Clearance and Utilization of Dicarbonyl-Modified LDL in Monkeys and Humans
Abstract
1. Introduction
2. Results
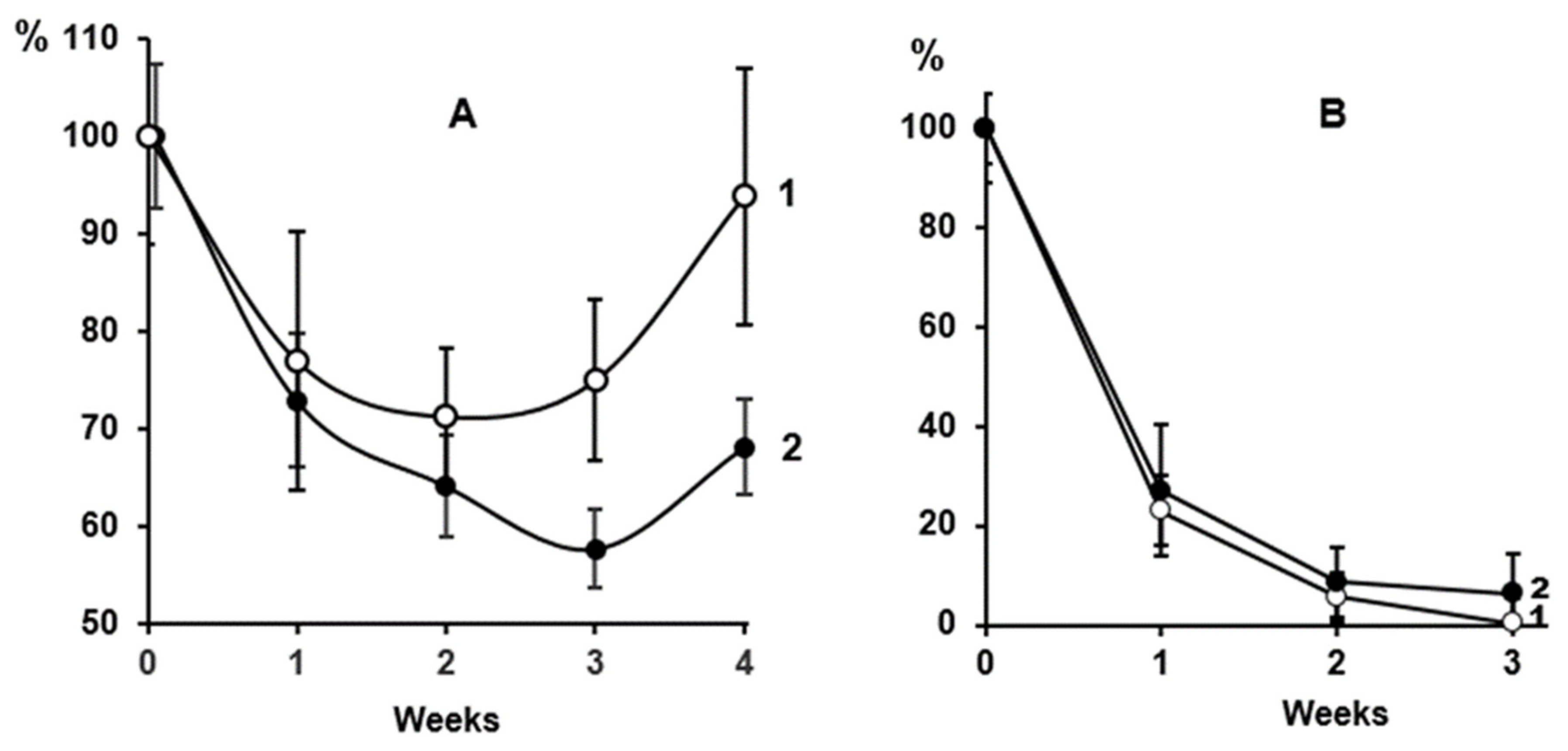
2.1. Clearance of Dicarbonyl-Modified LDL in Macaca Mulatta Monkeys
2.2. Effect of the PCSK9 Inhibitor Evolocumab on the Level of MDA-Modified LDL in the Bloodstream of CHD Patients
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. LDL Preparative Isolation
4.3. Preparation of FITC-Labeled LDL
4.4. Preparation of FITC-Labeled Dicarbonyl-Modified LDL
4.5. Examination of Clearance of Dicarbonyl-Modified LDL in Macaca Mulatta Monkeys
4.6. Clinical Study of the Effects of PCSK9 Inhibitor Evolocumab
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Witztum, J.L.; Steinberg, D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc. Med. 2001, 11, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K. Atherosclerosis as a free radical pathology and antioxidative therapy of this disease. In Free Radicals, NO and Inflammation; IOS Press: Amsterdam, The Netherlands, 2003; Volume 344, pp. 218–231. [Google Scholar]
- Niedowicz, D.M.; Daleke, D.L. The role of oxidative stress in diabetic complications. Cell Biochem. Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K. Role of oxidative stress in the genesis of atherosclerosis and diabetes mellitus: A personal look back on 50 years of research. Curr. Aging Sci. 2017, 10, 18–25. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Dicarbonyl-Dependent Modification of LDL as a Key Factor of Endothelial Dysfunction and Atherosclerotic Vascular Wall Damage. Antioxidants 2022, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Malondialdehyde as a Important Key Factor of Molecular Mechanisms of Vascular Wall Damage under Heart Diseases Development. J. Int. Mol. Sci. 2023, 24, 128. [Google Scholar] [CrossRef]
- Witz, G. Biological interactions of alpha,beta-unsaturated aldehydes. Free Rad. Biol. Med. 1989, 7, 333–349. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Kumskova, E.M. Macrophages actively accumulate malonyldialdehyde-modified but not enzymatically oxidized low density lipoprotein. Mol. Cell Biochem. 2012, 365, 93–98. [Google Scholar] [CrossRef]
- Fogelman, A.M.; Schechter, I.; Seager, J.; Hokum, M.; Child, J.S.; Edwards, P.E. Malondialdehyde alteration of ow density lipoproteins leads to the cholesteryl ester accumulation in human monocyte macrophages. Proc. Natl. Acad. Sci. USA 1980, 77, 2214–2218. [Google Scholar] [CrossRef]
