larson, R.A.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Boquimpani, C.; Pasquini, R.; Clark, R.E.; et al. Long-Term Outcomes with Frontline Nilotinib versus Imatinib in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase: ENESTnd 10-Year Analysis. Leukemia 2021, 35, 440–453. [Google Scholar] [CrossRef]
- Dulucq, S.; Astrugue, C.; Etienne, G.; Mahon, F.; Benard, A. Risk of Molecular Recurrence after Tyrosine Kinase Inhibitor Discontinuation in Chronic Myeloid Leukaemia Patients: A Systematic Review of Literature with a Meta-analysis of Studies over the Last Ten Years. Br. J. Haematol. 2020, 189, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Kirschner, K.; Copland, M. Improving Outcomes in Chronic Myeloid Leukemia through Harnessing the Immunological Landscape. Leukemia 2021, 35, 1229–1242. [Google Scholar] [CrossRef]
- Kitamura, H.; Tabe, Y.; Ai, T.; Tsuchiya, K.; Yuri, M.; Misawa, S.; Horii, T.; Kawaguchi, A.; Ohsaka, A.; Kimura, S. A New Highly Sensitive Real-Time Quantitative-PCR Method for Detection of BCR-ABL1 to Monitor Minimal Residual Disease in Chronic Myeloid Leukemia after Discontinuation of Imatinib. PLoS ONE 2019, 14, e0207170. [Google Scholar] [CrossRef] [Green Version]
- Gabert, J.; Beillard, E.; van der Velden, V.H.J.; Bi, W.; Grimwade, D.; Pallisgaard, N.; Barbany, G.; Cazzaniga, G.; Cayuela, J.M.; Cavé, H.; et al. Standardization and Quality Control Studies of ‘Real-Time’ Quantitative Reverse Transcriptase Polymerase Chain Reaction of Fusion Gene Transcripts for Residual Disease Detection in Leukemia—A Europe Against Cancer Program. Leukemia 2003, 17, 2318–2357. [Google Scholar] [CrossRef] [Green Version]
- Gerrard, G.; Mudge, K.; Stewart, R.; Foskett, P.; Stevens, D.; Khorashad, J.S.; Szydlo, R.; Rezvani, K.; Marin, D.; Reid, A.; et al. Duplex Quantitative PCR for Molecular Monitoring of BCR-ABL1-Associated Hematological Malignancies. Am. J. Hematol. 2011, 86, 313–315. [Google Scholar] [CrossRef]
- Ruiz, M.S.; Sánchez, M.B.; Vera Contreras, Y.M.; Agrielo, E.; Alonso, M.; Altuna, M.E.; Anchordoqui, M.S.; Asinari, M.; Bonetto, M.E.; Camargo, M.; et al. Programme for Harmonization to the International Scale in Latin America for BCR-ABL1 Quantification in CML Patients: Findings and Recommendations. Clin. Chem. Lab. Med. 2020, 58, 2025–2035. [Google Scholar] [CrossRef]
- Branford, S. Why Is It Critical to Achieve a Deep Molecular Response in Chronic Myeloid Leukemia? Haematologica 2020, 105, 2730–2737. [Google Scholar] [CrossRef]
