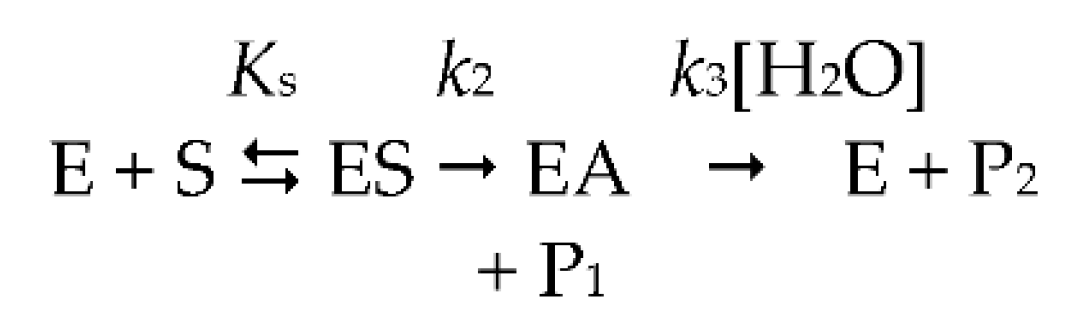Abstract
Cholinesterases (ChEs) display a non-michaelian behavior with positively charged substrates. In the steady-state rate equation, the b factor describes this behavior: if b > 1 there is substrate activation, if b < 1 there is substrate inhibition. The mechanistic significance of the b factor was investigated to determine whether this behavior depends on acylation, deacylation or on both steps. Kinetics of human acetyl- (AChE) and butyryl-cholinesterase (BChE) were performed under steady-state conditions and using a time-course of complete substrate hydrolysis. For the hydrolysis of short acyl(thio)esters, where acylation and deacylation are partly rate-limiting, steady-state kinetic analysis could not decide which step determines b. However, the study of the hydrolysis of an arylacylamide, 3-(acetamido)-N,N,N-trimethylanilinium (ATMA), where acetylation is rate-limiting, showed that b depends on the acylation step. The magnitude of b and opposite b values between AChE and BChE for the hydrolysis of acetyl(thio)- versus benzoyl-(thio) esters, then indicated that the productive adjustment of substrates in the active center at high concentration depends on motions of both the Ω and the acyl-binding loops. Benzoylcholine was shown to be a poor substrate of AChE, and steady-state kinetics showed a sudden inhibition at high concentration, likely due to the non-dissociation of hydrolysis products. The poor catalytic hydrolysis of this bulky ester by AChE illustrates the importance of the fine adjustment of substrate acyl moiety in the acyl-binding pocket. Molecular modeling and QM/MM simulations should definitively provide evidence for this statement.
1. Introduction
Cholinesterases (ChEs) are widely distributed in living organisms [1]. In animals, the main function of acetylcholinesterase (AChE, EC. 3.1.1.7) is to terminate the action of the neutrotransmitter acetylcholine (ACh) in the central nervous system (CNS), ganglions and at neuromuscular junctions (NMJ). In addition, AChE has non-cholinergic functions in cellular development [2]. The physiological functions of butyrylcholinesterase (BChE, EC. 3.1.1.8) are still debated. BChE may serve as a surrogate for AChE or as its backup under extreme physiological conditions, but likely also plays a constitutive role in the brain under normal conditions [3]. Other possible functions have been proposed. In particular, BChE deacylates ghrelin, the “hunger” hormone, an activity that may be relevant in fatty acid metabolism [4]. It was known for a long time that BChE hydrolyzes long-chain acylcholines, and recently it was shown that this property could modulate inflammatory processes such as the “cytokine storm” observed in severe forms of COVID-19 [5]. Furthermore, BChE displays a wide specificity towards esters. This includes ester-containing drugs such as aspirin [6], the myorelaxant succinylcholine [7], and poisonous carboxyl-esters like heroin and cocaine [8]. Both ChEs also display a promiscuous activity in hydrolyzing arylacylamides [9,10].
Both enzymes are irreversibly inhibited by poisonous organophosphates and carbamates, causing major cholinergic syndrome [11]. Thus, endogenous BChE is of toxicological importance in reacting with poisonous carbamyl-esters and phosphoryl-esters [12,13]. Endogenous BChE exerts protection of AChE in the cholinergic system in metabolizing or scavenging esters of natural or artificial origin. In particular, it was established that the administration of highly purified human BChE is an effective stoichiometric bioscavenger against the acute toxicity of organophosphate pesticides and nerve agents [12,14,15]. The inhibition (reversible and/or irreversible) of both ChEs has also been used in the treatment of Alzheimer’s disease [16]. The current and potential therapeutic uses of BChE in detoxification, in the treatment of obesity and in Alzheimer’s disease have recently been reviewed [17].
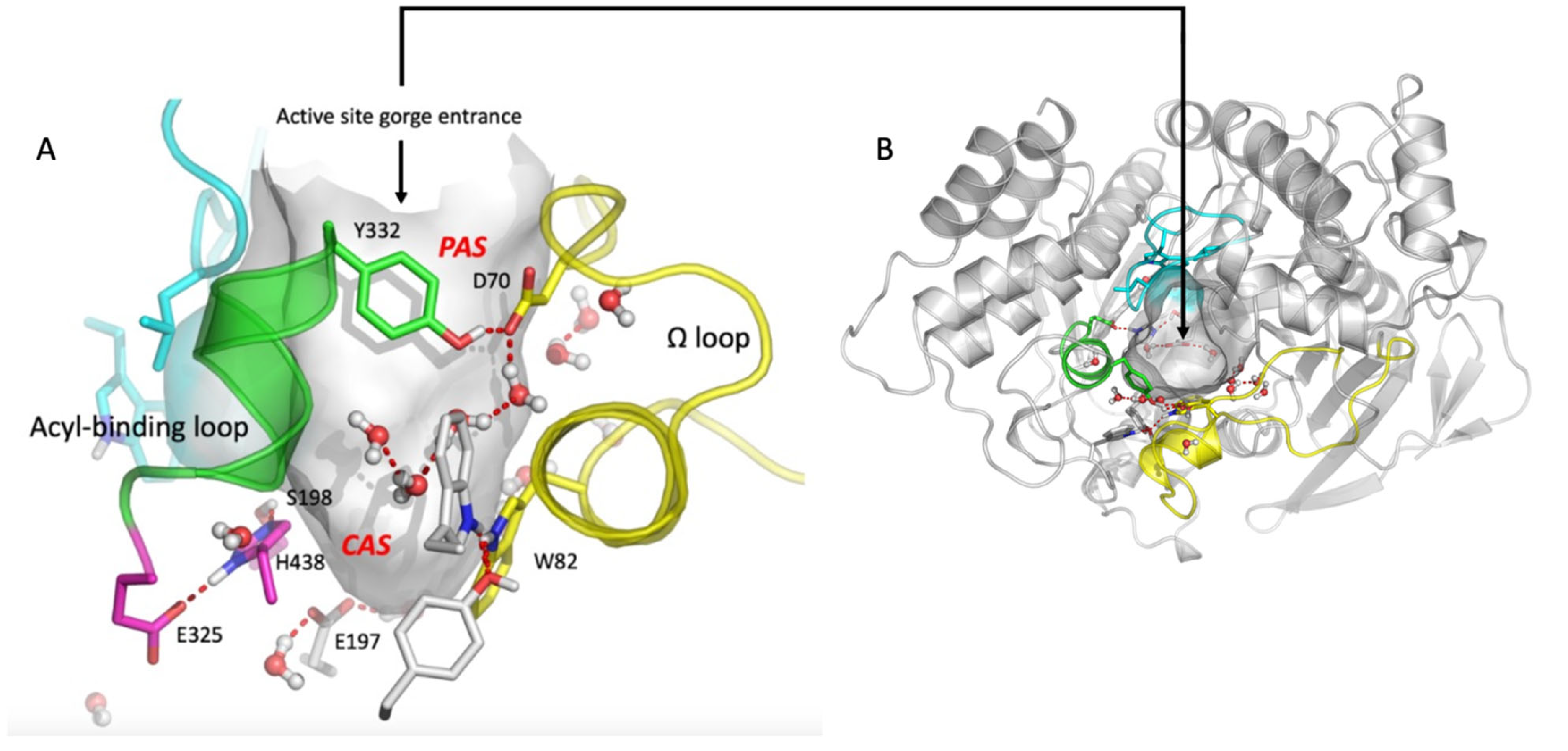
The 3D structure of human ChEs recombinant monomer was determined by X ray diffraction analysis (PDB 1PO1 for BChE and 4EY4 for AChE) [18,19] and that of naturally-occurring human plasma BChE tetramer by cryo-electron microscopy [20,21]. The 3D structure of membrane-anchored oligomeric forms of both ChEs has not yet been solved [22]. Monomers of both enzymes are single domain α/β fold proteins. In tetrameric structures, monomers are equivalent and show no cooperativity. In each monomer, the catalytic active site (CAS) is located at the bottom of a deep narrow gorge of 20 Å, just in the middle of the enzyme core (Figure 1). The main component of the CAS is the catalytic triad Ser-His-Asp (S198-H438-E325 in human BChE) surrounded by an oxyanion hole, plus a glutamate (D) adjacent to the catalytic serine (S). The protonation of this glutamate plays a key role in stabilizing the catalytic triad [23]. On both sides of the catalytic serine there are two binding sites: a π-cation binding site and an acyl-binding site. At the entrance of the active site gorge a peripheral anionic site (PAS) is located, which is the recognition binding site of substrates and ligands [18,19,24]. These molecules bind transiently to the PAS, and then slide down the gorge to the CAS. PAS and CAS are interconnected through the acyl-binding loop and the Ω loop (Figure 1). In AChE (D74 and Y334), as well as in BChE (D70 and Y332), part of these two loops are H-bonded at the rim of the active site gorge. The Ω loop interconnects the main residue of the PAS (D70 in human BChE, D74 in AChE) and the tryptophan (W) of the CAS (W82 in human BChE, W86 in AChE). This tryptophan establishes π-cation interactions with positively charged substrates and ligands. Therefore, any conformational change in the Ω loop induces a change in the binding of substrates and inhibitors in the CAS. The acyl-binding pocket loop interconnects the PAS with the acyl-binding pocket (ABP). The importance of this loop for the productive binding of substrate acyl moiety has long been recognized [25,26,27]. Recent crystallographic and small angle X-ray scattering (SAXS) data on AChE conjugates confirmed the conformational plasticity of this loop for the adaptative binding of various ligands, substrates and covalent inhibitors [28,29]. As a result, ChEs display a complex catalytic behavior particularly with positively charged substrates. The events determining this behavior are not completely understood. With neutral esters, the hydrolysis of substrates obeys the simple two-step Michaelis–Menten model. After the formation of the reversible michaelian complex ES, the active site serine is acylated (k2), and subsequently deacylated (k3), by the nucleophilic attack of water, acting as a co-substrate (Scheme 1).
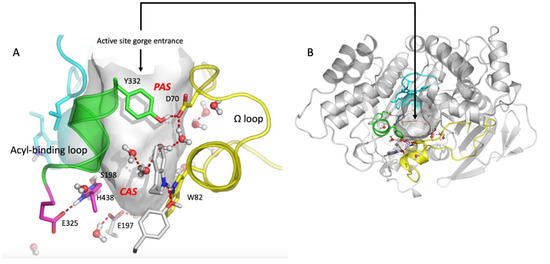
Figure 1.