- Spiteller, G. Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products. Ann. N. Y. Acad. Sci. 2008, 1126, 128–133. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Kapel’ko, V.I.; Shepel’kova, G.S.; Shumaev, K.B.; Panasenko, O.M.; Konovalova, G.G.; Belenkov, Y.N. Mechanisms of oxidative modification of low-density lipoproteins under conditions of oxidative and carbonyl stress. Biochemistry 2007, 72, 1081–1090. [Google Scholar] [CrossRef]
- Lankin, V.; Konovalova, G.; Tikhaze, A.; Shumaev, K.; Kumskova, E.; Viigimaa, M. The initiation of free radical peroxidation of low-density lipoproteins by glucose and its metabolite methylglyoxal: A common molecular mechanism of vascular wall injure in atherosclerosis and diabetes. Mol. Cell Biochem. 2014, 395, 241–252. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Shadyro, O.I.; Shumaev, K.B.; Tikhaze, A.K.; Sladkova, A.A. Non-enzymatic methylglyoxal formation from glucose metabolites and generation of superoxide anion radical during methylglyoxal-dependend cross-links reaction. J. Antioxid. Act. 2019, 1, 34–45. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low-density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic. Res. 2014, 48, 841–848. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshevet, Y.V. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell Physiol. Biochem. 2016, 38, 1851–1859. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Kanuri, S.H.; Mehta, J.L. Role of Ox-LDL and LOX-1 in atherogenesis. Curr. Med. Chem. 2019, 26, 1693–1700. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Gylling, H.; Vanhanen, H.; Ollus, A. Cholesterol absorption, elimination, and synthesis related to LDL kinetics during varying fat intake in men with different apoprotein E phenotypes. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 1044–1052. [Google Scholar] [CrossRef]
- Gylling, H.; Kontula, K.; Miettinen, T.A. Cholesterol absorption and metabolism and LDL kinetics in healthy men with different apoprotein E phenotypes and apoprotein B Xba I and LDL receptor Pvu II genotypes. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 208–213. [Google Scholar] [CrossRef]
- Turley, S.D.; Spady, D.K.; Dietschy, J.M. Role of liver in the synthesis of cholesterol and the clearance of low- density lipoproteins in the cynomolgus monkey. Lipid Res. 1995, 36, 67–79. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Stucchi, A.F.; Nicolosi, R.J.; Ginsberg, H.N.; Ramakrishnan, R.; Deckelbaum, R.J. Human plasma LDL cryopreserved with sucrose maintains in vivo kinetics indistinguishable from freshly isolated human LDL in cynomolgus monkeys. J. Lipid Res. 1994, 35, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Tikhaze, A.K.; Domogatsky, S.P.; Lankin, V.Z. Clearance of Carbonyl-Modified Low-Density Lipoproteins in Rabbits. Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 119–124. [Google Scholar] [CrossRef]