- Mahon, F.-X.; Etienne, G. Deep Molecular Response in Chronic Myeloid Leukemia: The New Goal of Therapy? Clin. Cancer Res. 2014, 20, 310–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boquimpani, C.M.; Abdo, A.N.R.; Martins, D.P.; de Abreu Lima, L.B.; Torriani, M.S.; Bendit, I. Inclusion of Molecular Monitoring (BCR-ABL1) in the Treatment of Chronic Myeloid Leukemia in the Brazilian Public Health System (SUS): An Urgent Need for Treatment Management. Hematol. Transfus. Cell Ther. 2021, 43, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.P.; Tomaz, J.P.; Lorand-Metze, I.; de Souza, C.A.; Vigorito, A.C.; Delamain, M.T.; Bendit, I.; Pereira, N.F.; Pagnano, K.B.B. Monitoring of BCR-ABL Levels in Chronic Myeloid Leukemia Patients Treated with Imatinib in the Chronic Phase. Rev. Bras. Hematol. Hemoter. 2011, 33, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Möbius, S.; Schenk, T.; Himsel, D.; Maier, J.; Franke, G.-N.; Saussele, S.; Pott, C.; Andrikovics, H.; Meggyesi, N.; Machova-Polakova, K.; et al. Results of the European Survey on the Assessment of Deep Molecular Response in Chronic Phase CML Patients during Tyrosine Kinase Inhibitor Therapy (EUREKA Registry). J. Cancer Res. Clin. Oncol. 2019, 145, 1645–1650. [Google Scholar] [CrossRef] [Green Version]
- Greiner, G.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Taghizadeh, H.; Mustafa, S.G.K.; Mitterbauer-Hohendanner, G.; Esterbauer, H.; Mannhalter, C.; Sperr, W.R.; et al. Comparison of BCR-ABL1 Quantification in Peripheral Blood and Bone Marrow Using an International Scale-Standardized Assay for Assessment of Deep Molecular Response in Chronic Myeloid Leukemia. Clin. Chem. Lab. Med. 2020, 58, 1214–1222. [Google Scholar] [CrossRef]
- Claudiani, S.; Gatenby, A.; Szydlo, R.; Nesr, G.; Abulafia, A.S.; Palanicawandar, R.; Nteliopoulos, G.; Khorashad, J.; Foroni, L.; Apperley, J.F.; et al. MR4 Sustained for 12 Months Is Associated with Stable Deep Molecular Responses in Chronic Myeloid Leukemia. Haematologica 2019, 104, 2206–2214. [Google Scholar] [CrossRef]
- Molica, M.; Breccia, M.; Colafigli, G.; Massaro, F.; Quattrocchi, L.; Mancini, M.; Diverio, D.; Latagliata, R.; Foà, R. Long-Term Impact of Molecular Response Fluctuations in Chronic Myeloid Leukaemia Patients Treated with Imatinib. Br. J. Haematol. 2018, 181, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Soverini, S.; Hochhaus, A.; Nicolini, F.E.; Gruber, F.; Lange, T.; Saglio, G.; Pane, F.; Müller, M.C.; Ernst, T.; Rosti, G.; et al. BCR-ABL Kinase Domain Mutation Analysis in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors: Recommendations from an Expert Panel on Behalf of European LeukemiaNet. Blood 2011, 118, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.H.; Baba, A.A.; Azlan, H.; Rosline, H.; Sim, G.A.; Padmini, M.; Fadilah, S.A.W.; Ankathil, R. BCR-ABL Kinase Domain Mutations, Including 2 Novel Mutations in Imatinib Resistant Malaysian Chronic Myeloid Leukemia Patients—Frequency and Clinical Outcome. Leuk. Res. 2014, 38, 454–459. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Xu, X.; Yi, S.; Meng, L. Mutations in the BCR-ABL1 Kinase Domain in Patients with Chronic Myeloid Leukaemia Treated with TKIs or at Diagnosis. Oncol. Lett. 2020, 20, 1071–1076. [Google Scholar] [CrossRef]