Human BChE active site gorge (PDB 1PO1 structure visualization, using PyMOL (v.2010) (pymol.org)). (A) Side view of the active site gorge of human BChE. The solvent-accessible volume (500 Å3) of the gorge is in grey. Key residues in PAS and CAS are represented as sticks with nitrogen atoms in dark blue and oxygen atoms in red. The Ω -loop (yellow) and the acyl-binding pocket (ABP) loop (green) are represented. A water molecule network interconnects key residues in the gorge. Pink color: residues of the CAS: S198, H438, E325. (B) Top view of the active site gorge entrance with complete structure of the α/β fold of monomer. Human AChE has a similar organization. However, its PAS is more extended and the solvent-accessible volume of the active site gorge is smaller (300 Å3).
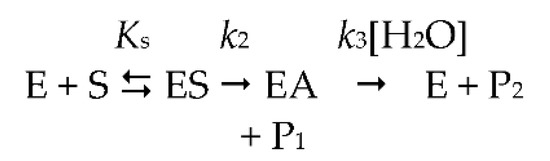
Scheme 1.
Michaelis-Menten two-step model.
As a result, ChEs display a complex catalytic behavior particularly with positively charged substrates. The events determining this behavior are not completely understood. With neutral esters, the hydrolysis of substrates obeys the simple two-step Michaelis–Menten model. After the formation of the reversible michaelian complex ES, the active site serine is acylated (k2), and subsequently deacylated (k3), by the nucleophilic attack of water, acting as a co-substrate (Scheme 1).
In Scheme 1, ES is the productive enzyme–substrate complex, EA is the acylated enzyme, P1 is the alcohol/phenol product and P2 is the acid product. For ester substrates, acylation and deacylation are partially rate-limiting (k2 ≥ k3) [9,30,31]. On the other hand, in the case of poor substrates, like arylacylamides, k2 << k3, then kcat = k2 and Km = Ks [9,10]. The kinetic Scheme 1 is described by the classical Michaelis–Menten rate equation (Equation (1)):
where
and
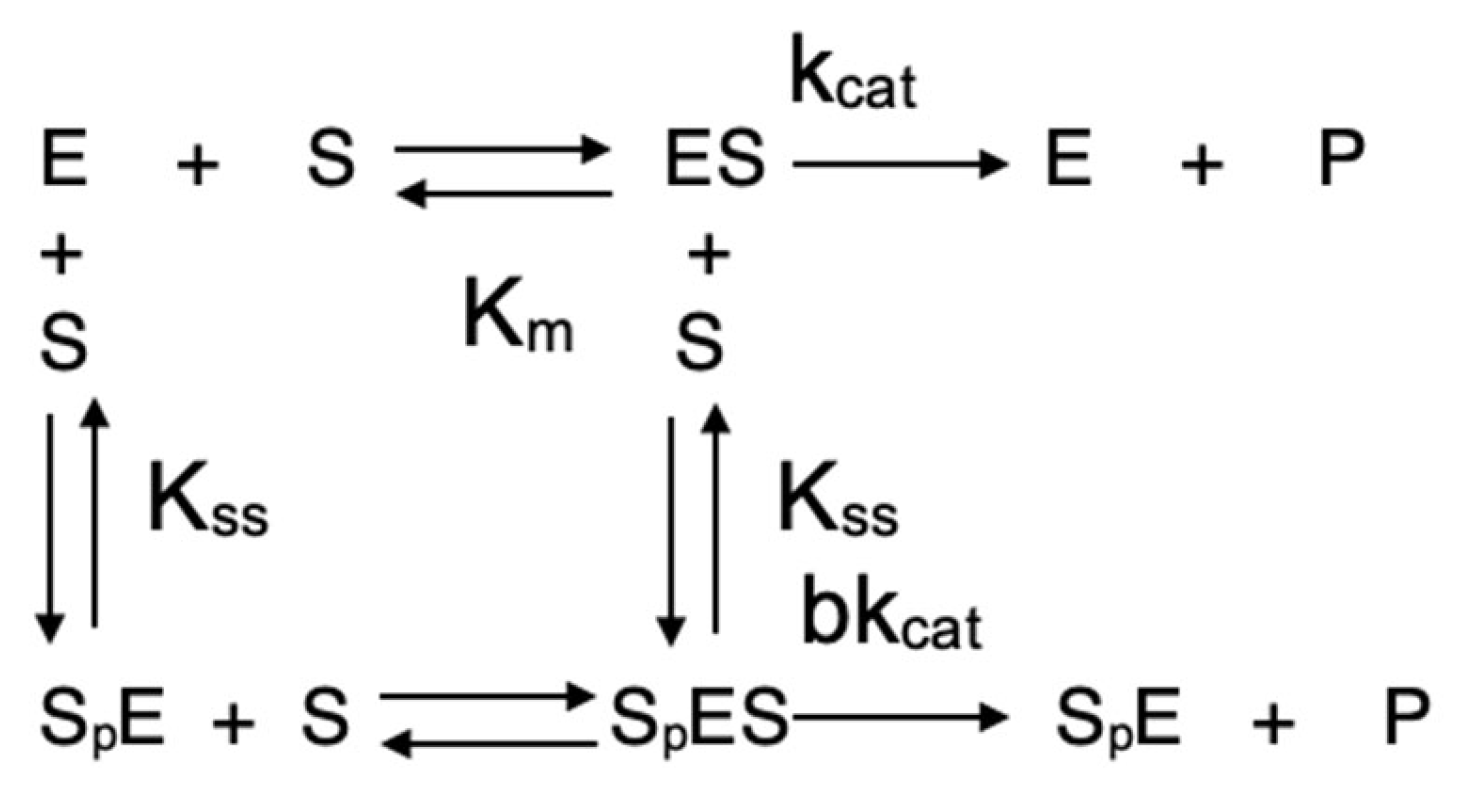
In Equation (3), Ks = (k−1 + k2)/k1 is the dissociation of the enzyme–substrate complex ES. However, with positively charged substrates, such as the natural substrate acetylcholine and, in fact, with the majority of known ChE substrates, ChEs deviate from the michaelian behavior at high substrate concentrations, being either activated or inhibited by excess substrate. With these substrates, the minimum catalytic mechanism is conveniently described by the Webb model (Scheme 2) [25]:
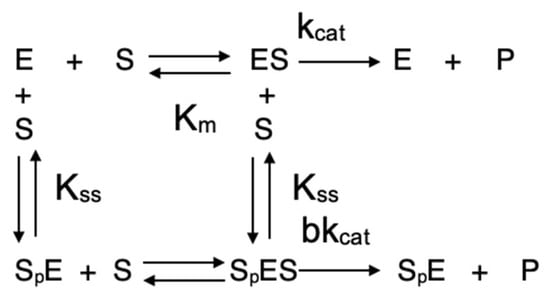
Scheme 2.
Webb-Radic model.
In Scheme 2, at high substrate concentrations, a second substrate molecule (Sp) binds to the (PAS, p), giving a ternary complex, SpES, characterized by a dissociation constant Kss. The binding of this second substrate molecule to the PAS triggers an allosteric effect via motions of both the Ω loop and the acyl-binding loop (Figure 1), causing an alteration in the catalytic constant kcat by a b factor. If b < 1, there is inhibition by excess substrate; on the contrary, if b > 1, there is activation by excess substrate. If b = 1, the catalytic behavior is michaelian. The non-michaelian behavior of ChEs is conveniently described by Equation (4), popularized by Radic [26]. This equation is now used by most researchers working on catalytic and inhibition mechanisms of cholinesterases.
As seen here, at low substrate concentration where [S] << Kss, => b → 1, Scheme 2 reduces to the simple Michalis–Menten model (Scheme 1) described by Equation (1). However, several issues remain unsolved. In particular, it is unclear whether the binding of a second substrate molecule (or a ligand) on the PAS affects acylation or deacylation. Thus, the b factor in Scheme 2 is essentially an overall phenomenological parameter.
The goal of the present work was to determine which catalytic step(s) determine the b factor. The b factor may indeed result from two additive contributions, a and d, acting on acylation (a) and deacylation (d), respectively. For this purpose, steady-state kinetics was performed, using (thio)esters and a positively charged arylacetylamide (ATMA). It was shown that the « a » contribution is the sole determinant of the catalytic behavior of ChEs at high substrate concentration, causing either an activation or inhibition by excess substrate with charged substrates. An analysis of the steady-state AChE-catalyzed hydrolysis of a bulky ester (BzCh), and of progress curves for competition between BzCh and BzTC, showed that the heteroatom (O vs. S) affects the productive binding and b. This indicates that the fine adjustment of the benzoyl moiety in the ABP results from the functional flexibility of this loop, making AChE capable of accommodating bulky substrates.
2. Results
2.1. Steady-State Kinetics of AChE and BChE with ATMA
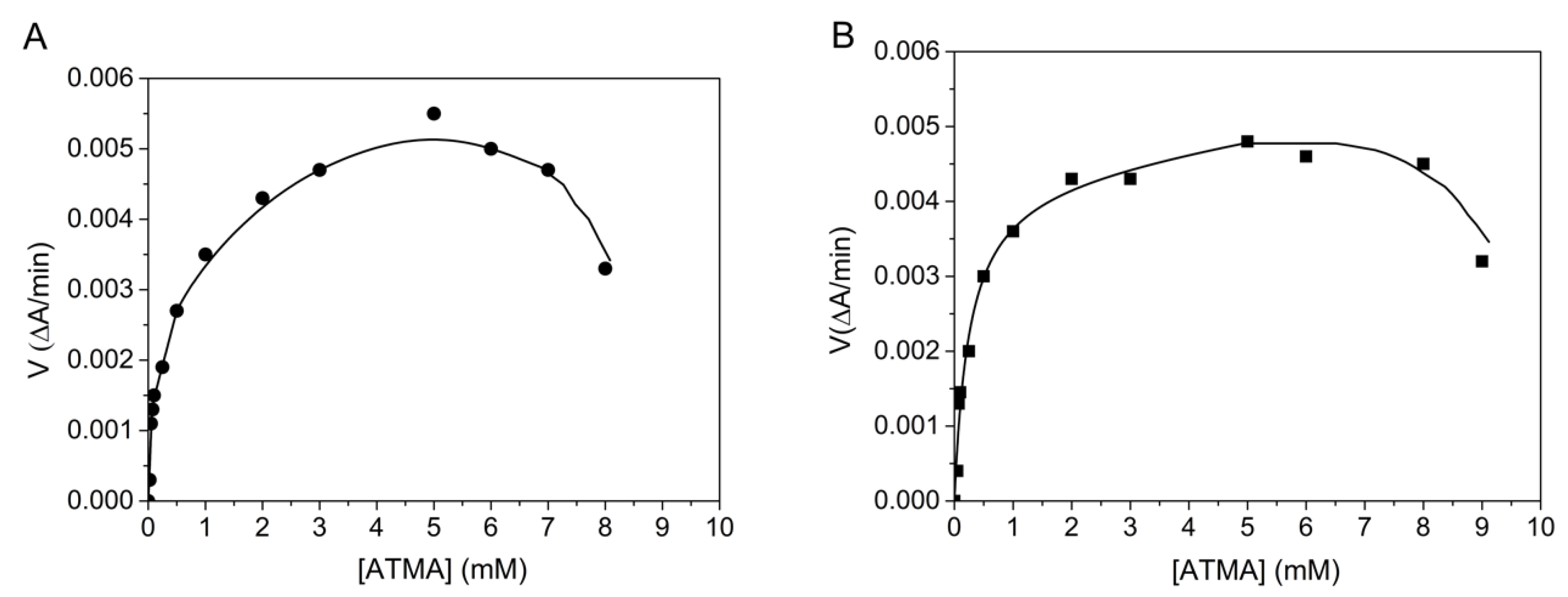
The BChE- and AChE-catalyzed hydrolysis of the arylacetylamide substrate ATMA is non-michaelian, showing activation by excess substrate up to 6 mM, and then inhibition for positively charged (thio)esters at very high concentrations (Figure 2). From the phase of activation by excess substrate, b was calculated using Equation (4). The b values are 1.8 ± 0.4 and 3.1 ± 0.6 for BChE and AChE, respectively. Because for this substrate, acylation is the rate-limiting step (k2 << k3) for both enzymes; Equation (9) is equal to 0 with a − b = 0, d = 1 and a = b. At high substrate concentrations up to 6 mM (Equation (8)), b∙kcat = a∙k2. Acylation is the sole contributor to the b factor, i.e., b = a. For both enzymes, the inhibitory phase, beyond 6 mM ATMA may correspond to product inhibition, forming an abortive complex SEP2. Such an inhibitory phase at very high substrate concentrations has been observed with all positively charged substrates of ChEs but has never, thus far, been thoroughly investigated.
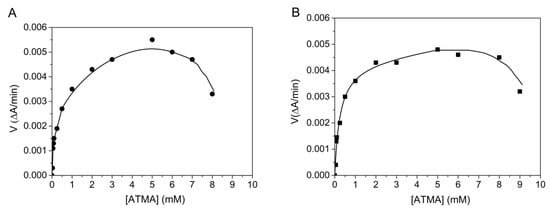
Figure 2.
Hydrolysis of ATMA by human AChE (A) in 0.1 M phosphate, pH 8.0 and human BChE (B) in 0.1 M phosphate, pH 7.0 at 25 °C. Computer fitting of data to Equation (2) gave Km = 0.07 ± 0.04 mM, Kss = 2.5 ± 1.5 mM, kcat = 200 ± 14 min−1 for AChE and Km = 0.14 ± 0.04 mM, Kss = 0.6 ± 0.1 mM and kcat = 322 ± 80 min−1 for BChE.
2.2. Steady-State Hydrolysis of BzCh and BzTC by Human AChE
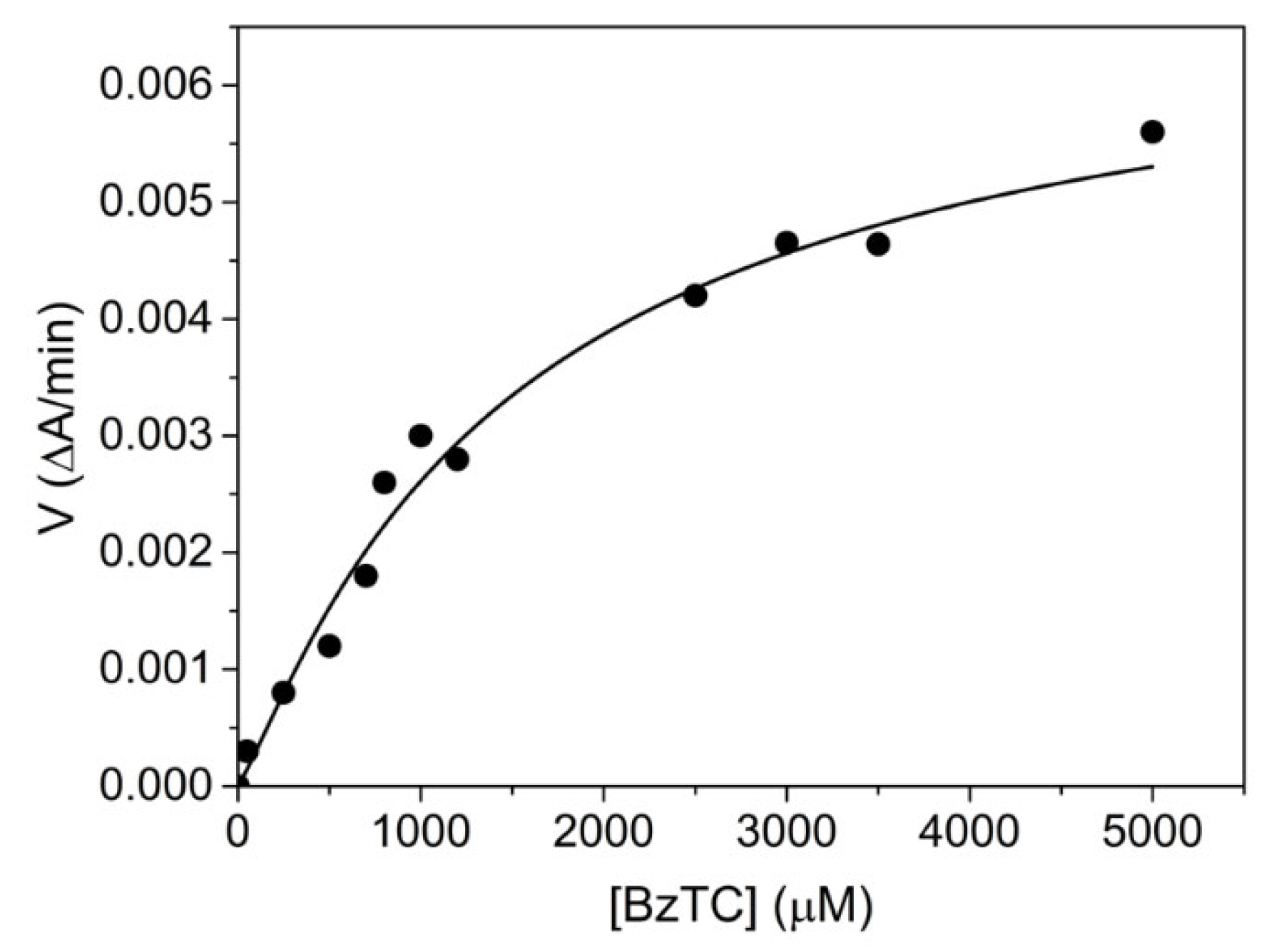
The human AChE-catalyzed hydrolysis of BzTC was found to be very slow and strongly activated by excess substrate (Figure 3). Data were fitted to Equation (4), giving Km = 0.32 ± 0.03 mM; Kss = 1.34 ± 0.4 mM; kcat = 18 ± 7 min−1 and b = 7.8 ± 0.5. These results are in agreement with rates reported by Hosea et al. [27] for mouse AChE (Table 1, footnote b).
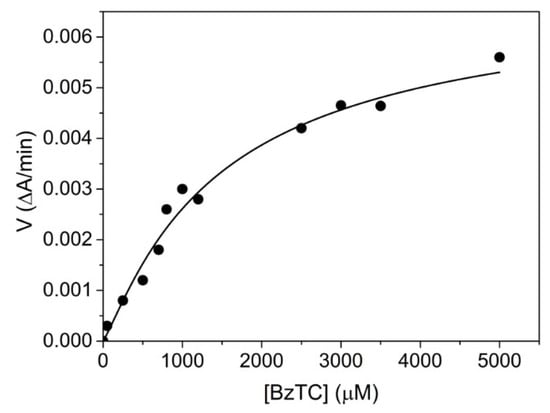
Figure 3.
Human AChE-catalyzed hydrolysis of BzTC up to 5 mM in 0.1M phosphate, pH 8.0, 25 °C.

Table 1.
b factor (average values) differences between human AChE and BChE (inhibition by excess substrate (b < 1) and activation by excess substrate (b > 1)).
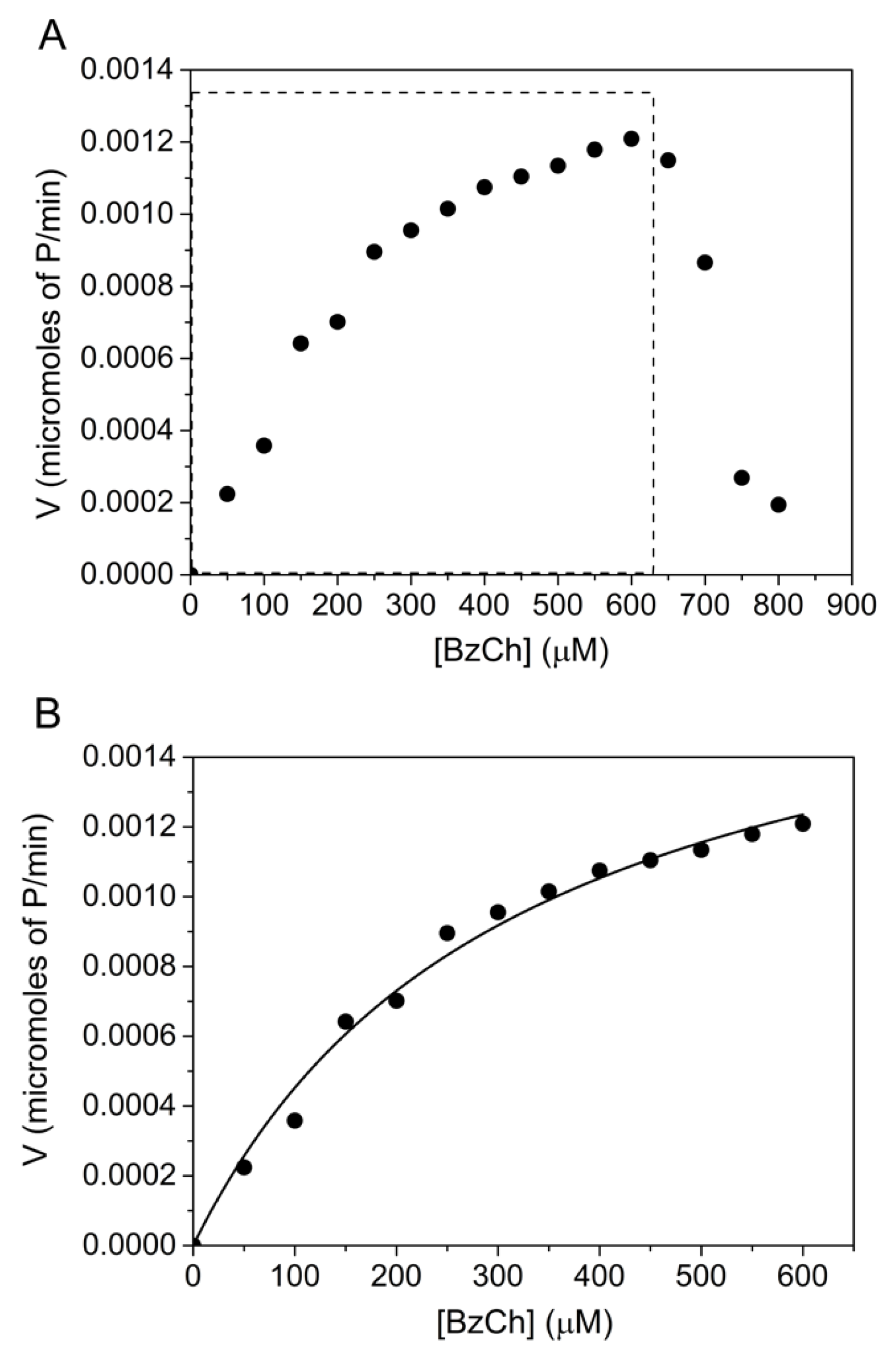
BzCh was also found to be a poor substrate of human AChE with Km = 0.3 ± 0.07 mM and kcat = 72 ± 4 min−1. This is in agreement with previously reported studies (Table 1). Although the b factor for the AChE-catalyzed hydrolysis of BzTC hydrolysis is high, it was impossible to determine the b factor for the hydrolysis of BzCh. Indeed, the steady-state AChE-catalyzed hydrolysis of BzCh, up to 800 μM, revealed unusual behavior beyond 500 μM (Figure 4A): a rapid drop of activity within a narrow interval of BzCh concentration. This behavior does not fit with the model described in Scheme 2 and Equation (4).