- Costa, H.Z.; Pereira, N.F.; Kaminski, L.; Pasquini, R.; Funke, V.A.M.; Mion, A.L.V. Mutations in the Breakpoint Cluster Region-Abelson Murine Leukemia 1 Gene in Brazilian Patients with Chronic Myeloid Leukemia. Hematol. Transfus. Cell Ther. 2018, 40, 363–367. [Google Scholar] [CrossRef]
- Pinto, L.C.; de Oliveira Sales, L.; de Brito Azevedo, T.C.; Moreira-Nunes, C.A.; Lemos, J.A.R. Análise de Mutações Do Domínio BCR-ABL Quinase Em Pacientes Com Leucemia Mielóide Crônica Refratários Ao Tratamento Com Mesilato de Imatinibe. Rev. Ciênc. Saúde 2020, 10, 77–84. [Google Scholar] [CrossRef]
- Lyczek, A.; Berger, B.-T.; Rangwala, A.M.; Paung, Y.; Tom, J.; Philipose, H.; Guo, J.; Albanese, S.K.; Robers, M.B.; Knapp, S.; et al. Mutation in Abl Kinase with Altered Drug-Binding Kinetics Indicates a Novel Mechanism of Imatinib Resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2111451118. [Google Scholar] [CrossRef]
- Mahon, F.-X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of Imatinib in Patients with Chronic Myeloid Leukaemia Who Have Maintained Complete Molecular Remission for at Least 2 Years: The Prospective, Multicentre Stop Imatinib (STIM) Trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and Efficacy of Imatinib Cessation for CML Patients with Stable Undetectable Minimal Residual Disease: Results from the TWISTER Study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.M.; Pagani, I.S.; Shanmuganathan, N.; Kok, C.H.; Seymour, J.F.; Mills, A.K.; Filshie, R.J.; Arthur, C.K.; Dang, P.; Saunders, V.A.; et al. Long-Term Treatment-Free Remission of Chronic Myeloid Leukemia with Falling Levels of Residual Leukemic Cells. Leukemia 2018, 32, 2572–2579. [Google Scholar] [CrossRef]
- Goni, D.; Jain, A.; Singh, C.; Jindal, N.; Nampoothiri, R.; Jandial, A.; Lad, D.; Khadwal, A.; Prakash, G.; Naseem, S.; et al. Feasibility of Treatment-Free Remission with Generic Imatinib: Results of Generic Imatinib-Free Trial-in-Chronic Myeloid Leukaemia-Chronic Phase Study. Indian J. Med. Res. 2023, 157, 87–89. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Guo, J.; Zou, J.; He, W.; Han, D.; Cheng, F.; Zhang, Y.; Li, W. Successful Treatment Discontinuation in CML Patients with Full-Dose and Low-Dose TKI: Results from Real-World Practice. Front. Pharm. 2023, 14, 1101743. [Google Scholar] [CrossRef]
- Di Giusto, S.; Toffoletti, E.; Bonifacio, M.; Binotto, G.; Miggiano, M.C.; Calistri, E.; Stulle, M.; Ermacora, A.; Stella, R.; Scaffidi, L.; et al. BCR::ABL1 Levels at First Month after TKI Discontinuation Predict Subsequent Maintenance of Treatment-free Remission: A Study from the “GRUPPO TRIVENETO LMC”. Cancer Med. 2023, 12, 3180–3184. [Google Scholar] [CrossRef]