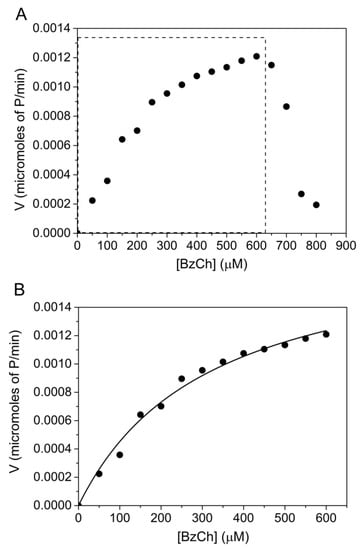
Figure 4.
AChE-catalyzed hydrolysis of BzCh up to 800 μM. (A) Catalysis over the full BzCh concentration range; (B) BzCh hydrolysis up to 600 μM (non-linear fitting of Michaelis–Menten rate equation, (Equation (1)), providing estimates of Km = 0.3 ± 0.07 mM and kcat = 72 ± 4 min−1.
After conversion, the initial rates, ranging from 0.0016 to 0.0082 ΔA240/min, were expressed in terms of μmol./min of released product: −d[BzCh]/dt = d[P1]/dt = d[P2]/dt.
A statistical analysis of the residuals was used to discriminate between the two models that describe the catalytic mechanisms of ChEs (Scheme 1 and Scheme 2). For this purpose, we compared the sum of square Q2 (for details, see Supplementary Materials, Section S3—Residual analysis). Plots of residuals, as a function of predicted velocities, were calculated by fitting the experimental data of Figure 4 to the Michaelis–Menten (Scheme 1), and Webb (Scheme 2) models are reported (Figures S8 and S9). The narrowly scattered distribution of residuals around the horizontal axis, up to 600 μM BzCh, indicates that the kinetics obey the Michaelis–Menten model up to this concentration. However, the use of the Michaelis–Menten model and the Webb–Radic model, for BzCh concentrations up to 800 μM, shows an abnormal distribution of residuals (Figures S10 and S11). Errors in estimates of rates are not the result of experimental measurements but reveal a change in the catalytic behavior. In particular, the sudden collapse of residuals above 600 μM indicates that the kinetics follows neither of the two Schemes beyond this concentration.
To interpret this phenomenon, several hypotheses were considered. Firstly, because of the structural analogy of BzCh with cationic denaturing agents, such as benzalkonium, we speculated whether concentrations of BzCh > 600 μM could induce the unfolding of AChE. ChEs are very sensitive to chemical denaturants and undergo irreversible denaturation in the presence of such agents [35]. In fact, after the microdialysis or dilution of AChE samples subjected to high concentrations of BzCh, the catalytic activity was fully recovered. Thus, BzCh does not denature AChE. Then, two alternative hypotheses were proposed: (a) at high BzCh concentration, substrate molecules arrange as dimer and multimers to form large self-assembling bilayers, organized by electrostatic interactions, that cannot enter into the active site gorge of the enzyme (monomeric substrate depletion hypothesis); (b) at high concentration, the hydrolysis reaction products, P1 (choline) and/or P2 (benzoic acid), either do(es) not dissociate from the enzyme active center, causing product inhibition [36], or accumulate(s) inside the active site gorge where they/it may inhibit the entrance of new BzCh molecules (traffic jam hypothesis) or cause local pH decrease (benzoic acid).
Unlike the BChE-catalyzed hydrolysis of BzCh and related long-alkyl chain derivatives, that display damped [33,37] or stochastic [36] oscillations in the first minutes of steady-state hydrolysis in 0.1–0.2 M phosphate, pH 6.0 or 7.0, the AChE-catalyzed hydrolysis of BzCh was linear in 0.1 M phosphate buffer, pH 8.0. In the case of BChE, at low substrate concentration, oscillations at the beginning of the BChE-catalyzed hydrolysis of BzCh were interpreted in terms of slow equilibria between multiple molecular associates of BzCh molecules [37]. In the case of AChE, no oscillations were observed. Nevertheless, the first hypothesis (a) was checked by 1H-NMR. The 1H-NMR spectra of BzCh at different concentrations did not provide evidence either for dimer of BzCh molecules (π−cation interactions between choline and benzoic ring) or multimeric and micellar associates (Figure S3). The determination of the self-diffusion coefficients, by means of Fourier transform-pulsed gradients spin-echo (FT-PGSE) NMR, is known as a powerful tool for the characterization of supramolecular systems in solution. The self-diffusion coefficients (Ds) do not change with increasing BzCh concentration (Figure S4, Table S1). Moreover, the results of tensiometry, spectrophotometry and DLS studies do not support the formation of such molecular associations. We see that BzCh does not decrease the surface activity on the air–water interfaces (Figure S5). The investigation of the concentration-dependent absorption spectra of the molecular state does not show any shift (Figure S6A) or change in absorbance intensity with increasing of concentration (Figure S6B). The DLS method revealed the formation of large structures with diameters of about 200 and 300 nm and a polydispersity index around 0.4 (Figure S7). Since spectrophotometry data about the solubilization of hydrophobic dye Sudan I did not reveal hydrophobic zones capable of solubilizing the dye, and data obtained from other methods denied the formation of self-assemblies, the DLS method cannot correctly reflect the formation of micelles. We may, therefore, refute the hypothesis that the formation of micelles impaired the penetration of single BzCh molecules into the active site gorge.
The second hypothesis (b) was checked by performing the steady-state hydrolysis of BzCh in the presence of choline and benzoic acid, the hydrolysis products P1 and P2, respectively, of BzCh hydrolysis. Moreover, 1H-NMR of BzCh solutions showed that a small fraction (2%) of BzCh was spontaneously hydrolyzed in choline and benzoic acid in highly concentrated solutions (Figure S3). The inhibitory action of choline on AChE was previously investigated by steady-state kinetic analysis and reported to be a weak reversible competitive inhibition (Ki = 3.2 ± 0.4 mM) [33]. Moreover, there was no non-linear dependence of inhibition that could have suggested either allostery or partial inhibition. This linear and low inhibitory potency suggests that the presence of 2% choline in substrate solutions cannot significantly inhibit the enzyme.
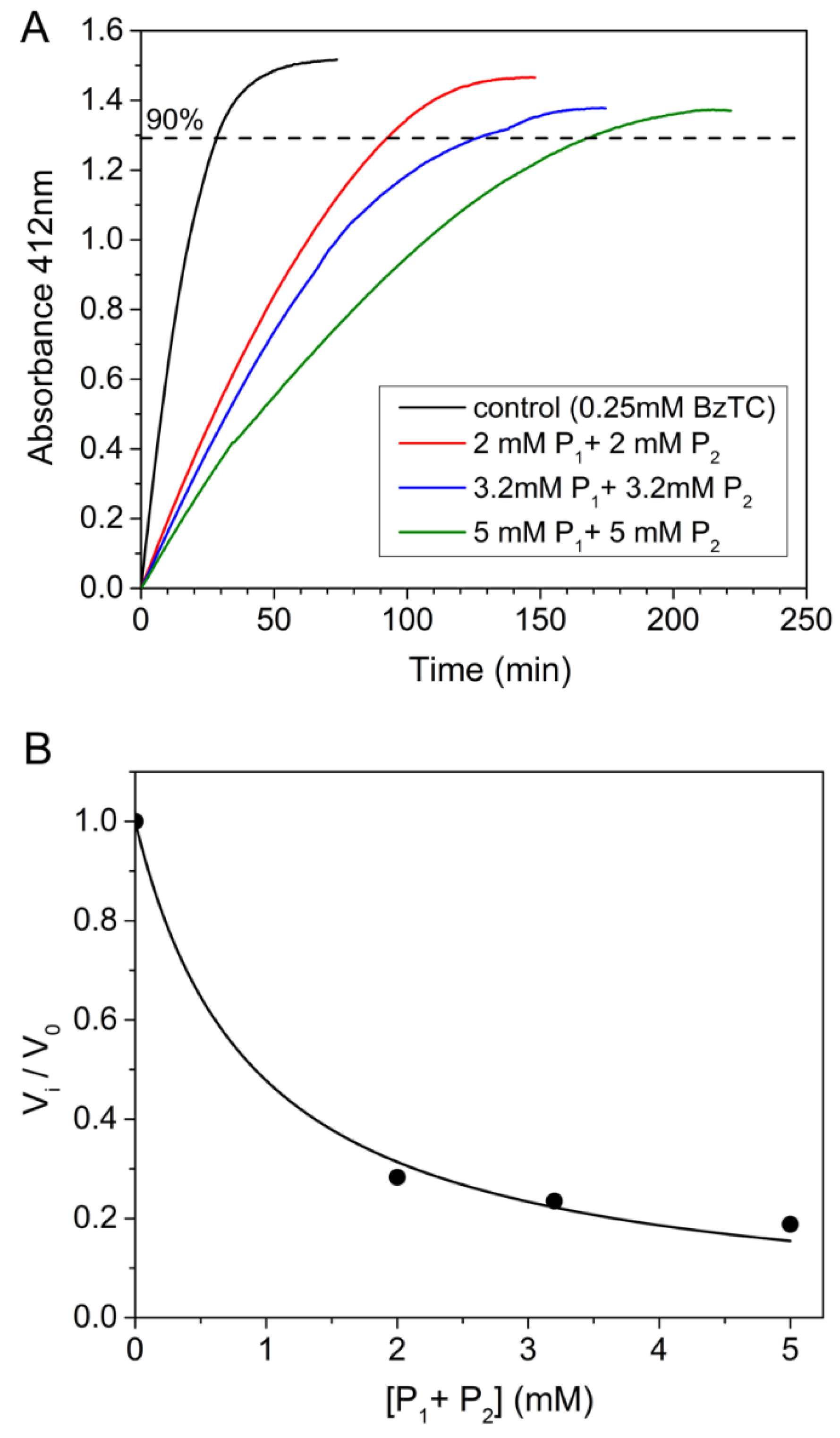
Competing substrate kinetics of the AChE-catalyzed hydrolysis between BzCh and BzTC were performed in the presence of high concentrations (2; 3.2; 5 mM) of benzoic acid or choline and of both choline and benzoic acid. High concentrations of benzoic acid caused a slight decrease in pH buffer, e.g., pH = 7.6 in the presence of 5 mM benzoic acid. Such a pH decrease cannot explain the sudden drop of AChE activity observed beyond 600 μM BzCh (Figure 4A). Finally, the time-course hydrolysis of the AChE-catalyzed hydrolysis of BzTC, in the presence of high concentrations of choline, benzoic acid or both products (Figure 5), also showed that products do not impair the catalysis and cause only an increase in the time needed for the full completion of the substrate, acting like a reversible competitive inhibitor with an apparent overall Ki = 0.5 ± 0.04 mM.