- Kim, D.D.H.; Kim, T.S.; Atenafu, E.G.; Novitzky Basso, I.; Forrest, D.; Bence-Bruckler, I.; Savoie, L.; Busque, L.; Keating, M.; Delage, R.; et al. BCR–ABL1 Transcript Doubling Time as a Predictor for Treatment-free Remission Failure after Imatinib Discontinuation in Chronic Myeloid Leukaemia in Chronic Phase. Br. J. Haematol. 2022, 196, 136–145. [Google Scholar] [CrossRef]
- Kockerols, C.C.B.; Valk, P.J.M.; Levin, M.-D.; Pallisgaard, N.; Cornelissen, J.J.; Westerweel, P.E. Digital PCR for BCR-ABL1 Quantification in CML: Current Applications in Clinical Practice. Hemasphere 2020, 4, e496. [Google Scholar] [CrossRef]
- Bochicchio, M.T.; Petiti, J.; Berchialla, P.; Izzo, B.; Giugliano, E.; Ottaviani, E.; Errichiello, S.; Rege-Cambrin, G.; Venturi, C.; Luciano, L.; et al. Droplet Digital PCR for BCR–ABL1 Monitoring in Diagnostic Routine: Ready to Start? Cancers 2021, 13, 5470. [Google Scholar] [CrossRef] [PubMed]
- Colafigli, G.; Scalzulli, E.; Di Prima, A.; Pepe, S.; Loglisci, M.G.; Diverio, D.; Martelli, M.; Foà, R.; Breccia, M. Digital Droplet PCR as a Predictive Tool for Successful Discontinuation Outcome in Chronic Myeloid Leukemia: Is It Time to Introduce It in the Clinical Practice? Crit. Rev. Oncol. Hematol. 2021, 157, 103163. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Hur, M.; Yoon, S.; Hwang, K.; Lim, H.-S.; Kim, H.; Moon, H.-W.; Yun, Y.-M. Performance Evaluation of the QXDx BCR-ABL %IS Droplet Digital PCR Assay. Ann. Lab. Med. 2020, 40, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Shelton, D.N.; Bhagavatula, P.; Sepulveda, N.; Beppu, L.; Gandhi, S.; Qin, D.; Hauenstein, S.; Radich, J. Performance Characteristics of the First Food and Drug Administration (FDA)-Cleared Digital Droplet PCR (DdPCR) Assay for BCR::ABL1 Monitoring in Chronic Myelogenous Leukemia. PLoS ONE 2022, 17, e0265278. [Google Scholar] [CrossRef] [PubMed]
| BCR::ABL1 | ABL1 | |||
|---|---|---|---|---|
| RNA (ng) | Ct (SD) | rRSD% | Ct (SD) | rRSD% |
| 200 | 17.84 (0.207) | 1.16 | 20.47 (0.771) | 3.766 |
| 100 | 18.92 (0.063) | 0.333 | 21.62 (0.378) | 1.748 |
| 50 | 19.94 (0.116) | 0.582 | 22.61 (0.484) | 2.141 |
| 25 | 21.05 (0.136) | 0.646 | 23.81 (0.505) | 2.121 |
| 12.5 | 22.16 (0.307) | 1.385 | 24.87 (0.626) | 2.517 |
| Sample | BCR::ABL1 % | |
|---|---|---|
| DualQuant | Reference | |
| 1 | ND | Below LoQ |
| 2 | 0.0285 | 0.0064 |
| 3 | 0.0642 | 0.0112 |
| 4 | 0.0404 | 0.0155 |
| 5 | 0.0199 | 0.0158 |
| 6 | 0.0189 | 0.017 |
| 7 | 0.0607 | 0.0284 |
| 8 | 0.0659 | 0.0313 |
| 9 | 0.1409 | 0.0375 |
| 10 | 0.1166 | 0.0593 |
| 11 | 0.074 | 0.108 |
| 12 | 0.4565 | 0.169 |
| 13 | 0.7138 | 0.2744 |
| 14 | 1.3648 | 0.7119 |
| 15 | 8.4595 | 9.6036 |
| 16 | Below LoQ | Below LoQ |
| 17 | ND | Below LoQ |