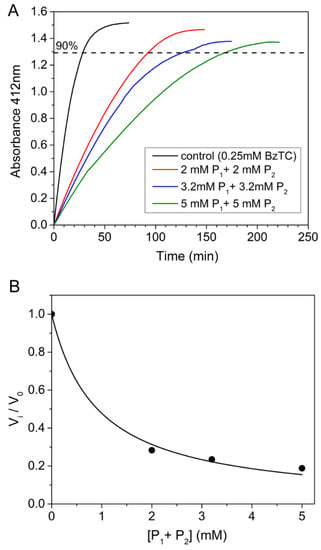
Figure 5.
Time-course of AChE-catalyzed hydrolysis of BzTC (0.25 mM) in the absence and presence of high concentrations of choline and benzoic acid (2; 3.2; 5 mM of P1 and P2). (A) Competing kinetic profiles vs. time; (B) ratio of initial velocities (vi/v0) for BzTC hydrolysis in the absence (v0) and presence of P1 + P2 up to 5 mM (vi).
Such concentrations are much higher than the highest concentrations (<1 mM) of BzCh that we used in the steady-state kinetic experiments. However, if we state that the hydrolysis products of BzCh, namely choline (P1) and benzoic acid (P2), remain bound in the active site gorge where they accumulate, their local concentration rapidly increases in the 300 Å3 volume of the enzyme active site gorge.
According to Equation (5), with [E] = 10−8 M and an apparent BzCh turnover of 72 min−1, at BzCh concentration 600 μM, much higher than Km, it would take about t = 800 min for the hydrolysis products to reach such a concentration if they accumulate in the active site gorge of AChE (volume = 300 Å3). Moreover, if benzoic acid (pKa = 4.2) is dissociated at pH 8.0, and protons are also released with P1 and P2, then the local pH would drop below the enzyme pKa if protons were not evacuated from the active site gorge; we observed only a modest pH decrease in spectrophotometer cuvettes at the highest BzCh concentrations. This situation is very different from the pH drop effect on enzyme velocity that was observed for the BChE-catalyzed hydrolysis of aspirin [6]. Thus, the time-dependent product accumulation hypothesis is not realistic.
Therefore, the unpredicted abnormal behavior of AChE at high concentrations of BzCh cannot be simply explained. A thorough investigation of the ChE-catalyzed hydrolysis of substrates at high concentrations is needed for all types of substrates to determine the catalytic mechanism over a large range of substrate concentrations. Regarding BzCh, the most likely explanation for the observed BzCh inhibition of AChE beyond 600 μM BzCh is that hydrolysis products, P1 or P2, remain bound to the CAS, leading to a catalytically unproductive ternary complex, SEP. Such an inhibitory phenomenon, due to the blockade of product dissociation from the CAS, was carefully investigated with AChE [38] and for haloalkane dehalogenase [39] with certain substrates. More detailed studies should be carried out on both ChEs with BzCh and also with the natural substrate acetylcholine. Under certain circumstances, i.e., the accumulation of acetylcholine or an exogenous substrate (poisonous or medicinal esters) in synaptic clefts and/or at neuromuscular junctions, the sudden inhibition of ChEs by a very large excess of substrate may have physio-pathological, toxicological or pharmacological significance and consequences.
2.3. Competing Substrate Kinetics of AChE
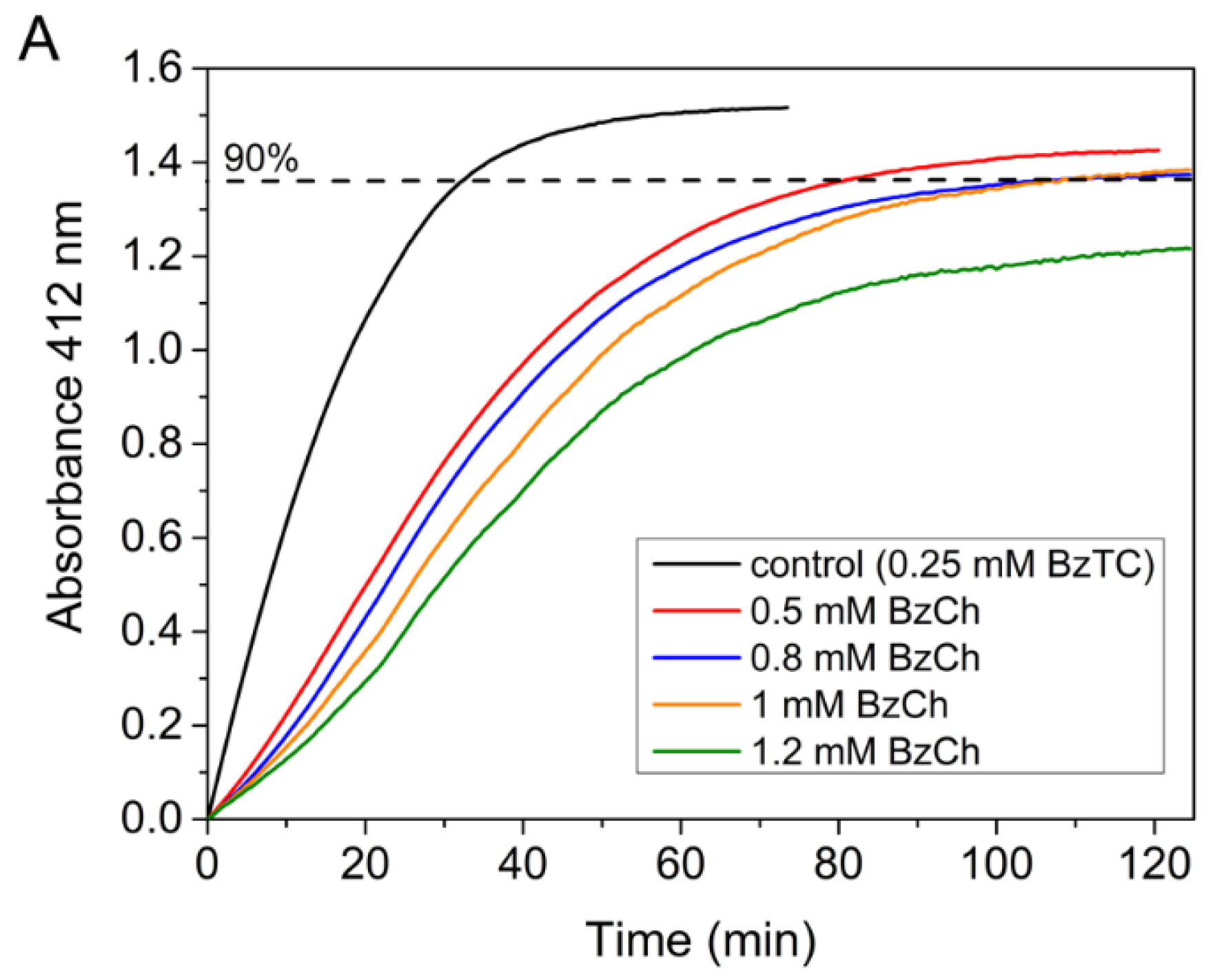
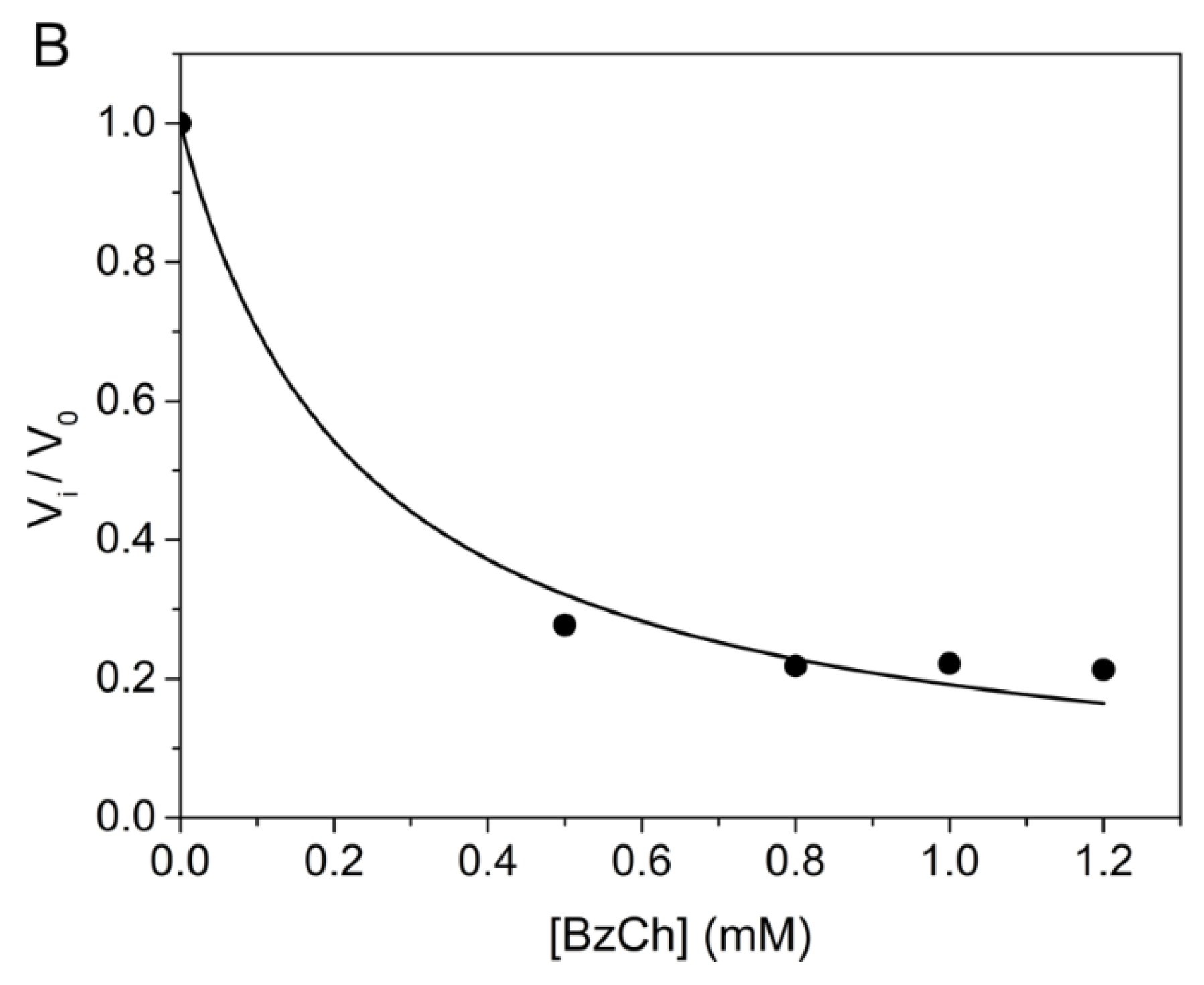
Competing substrate kinetics between low concentrations (0.25 mM) of BzTC as the reporter substrate, and increasing concentrations (from 0.5 to 1.2 mM) of BzCh as the blind substrate, was also performed in order to check the above-mentioned hypothesis. Kinetics were performed in 0.1 M phosphate buffer, pH 8.0 at 25 °C, according to [33]. The results showed that the time-course of competing progress curves are sigmoidal and that their plateau for maximum hydrolysis of the reporter substrate (BzTC) decreases with the concentration of competing substrate (BzCh) (Figure 6A). The sigmoidal shape indicates that the competing blind substrate is significantly hydrolyzed during the time course of the experiment [33,40]. The fact that vi/v0 curves vs. BzCh concentration does not reach 0 at high BzCh concentration (Figure 6B) also indicates that the inhibition is partial.
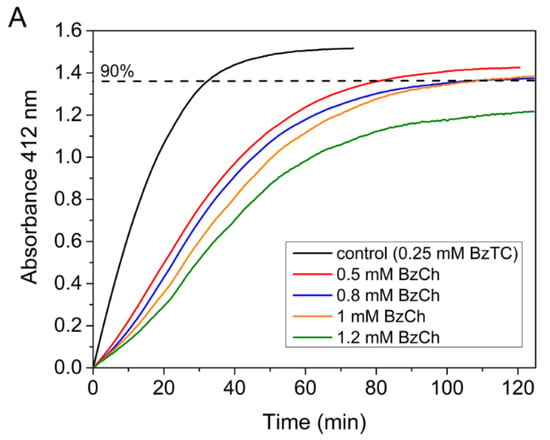
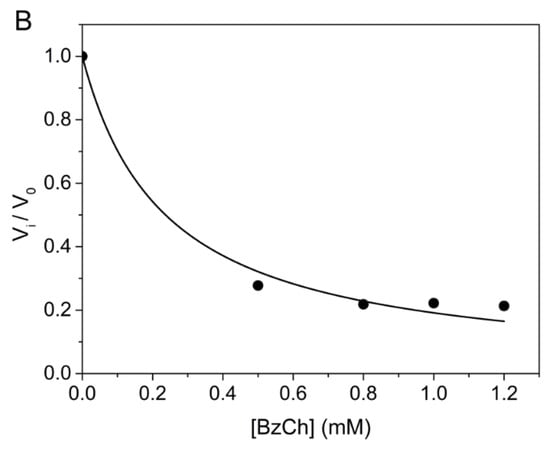
Figure 6.
Competing substrate kinetics of AChE between BzTC and BzCh in 0.1M phosphate, pH 8.0 at 25 °C. (A) Time-course of BzTC (0.25 mM) hydrolysis in the absence and presence of BzCh; (B) ratio of initial velocities (vi/v0) of BzTC hydrolysis in the absence (v0) and presence of BzCh (vi).
3. Discussion
The present results clearly show that the binding of a second positively charged substrate molecule on the PAS affects the acylation step when substrates are charged (thio)esters or an arylacylamide, e.g., acetyl-esters, benzoyl(thio)choline or arylacetyl-amides like ATMA. This confirms our hypothesis that a > d (cf. end of Section 4.3.1)
This effect can be the activation or inhibition of the acylation step. These mechanisms involve motions of both the Ω loop and the acyl-binding (ABP) loop. At high substrate concentration, the simulation of the b factor changes as a function of three variables k2/k3, a and d (cf Equation (7)), showing that, for the BChE catalysis of an ester like BTC (b = 3, cf Table 1) where k2 and k3 are partly rate-limiting, the contribution of the factor a is dominating (a = 4.5, d = 2.4). A similar conclusion can be drawn for AChE, where b < 1 with this substrate or ATC. However, in the case of an arylacylamide (k2 << k3), for both enzymes, the factor a is the sole contribution to b, i.e., a = b.
Several unanswered questions remain. In particular, it is not clear why both enzymes display opposite behavior with most positively charged esters but not with ATMA (Table 1). Site-directed mutagenesis studies on AChE showed, also, that mutations on F297 and around this position in the acyl-binding pocket (ABP) impact the value of the b factor in an opposite way [26,27,41,42]. In silico simulations, using molecular docking and QM/MM, should provide a definitive answer to this question.
We must point out that a thorough kinetic study showed that high concentrations of acetylcholine-, ATC- or a positively charged analog accelerate the decarbamylation of Drosophila AChE, carbamylated by a neutral carbamyl-ester (k3 >> k2). The acceleration of deacylation (decarbamylation) results from the binding of the second molecule (acetylcholine, ATC, substrate analog) at the rim of the active site gorge, i.e., near the PAS [43]. Our results, showing that a > d for carboxyl-esters (k3 ≈ k2) and arylacylamides (k2 << k3), do not follow this explanation. Indeed, in the case of the acceleration of the hydrolysis of a carbamyl-ester, d.k3 is increased by high concentrations of a positively charged ligand, because k2 >> k3, d.k3 is always smaller than a.k2 (even if a is increased too).
This result also highlights that reversible and irreversible inhibitors of pharmacological and toxicological interest may interfere with the complex catalytic mechanisms of ChEs. Irreversible inhibitors like carbamates and organophosphates alkylate the CAS serine, then decrease the free active enzyme concentration; they may also bind reversibly to the PAS and modulate the reactivity of the CAS (cf. interaction of VX with the PAS of AChE [44] or acceleration of AChE inhibition by PAS ligands [45]) Ligands forming non-covalent complexes act as reversible competitive, non-competitive, mixed-type or uncompetitive inhibitors. Reversible inhibition is fast with most ligands (equilibrium is reached within microseconds). However, it can be slow, in particular with bulky ligands [46,47]. Exclusive binding on PAS makes ternary complexes (IpES), determining uncompetitive inhibition. Reversible inhibition can be total (linear inhibition) or, less frequently, partial (hyperbolic inhibition) [48]. Inhibitors that bind on both CAS and PAS determine more complex reversible inhibition patterns. All reversible and irreversible inhibitors are either potent toxicants or important drugs used for the treatment of various diseases, in particular Alzheimer’s disease [49,50]. The 3D structures of numerous covalent conjugates and non-covalent complexes have been solved in the past 25 years. Although binding kinetics and inhibition mechanisms are still puzzling for certain of these molecules [46], adaptative conformational changes, upon the binding of various ligands [28], shed light on the complex interplay between the activity, binding and inhibition of ChEs. Thus, the effects of substrate/ligand binding to PAS on the acylation step of both ChEs has important functional implications with respect to the cholinergic mechanisms and pharmacological/toxicological actions of ChE ligands. Since the catalytic response of AChE and BChE to the binding of a second substrate molecule on the PAS are not systematically opposite, the response depends on the chemical structure of the substrate (Table 1) and its productive adjustment in the CAS (ES ES≠), leading to enzyme acylation (EA). Thus, a simple kinetic analysis shows its limitation.
4. Materials and Methods
4.1. Chemicals
Acetylthiocholine iodide (ATC), butyrylthiocholine iodide (BTC), benzoylcholine chloride (BzCh) and dithio-bisnitrobenzoic acid (DTNB) were purchased from Sigma–Aldrich (Saint Louis, MO, USA) and benzoylthiocholine chloride (BzTC) from ICN (Tokyo, Japan). N3-(acetamido)-N,N,N-trimethylanilinium iodide (ATMA) was synthesized according to the method described by Johnson et al. [32]. Based on NMR spectra, purity of ATMA was >99%. 1H- and 13C-NMR spectral data of ATMA are in Supplementary Materials (Section S1—Synthesis of ATMA: 1H- and 13C-NMR spectra, Figures S1 and S2). Stock solution of 0.1 M ATMA was in water. Stock solutions of ATC, BTC, BzCh and BzTC (0.1 M) were in water and stored at −20 °C. Echothiophate iodide was from Biobasal AG (Basel, Switzerland). Stock solution of 0.1 M echothiophate was in water and stored at −20 °C. Choline chloride was from Acros Organics (Geel, Belgium). Benzoic acid was from Vecton (Saint Petersburg, Russia). All other chemicals were of biochemical grade.
4.2. Enzymes
Human BChE tetrameric form (MW = 340 kDa), highly purified from human plasma Cohn fraction IV-4 [51], was a gift from Dr. O. Lockridge (UNMC, Omaha, NE, USA). The enzyme was diluted in 0.1 M sodium phosphate buffer, pH 7.0, to an activity of 45 units/mL with 1 mM BTC as the substrate at 25 °C (one unit corresponds to the number of micromoles of substrate hydrolyzed per minutes). The diluted enzyme was titrated according to Leuzinger [52], using echothiophate as the titrant. The active site concentration of this preparation was 1.9 × 10−7 M.
Highly purified (>95% pure) recombinant human AChE monomer (MW = 70 kDa) was in solution in 10 mM HEPES, pH 7.5, containing 10 mM NaCl [53]. AChE concentration was 14.7 mg/mL based on absorbance at 280 nm (for highly purified BChE, A280 = 1.7 corresponds to mass concentration of 1 mg/mL). The enzyme was stabilized by bovine serum albumin (1 mg/mL w/v). A second preparation, 53.4 mg/mL, was stabilized by a polysacharide, citric pectine at 1 mg/mL w/v. The active site titration of stabilized AChE preparations, using echothiophate as the titrating agent, provided active site concentrations of 1.1 × 10−3 M and 4 × 10−2 M, respectively.
During the titration processes, enzyme activity was checked using the method of Ellman [54] with 1 mM BTC in 0.1 M phosphate buffer pH 7.0 for BChE and 1 mM ATC in 0.1 M phosphate buffer pH 8.0 for AChE.
4.3. Steady-State Kinetic Analysis
4.3.1. Rationale
For simulation of steady-state kinetics of substrate hydrolysis by AChE and BChE, substrates were chosen so that the acyl-intermediates were the same for each enzyme, i.e, same k3 for each enzyme regardless of the substrate used. For hydrolysis of esters, both chemical steps, acylation and diacylation, are partly rate-limiting, i.e., k2 is of the same order of magnitude as k3. Then, Equation (2) can be re-written as:
For the arylacetylamide substrate ATMA, acylation is the rate-limiting step, i.e., k2 << k3 and thus, kcat = k2. This kind of substrate simplifies the analysis.
From Equation (4), it follows that at high substrate concentration (ester), v = b·kcat·[E]. The b factor may be regarded as a phenomenological composite variable resulting from the contribution of two components: “a” that alters the acylation rate (a·k2) and “d” that alters the deacylation rate (d·k3). Then, considering the catalytic constant at high substrate concentration as b·kcat, Equation (6) leads to the following expression for this catalytic rate constant for hydrolysis of a (thio)ester:
and, for hydrolysis of an arylacylamide substrate like ATMA, bkcat = ak2.
Combining Equations (2) and (7) leads to:
When the mechanism obeys the Michaelis–Menten model, b = 1 and therefore, a = d = 1. On the other hand, when there is activation by excess substrate, b > 1, a > 1 and d > 1. When there is inhibition by excess substrate, b < 1, a < 1 and d < 1. Therefore, with partly rate-limiting ChE-catalyzed hydrolysis of charged substrates (b ≠ 1), the ratio k2/k3 at high substrate concentration can be expressed by:
However, because of the allosteric effects, caused by motion of both the Ω loop and the acyl loop upon binding of Sp (see Scheme 1), it can be reasonably hypothesized that these effects are more pronounced on acylation than on deacylation. Thus, it is expected that a > d.
4.3.2. Steady-State Kinetics of Substrate Hydrolysis
Steady-state of ATMA, BzCh and BzTC hydrolysis by AChE and BChE were performed at 25 °C, at optimum pH of enzymes, i.e., in 0.1 M sodium phosphate buffer, pH 8.0, for AChE and 0.1 M sodium phosphate buffer, pH 7.0, for BChE. The active site enzyme concentration in assays, [E]0, was 10−8–10−9 M for AChE and 5 × 10−9 M for BChE.