| 18 | Below LoQ | Below LoQ |
| 19 | Below LoQ | ND |
| 20 | ND | ND |
| 21 | ND | ND |
| 22 | 0.0428 | ND |
| 23 | 0.7309 | ND |
| 24 | ND | ND |
| DualQuant | Mobius | |||||
|---|---|---|---|---|---|---|
| Samples | BCR::ABL1 Ct | ABL1 Ct | % | BCR::ABL1 Ct | ABL1 Ct | % |
| 1 | 28.79 | 21.87 | 0.074 | 31.52 | 23.11 | 0.085 |
| 2 | 31.44 | 24.14 | 0.066 | 34.69 | 24.83 | Below LoQ |
| 3 | 32.37 | 24.28 | 0.040 | 35.28 | 24.98 | Below LoQ |
| 4 | 29.27 | 21.65 | 0.061 | 32.08 | 22.6 | 0.041 |
| 5 | 32.1 | 23.14 | 0.019 | 35.01 | 24.15 | Below LoQ |
| 6 | ND | 25.75 | NA | 37.97 | 26.59 | Below LoQ |
| 7 | 24.08 | 23.8 | 8.459 | 26.35 | 24.83 | 9.377 |
| 8 | ND | 21.77 | NA | ND | 22.67 | NA |
| 9 | ND | 23.8 | NA | ND | 24.88 | NA |
| 10 | 28.4 | 22.64 | 0.457 | 31.15 | 24.12 | 0.218 |
| 11 | 34.78 | 26.03 | Below LoQ | 36.63 | 24.13 | Below LoQ |
| 12 | 33.96 | 29.75 | Below LoQ | ND | 37.98 | NA |
| 13 | 30 | 25.28 | 0.714 | 32.55 | 26.05 | 0.309 |
| 14 | 28.16 | 24.46 | 1.365 | 29.17 | 24.56 | 1.136 |
| 15 | 31.39 | 23.74 | 0.141 | 32.91 | 24.07 | 0.063 |
| 16 | 28.84 | 22.66 | 0.117 | 32.35 | 23.64 | 0.069 |
| 17 | 31.27 | 22.75 | 0.029 | 34.87 | 23.85 | Below LoQ |
| 18 | ND | 23.35 | NA | ND | 24.37 | NA |
| 19 | 24.96 | 25.05 | 5.890 | 26.82 | 24.23 | 4.531 |
| Year | Unique Patients (n) | %IS > 1 n (%) | CCR (IS ≤ 1%) n (%) | MMR 3 (IS ≤ 0.1%) n (%) | MMR 4 (IS ≤ 0.01%) n (%) | MMR 4.5 (IS ≤ 0.0032%) n (%) | MMR 5 (IS ≤ 0.001%) or Undetectable n (%) | DMR (≤MMR 4.0) n (%) |
|---|---|---|---|---|---|---|---|---|
| 2020 | 60 | 11 (18.3%) | 7 (11.7%) | 16 (26.7%) | 5 (6.7%) | 0 (0%) | 22 (36.6%) | 26 (43.3%) |
| 2021 | 124 | 17 (13.7%) | 16 (12.9%) | 30 (24.2%) | 4 (3.2%) | 0 (0%) | 57 (46%) | 61 (49.2%) |
| 2022 | 126 | 14 (11.1%) | 10 (7.9%) | 33 (26.2%) | 9 (7.1%) | 2 (1.6%) | 58 (46.1%) | 69 (54.7%) |
| 2023 | 23 | 5 (21.7%) | 3 (13%) | 11 (47.8%) | 0 (0%) | 0 (0%) | 4 (17.4%) | 4 (17.4%) |
| Total (n) | 334 | 47 | 36 | 90 | 18 | 2 | 141 | 161 |
| 2020–2023 | IS > 1% | CCR (<1%) | MMR 3 (<0.1%) | MMR 4.0 (<0.01%) | MMR4.5 (<0.0032%) | MMR 5.0 (<0.001%) or ND | DMR (≤MMR 4.0) |
|---|---|---|---|---|---|---|---|
| n | 17 | 17 | 45 | 13 | 1 | 85 | 99 |
| % | 9.5% | 9.5% | 25.3% | 7.3% | 0.6% | 47.8% | 55.6% |
| BCR::ABL1 | ||
|---|---|---|
| dPCR Copies/Reaction | qPCR %IS | |
| Patient 1 * | 0.188 | 0 |
| Patient 2 | 0.196 | 0.009 |
| Patient 3 | 0 | 0 |
| Patient 4 | 0 | 0 |
| Patient 5 | 0 | 0 |
| Patient 6 * | 0.2 | 0 |
| Patient 7 | 0 | 0 |
| Patient 8 * | 0.208 | 0 |
| Patient 9 | 0.204 | 0.065 |
| Patient 10 * | 0.2 | 0 |
| Patient 11 * | 0.208 | 0 |
| Patient 12 * | 0.204 | 0 |
| Patient 13 | 0 | 0 |
| Patient 14 | 0 | 0 |
| Patient 15 * | 0.204 | 0 |
| Patient 16 # | 0 | 0.008 |
| Patient 17 # | 0 | 0.005 |
| Patient 18 * | 0.408 | 0 |
| Patient 19 * | 0.204 | 0 |