For ChE-catalyzed hydrolysis of ATMA, the concentration of ATMA ranged from 0.05 to 9 mM. Hydrolysis of ATMA was monitored by the absorbance change at 290 nm (εTMA = 1850 M−1 cm−1) [32]. For hydrolysis of BzCh, the BzCh concentration ranged from 1 to 800 μM. Hydrolysis kinetics of BzCh was monitored by recording the decrease in absorbance at 240 nm (the difference in the extinction coefficient between substrate and products, Δε, is 6700 M−1 cm−1 at 240 nm in phosphate buffer [37,55]). For the thioester BzTC, hydrolysis was followed according to the method of Ellman et al. [37,54] with 0.33 mM dithio-bis-nitro-benzoate (DTNB) as the chromogenic reagent, by recording the increase in absorbance at 412 nm of 5-thio-2-nitrobenzoate (ε = 13,300 M−1 cm−1), resulting from the reduction in DTNB by thiocholine, the substrate hydrolysis product P1. The concentration in BzTC ranged from 5 μM to 5 mM.
Kinetic runs were performed at least in triplicate and catalytic parameters were determined by weighted non-linear fitting of rate equations (Equations (1) and (4)), using Origin (Originlab Co., Northampton, MA, USA). Kinetic and binding parameters are provided with standard errors of the mean. Discrimination between steady-state kinetic models (Michaelis–Menten (Scheme 1) vs. Webb model (Scheme 2)) were made by using the statistical analysis of residuals proposed by Bartfai and Mannervik [56]. Principles of this procedure, thoroughly expanded by Cornish-Bowden [57], are given in Supplementary Materials (Section S3—Residual Analysis).
4.3.3. Possible Inhibition of AChE-Catalyzed BzCh Hydrolysis by Reaction Products
These studies were only performed with AChE that displays a very low activity with BzCh as the substrate at pH 8.0 and 25 °C. At high concentrations of BzCh (above 500 μM), the possibility that AChE was inhibited by released hydrolysis products was considered. The reaction products P1 (choline) and/or P2 (benzoic acid) were added to the medium either for steady-state kinetic analysis or for time-course of competing substrates kinetics. At the same time, for kinetics in the presence of benzoic acid (pKa = 4.2) possible pH decrease was controlled.
- Steady-state kinetics
Steady-state kinetics of BzCh hydrolysis at 3 different concentrations, 100, 250 and 500 μM, was performed in the absence and presence of choline (product P1) at one concentration: 3.5 mM. Because the product P2 (benzoic acid) adsorption at 240 nm, study of the effect of P2 was performed by competing substrate kinetics (next section).
- Time-course of competing substrate kinetics
Time-course of complete AChE-catalyzed hydrolysis of the reporter substrate (BzTC) at low concentration (0.25 mM, i.e., less than Km) was performed in the absence and presence of BzCh as the blind substrate at various concentrations (0.5, 0.8, 1, 1.2 mM). Certain kinetic runs were also performed in the presence of choline and/or benzoic acid, the hydrolysis products P1 and P2 of BzCh, at concentrations 2, 3.2, 5 mM. Analysis of progress curves was performed according to the method we previously developed [33,40].
4.4. 1H-NMR of BzCh Solutions
To check whether BzCh at high concentrations can form multiple associates from non-covalent dimers to different type of micelles, 1H-NMR spectra of BzCh chloride solutions from 0.25 to 50 mM in 0.1 M phosphate buffer, pH 8.0, were performed at 30 °C. 1H NMR spectra were recorded on a Bruker AvanceIII-500 spectrometer working at 500.1 MHz in 1H and 125.8 MHz in 13C experiments. Chemical shifts were reported in ppm relative to residual signals of protons of deuterated solvents. D2O-d6 were used as NMR solvents. (Supplementary Materials, Section S2—Benzoylcholine chloride solutions, Figures S3 and S4).
4.5. Tensiometry of BzCh Solutions
Surface tension measurements of BzCh solutions were performed using the du Nouy ring detachment method (Kruss K6 Tensiometer, Hamburg, Germany). Briefly, the spherical ring was placed parallel to the air/solvent interface. Between two surface tension analyses, the ring was cleaned with ultra-purified water, followed by soaking in ethanol and drying. Temperature was maintained at a constant at 25 °C during all measurements. (Supplementary Materials, Section S2—Benzoylcholine chloride solutions, Figure S5).
4.6. Dynamic Light Scattering
Size and polydispersity index of BzCh solutions were determined by dynamic light scattering (DLS) measurements, using the Malvern Instrument Zetasizer Nano (Worcestershire, UK). Measured autocorrelation functions were analyzed by Malvern M1 DTS software v.7.13, applying the second-order cumulant expansion methods. The effective hydrodynamic radius (RH) was calculated according to the Einstein–Stokes equation D = kBT/6πηRH, where D is the diffusion coefficient, kB is the Boltzmann constant, T is the absolute temperature and η is the viscosity. The diffusion coefficient was measured at least in triplicate for each sample. The average error of measurements was ±4%. (Supplementary Materials, Section S2—Benzoylcholine chloride solutions, Figure S7).
4.7. UV Spectrophotometry and Dye Solubilization
The concentration-dependent absorption spectra of BzCh solutions were measured using PerkinElmer λ35 (PerkinElmer Instruments, Waltham, MA, USA). Solubilization of the dye (Sudan I) was performed by adding an excess of crystalline Sudan I to BzCh solutions. These solutions were allowed to equilibrate for about 48 h at constant temperature (25 °C), followed by filtration. UV absorbance was measured at 485 nm (for Sudan I). Quartz cuvettes (1 cm-path) containing sample were used. (Supplementary Materials, Section S2—Benzoylcholine chloride solutions, Figure S6).
5. Conclusions
The purpose of this work was to interpret the phenomenological b factor in terms of its acylation vs. deacylation contributions to the catalytic constant of ChEs at high concentrations of positively charged substrates. The magnitude and opposite values of b between AChE and BChE indicated that the productive adjustment of substrates in the active center depends on the motions of both the Ω and the acyl-binding loops, resulting from the occupancy of the PAS by a second substrate molecule. Remembering that the active site gorge of AChE is 300 Å3 against 500 Å3 for BChE, the poor catalytic hydrolysis efficiency of AChE against the bulky ester benzoylcholine illustrates the importance of the fine adjustment of the substrate acyl moiety in the acyl-binding pocket. Bulkier esters are not hydrolyzed by AChE while they are substrates of BChE. This property of BChE, capable of accommodating large molecules in its CAS, has important toxicological and pharmacological implications for the metabolism of ester-containing drugs.
Now, to understand the intimate mechanism of the activation versus inhibition of ChEs at high substrate (or ligand) concentrations, a thorough analysis of the catalytic pathway, including the cross-talk between PAS, CAS and ABP, is needed. For this purpose, QM/MM simulations of substrate hydrolysis should confirm that b depends on the effect of PAS occupancy on the dissociation of the bound substrate tetrahedral intermediate into the acetylated enzyme and alcohol/phenol product P1. Moreover, in silico studies should shed light on the effect of the size of the acyl moiety of the substrates on the stabilization of ES≠. Finally, these works are expected to support the recent findings of Radic’s group on X-ray and neutron diffraction/scattering and on MD simulations of AChE conjugates [28,29,58,59].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310472/s1.
Author Contributions
P.M., conceptualization, supervision and writing; A.R.M., kinetic studies, analysis and data curation; A.V.N., synthesis of chemicals; V.V.S., NMR analyses; T.N.P. physicochemical analyses. All authors have read and agreed to the published version of the manuscript.
Funding
The work of P. Masson was carried out in accordance with the Strategic Academic Leadership Program “Priority 2030” of the Kazan Federal University of the Government of the Russian Federation. A.V. Nemtarev (synthesis of ATMA), T.N. Pashirova (self-assembly study) and V. Sykaev (NMR study) acknowledge the financial support received from the government assignment for the FRC Kazan Scientific Center of Russian Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are indebted to O. Lockridge (UNMC, Omaha, NE, USA) for the gift of the highly purified human plasma BChE tetramer. The authors thank the Spectral and Analytical Joint Center (Kazan Scientific Center, Russian Academy of Sciences) for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ACh, acetylcholine; AChE, acetylcholinesterase; APB, acyl-binding pocket; ATC, acetylthiocholine; ATMA, 3-(acetamido)-N,N,N-trimethylanilinium; BChE, butyrylcholinesterase; BzCh, benzoylcholine; BzTC, benzoylthiocholine; CAS, catalytic active site; ChE, cholinesterase; DTNB, dithio-bis-nitrobenzoic acid; PAS, peripheral anionic site; QM/MM, quantum mechanics/molecular mechanics.