| Patient 20 | 0.208 | 0.004 |
| Patient 21 | 0.2 | 0 |
| Patient 22 | 0 | 0 |
| T315I | |||||
|---|---|---|---|---|---|
| Patient ID | Sample ID | qPCR | dPCR | %BCR::ABL1 (IS) | Treatment |
| Patient 1 | Sample 1 | WT | WT | 0.054 | Nilotinib |
| Sample 2 | T315I | T315I | 100 | ||
| Patient 2 | Sample 1 | WT | WT | 22.92 | BMT |
| Sample 2 | WT | T315I | NA | ||
| Patient 3 | Sample 1 | WT | T315I | 0.281 | BMT |
| Patient 4 | Sample 1 | WT | T315I | 0.315 | Dasatinib |
| Patient 5 | Sample 1 | WT | T315I | 8.9 | Dasatinib |
| Patient 6 | Sample 1 | WT | T315I | 1,38 | Imatinib |
| E255K | |||||
| Patient ID | Sample ID | qPCR | dPCR | %BCR::ABL1 (IS) | Treatment |
| Patient 7 | Sample 1 | WT | WT | 61.12 | Imatinib |
| Sample 2 | WT | E255K | 72.7 | ||
| Sample 3 | E255K | E255K | 80.6 | ||
| Sample 4 | WT | WT | 8.19 | Dasatinib | |
| V359F | |||||
| Patient ID | Sample ID | qPCR | dPCR | %BCR::ABL1 (IS) | treatment |
| Patient 8 | Sample 1 | WT | WT | 47.3 | None |
| Sample 2 | V359F | V359F | 43.2 | Nilotinib |
| Group | TFR Patients | TFR Success (65%) (a) | Monthly Cost (USD) | Annual Cost/Patient (b) | Annual Cost (USD) (a,b) |
|---|---|---|---|---|---|
| 1 (immediately) | 19 | 12 | 1200 | 14,400 | 172,800 |
| 2 (In one year) | 14 | 9 | 129,600 | ||
| In two years | 33 | 21 | 302,400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, A.M.; Wosniaki, D.K.; Sanchuki, H.B.S.; Munhoz, E.C.; Nardin, J.M.; Soares, G.S.; Espinace, D.C.; de Holanda Farias, J.S.; Veroneze, B.; Becker, L.F.; et al. Molecular BCR::ABL1 Quantification and ABL1 Mutation Detection as Essential Tools for the Clinical Management of Chronic Myeloid Leukemia Patients: Results from a Brazilian Single-Center Study. Int. J. Mol. Sci. 2023, 24, 10118. https://doi.org/10.3390/ijms241210118
Marin AM, Wosniaki DK, Sanchuki HBS, Munhoz EC, Nardin JM, Soares GS, Espinace DC, de Holanda Farias JS, Veroneze B, Becker LF, et al. Molecular BCR::ABL1 Quantification and ABL1 Mutation Detection as Essential Tools for the Clinical Management of Chronic Myeloid Leukemia Patients: Results from a Brazilian Single-Center Study. International Journal of Molecular Sciences. 2023; 24(12):10118. https://doi.org/10.3390/ijms241210118
Chicago/Turabian StyleMarin, Anelis Maria, Denise Kusma Wosniaki, Heloisa Bruna Soligo Sanchuki, Eduardo Cilião Munhoz, Jeanine Marie Nardin, Gabriela Silva Soares, Dhienifer Caroline Espinace, João Samuel de Holanda Farias, Bruna Veroneze, Luiz Felipe Becker, and et al. 2023. "Molecular BCR::ABL1 Quantification and ABL1 Mutation Detection as Essential Tools for the Clinical Management of Chronic Myeloid Leukemia Patients: Results from a Brazilian Single-Center Study" International Journal of Molecular Sciences 24, no. 12: 10118. https://doi.org/10.3390/ijms241210118