References
- Pezzementi, L.; Chatonnet, A. Evolution of Cholinesterases in the Animal Kingdom. Chem. Biol. Interact. 2010, 187, 27–33. [Google Scholar] [CrossRef]
- Silman, I. The Multiple Biological Roles of the Cholinesterases. Prog. Biophys. Mol. Biol. 2021, 162, 41–56. [Google Scholar] [CrossRef]
- Mesulam, M.-M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase Knockouts Establish Central Cholinergic Pathways and Can Use Butyrylcholinesterase to Hydrolyze Acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Schuhmacher, L.-N.; Roberts, M.; Kakugawa, S.; Bineva-Todd, G.; Howell, S.; O’Reilly, N.; Perret, C.; Snijders, A.P.; Vincent, J.-P.; et al. Notum Deacylates Octanoylated Ghrelin. Mol. Metab. 2021, 49, 101201. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, J.M.; Mohney, R.P.; Pappan, K.L. Long-Chain Acylcholines Link Butyrylcholinesterase to Regulation of Non-Neuronal Cholinergic Signaling. J. Proteome Res. 2022, 21, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Froment, M.T.; Fortier, P.L.; Visicchio, J.E.; Bartels, C.F.; Lockridge, O. Butyrylcholinesterase-Catalysed Hydrolysis of Aspirin, a Negatively Charged Ester, and Aspirin-Related Neutral Esters. Biochim. Biophys. Acta 1998, 1387, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Legrand, P.; Bartels, C.F.; Froment, M.-T.; Schopfer, L.M.; Lockridge, O. Role of Aspartate 70 and Tryptophan 82 in Binding of Succinyldithiocholine to Human Butyrylcholinesterase. Biochemistry 1997, 36, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O. Review of Human Butyrylcholinesterase Structure, Function, Genetic Variants, History of Use in the Clinic, and Potential Therapeutic Uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef]
- Quinn, D.M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Masson, P.; Froment, M.-T.; Gillon, E.; Nachon, F.; Darvesh, S.; Schopfer, L.M. Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Acetanilides. Biochim. Biophys. Acta 2007, 1774, 1139–1147. [Google Scholar] [CrossRef]
- Eddleston, M. Novel Clinical Toxicology and Pharmacology of Organophosphorus Insecticide Self-Poisoning. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Lockridge, O. Butyrylcholinesterase for Protection from Organophosphorus Poisons: Catalytic Complexities and Hysteretic Behavior. Arch. Biochem. Biophys. 2010, 494, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Lushchekina, S. Catalytic Bioscavengers: The New Generation of Bioscavenger-Based Medical Countermeasures. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1199–1229. [Google Scholar]
- Saxena, A.; Myers, T.M.; Sipos, M.L. Conjugates of Human Serum Butyrylcholinesterase and Nerve Agents Are Behaviorally Safe in Rhesus Macaques. Chem. Biol. Interact. 2021, 344, 109499. [Google Scholar] [CrossRef] [PubMed]
- Cerasoli, D.M.; Armstrong, S.J.; Reeves, T.E.; Hodgins, S.M.; Kasten, S.A.; Lee-Stubbs, R.B.; Cadieux, C.L.; Otto, T.C.; Capacio, B.R.; Lenz, D.E. Butyrylcholinesterase, a Stereospecific in Vivo Bioscavenger against Nerve Agent Intoxication. Biochem. Pharmacol. 2020, 171, 113670. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. The Biological Activities of Butyrylcholinesterase Inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- Xing, S.; Li, Q.; Xiong, B.; Chen, Y.; Feng, F.; Liu, W.; Sun, H. Structure and Therapeutic Uses of Butyrylcholinesterase: Application in Detoxification, Alzheimer’s Disease, and Fat Metabolism. Med. Res. Rev. 2021, 41, 858–901. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Leung, M.R.; van Bezouwen, L.S.; Schopfer, L.M.; Sussman, J.L.; Silman, I.; Lockridge, O.; Zeev-Ben-Mordehai, T. Cryo-EM Structure of the Native Butyrylcholinesterase Tetramer Reveals a Dimer of Dimers Stabilized by a Superhelical Assembly. Proc. Natl. Acad. Sci. USA 2018, 115, 13270–13275. [Google Scholar] [CrossRef]
- Boyko, K.M.; Baymukhametov, T.N.; Chesnokov, Y.M.; Hons, M.; Lushchekina, S.V.; Konarev, P.V.; Lipkin, A.V.; Vasiliev, A.L.; Masson, P.; Popov, V.O.; et al. 3D Structure of the Natural Tetrameric Form of Human Butyrylcholinesterase as Revealed by CryoEM, SAXS and MD. Biochimie 2019, 156, 196–205. [Google Scholar] [CrossRef]
- Leung, M.R.; Zeev-Ben-Mordehai, T. Cryo-Electron Microscopy of Cholinesterases, Present and Future. J. Neurochem. 2021, 158, 1236–1243. [Google Scholar] [CrossRef]
- Wang, J.; Lai, S.; Kong, Y.; Yao, W.; Chen, X.; Liu, J. The Protonation State of Glu202 in Acetylcholinesterase. Proteins 2022, 90, 485–492. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic Structure of Acetylcholinesterase from Torpedo Californica: A Prototypic Acetylcholine-Binding Protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Reiner, E.; Simeon-Rudolf, V. Cholinesterase: Substrate Inhibition and Substrate Activation. Pflug. Arch. 2000, 440 (Suppl. S1), R118–R120. [Google Scholar] [CrossRef] [PubMed]
- Radic, Z.; Pickering, N.A.; Vellom, D.C.; Camp, S.; Taylor, P. Three Distinct Domains in the Cholinesterase Molecule Confer Selectivity for Acetyl- and Butyrylcholinesterase Inhibitors. Biochemistry 1993, 32, 12074–12084. [Google Scholar] [CrossRef] [PubMed]
- Hosea, N.A.; Berman, H.A.; Taylor, P. Specificity and Orientation of Trigonal Carboxyl Esters and Tetrahedral Alkylphosphonyl Esters in Cholinesterases. Biochemistry 1995, 34, 11528–11536. [Google Scholar] [CrossRef]
- Radić, Z. Shifts in Backbone Conformation of Acetylcholinesterases upon Binding of Covalent Inhibitors, Reversible Ligands and Substrates. Crystals 2021, 11, 1557. [Google Scholar] [CrossRef]
- Radić, Z. Connectivity between Surface and Interior in Catalytic Subunits of Acetylcholinesterases Inferred from Their X-ray Structures. J. Neurochem. 2023, in press. [Google Scholar] [CrossRef]
- Froede, H.C.; Wilson, I.B. Direct Determination of Acetyl-Enzyme Intermediate in the Acetylcholinesterase-Catalyzed Hydrolysis of Acetylcholine and Acetylthiocholine. J. Biol. Chem. 1984, 259, 11010–11013. [Google Scholar] [CrossRef]
- Masson, P.; Froment, M.-T.; Gillon, E.; Nachon, F.; Lockridge, O.; Schopfer, L.M. Hydrolysis of Oxo- and Thio-Esters by Human Butyrylcholinesterase. Biochim. Biophys. Acta BBA-Proteins Proteom. 2007, 1774, 16–34. [Google Scholar] [CrossRef]
- Johnson, J.L.; Cusack, B.; Davies, M.P.; Fauq, A.; Rosenberry, T.L. Unmasking Tandem Site Interaction in Human Acetylcholinesterase. Substrate Activation with a Cationic Acetanilide Substrate. Biochemistry 2003, 42, 5438–5452. [Google Scholar] [CrossRef] [PubMed]
- Mukhametgalieva, A.R.; Aglyamova, A.R.; Lushchekina, S.V.; Goličnik, M.; Masson, P. Time-Course of Human Cholinesterases-Catalyzed Competing Substrate Kinetics. Chem.-Biol. Interact. 2019, 310, 108702. [Google Scholar] [CrossRef] [PubMed]
- Boeck, A.T.; Schopfer, L.M.; Lockridge, O. DNA Sequence of Butyrylcholinesterase from the Rat: Expression of the Protein and Characterization of the Properties of Rat Butyrylcholinesterase. Biochem. Pharmacol. 2002, 63, 2101–2110. [Google Scholar] [CrossRef]
- Masson’, P.; Lushchekina, S. Conformational Stability and Denaturation Processes of Proteins Investigated by Electrophoresis under Extreme Conditions. Molecules 2022, 27, 6861. [Google Scholar] [CrossRef] [PubMed]
- Hrabovská, A.; Debouzy, J.-C.; Froment, M.-T.; Devínsky, F.; Pauliková, I.; Masson, P. Rat Butyrylcholinesterase-Catalysed Hydrolysis of N-Alkyl Homologues of Benzoylcholine. FEBS J. 2006, 273, 1185–1197. [Google Scholar] [CrossRef]
- Masson, P.; Goldstein, B.N.; Debouzy, J.-C.; Froment, M.-T.; Lockridge, O.; Schopfer, L.M. Damped Oscillatory Hysteretic Behaviour of Butyrylcholinesterase with Benzoylcholine as Substrate. Eur. J. Biochem. 2004, 271, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L. Strategies to Resolve the Catalytic Mechanism of Acetylcholinesterase. J. Mol. Neurosci. 2010, 40, 32–39. [Google Scholar] [CrossRef]
- Kokkonen, P.; Beier, A.; Mazurenko, S.; Damborsky, J.; Bednar, D.; Prokop, Z. Substrate Inhibition by the Blockage of Product Release and Its Control by Tunnel Engineering. RSC Chem. Biol. 2021, 2, 645–655. [Google Scholar] [CrossRef]
- Goličnik, M.; Masson, P. Time-Course of Enzyme-Catalyzed Competing Substrate Degradation for Michaelian Behavior and for Enzymes Showing Activation/Inhibition by Excess Substrate. Chem.-Biol. Interact. 2019, 309, 108704. [Google Scholar] [CrossRef]
- Vellom, D.C.; Radić, Z.; Li, Y.; Pickering, N.A.; Camp, S.; Taylor, P. Amino Acid Residues Controlling Acetylcholinesterase and Butyrylcholinesterase Specificity. Biochemistry 1993, 32, 12–17. [Google Scholar] [CrossRef]
- Kaplan, D.; Ordentlich, A.; Barak, D.; Ariel, N.; Kronman, C.; Velan, B.; Shafferman, A. Does “Butyrylization” of Acetylcholinesterase through Substitution of the Six Divergent Aromatic Amino Acids in the Active Center Gorge Generate an Enzyme Mimic of Butyrylcholinesterase? Biochemistry 2001, 40, 7433–7445. [Google Scholar] [CrossRef]
- Brochier, L.; Pontié, Y.; Willson, M.; Estrada-Mondaca, S.; Czaplicki, J.; Klaébé, A.; Fournier, D. Involvement of Deacylation in Activation of Substrate Hydrolysis by Drosophila Acetylcholinesterase. J. Biol. Chem. 2001, 276, 18296–18302. [Google Scholar] [CrossRef] [PubMed]
- Friboulet, A.; Rieger, F.; Goudou, D.; Amitai, G.; Taylor, P. Interaction of an Organophosphate with a Peripheral Site on Acetylcholinesterase. Biochemistry 1990, 29, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Radić, Z.; Taylor, P. Peripheral Site Ligands Accelerate Inhibition of Acetylcholinesterase by Neutral Organophosphates. J. Appl. Toxicol. 2001, 21 (Suppl. S1), S13–S14. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, S.V.; Masson, P. Slow-Binding Inhibitors of Acetylcholinesterase of Medical Interest. Neuropharmacology 2020, 177, 108236. [Google Scholar] [CrossRef]
- Lamba, D.; Pesaresi, A. Kinetic Modeling of Time-Dependent Enzyme Inhibition by Pre-Steady-State Analysis of Progress Curves: The Case Study of the Anti-Alzheimer’s Drug Galantamine. Int. J. Mol. Sci. 2022, 23, 5072. [Google Scholar] [CrossRef]
- Mukhametgalieva, A.R.; Lushchekina, S.V.; Aglyamova, A.R.; Masson, P. Steady-State Kinetic Analysis of Human Cholinesterases over Wide Concentration Ranges of Competing Substrates. Biochim. Biophys. Acta Proteins Proteom. 2022, 1870, 140733. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Llanes, L.C.; Kuehlewein, I.; de França, I.V.; da Silva, L.V.; da Cruz Junior, J.W. Anticholinesterase Agents For Alzheimer’s Disease Treatment: An Updated Overview. Curr. Med. Chem. 2023, 30, 701–724. [Google Scholar] [CrossRef]
- Schopfer, L.M.; David, E.; Hinrichs, S.H.; Lockridge, O. Human Butyrylcholinesterase in Cohn Fraction IV-4 Purified in a Single Chromatography Step on Hupresin. PLoS ONE 2023, 18, e0280380. [Google Scholar] [CrossRef]
- Leuzinger, W. The Number of Catalytic Sites in Acetylcholinesterase. Biochem. J. 1971, 123, 139–141. [Google Scholar] [CrossRef]
- Carletti, E.; Colletier, J.-P.; Dupeux, F.; Trovaslet, M.; Masson, P.; Nachon, F. Structural Evidence That Human Acetylcholinesterase Inhibited by Tabun Ages through O-Dealkylation. J. Med. Chem. 2010, 53, 4002–4008. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O. Genetic Variants of Human Serum Cholinesterase Influence Metabolism of the Muscle Relaxant Succinylcholine. Pharmacol. Ther. 1990, 47, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Bartfal, T.; Mannervik, B. A Procedure Based on Statistical Criteria for Discrimination between Steady State Kinetic Models. FEBS Lett. 1972, 26, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Gerlits, O.; Blakeley, M.P.; Keen, D.A.; Radić, Z.; Kovalevsky, A. Room Temperature Crystallography of Human Acetylcholinesterase Bound to a Substrate Analogue 4K-TMA: Towards a Neutron Structure. Curr. Res. Struct. Biol. 2021, 3, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Fajer, M.; Cheng, X.; Blumenthal, D.K.; Radić, Z.; Kovalevsky, A. Structural and Dynamic Effects of Paraoxon Binding to Human Acetylcholinesterase by X-Ray Crystallography and Inelastic Neutron Scattering. Structure 2022, 30, 1538–1549.e3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).