- Afanasieva, O.; Ezhov, M.V.; Klesareva, E.; Razova, O.; Chubykina, U.; Egiazaryan, M.; Sherstyuk, E.; Afanasieva, M.; Utkina, E.; Pokrovsky, S. Effect of Evolocumab on Lipoprotein(a) and PCSK9 in Healthy Individuals with Elevated Lipoprotein(a) Level. J. Cardiovasc. Dev. Dis. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Khlebus, E.; Kutsenko, V.; Meshkov, A.; Ershova, A.; Kiseleva, A.; Shcherbakova, N.; Zharikova, A.; Drapkina, O.; Shevtsov, A.; Yarovaya, E.; et al. Multiple rare and common variants in APOB gene locus associated with oxidatively modified low-density lipoprotein levels. PLoS ONE 2019, 14, e0217620. [Google Scholar] [CrossRef]
- Ragusa, R.; Basta, G.; Neglia, D.; De Caterina, R.; Del Turco, S.; Caselli, C. PCSK9 and atherosclerosis:Looking beyond LDL regulation. Eur. J. Clin. Invest. 2021, 51, e13459. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial. Circulation 2018, 23, 338–350. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 11, 1109–1119. [Google Scholar] [CrossRef]
- Ganesan, R.; Henkels, K.M.; Wrenshall, L.E.; Kanaho Ya Di Paolo, G.; Frohman, M.A.; Gomez-Cambronero, J. Oxidized LDL phagocytosis during foam cell formation in atherosclerotic plaques relies on a PLD2-CD36 functional interdependence. J. Leukoc. Biol. 2018, 103, 867–883. [Google Scholar] [CrossRef]
- Tertov, V.V.; Kaplun, V.V.; Dvoryantsev, S.N.; Orekhov, A.N. Apolipoprotein B-bound lipids as a marker for evaluation of low density lipoprotein oxidation in vivo. Biochem. Biophys. Res. Commun. 1995, 214, 608–613. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Staines, W.A.; Meister, B.; Melander, T.; Nagy, J.I.; Hokfelt, T.J. Three-color immunofluorescence histochemistry allowing triple labeling within a single section. J. Histochem. Cytochem. 1988, 36, 145–151. [Google Scholar] [CrossRef]
- Requena, J.R.; Fu, M.X.; Ahmed, M.U.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997, 322, 317–325. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Vermeer, M.A.; Stehouwer, C.D.; te Koppele, J.; Princen, H.M.; van Hinsbergh, V.W. Effect of methylglyoxal on the physico-chemical and biological properties of low-density lipoprotein. Biochim. Biophys. Acta 1998, 1394, 187–198. [Google Scholar] [CrossRef]

| Groups | Time | |
|---|---|---|
| 6 min | 90 min | |
| 1. Natural LDL | 10.33 ± 1.34 | 10.07 ± 1.50 |
| 2. Glyoxal-modified LDL | 11.62 ± 0.53 | 11.36 ± 1.45 |
| 3. Methylglyoxal-modified LDL | 12.42 ± 4.15 | 9.36 ± 1.64 |
| 4. MDA-modified LDL | 1.27 ± 0.20 * | 1.04 ± 0.16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lankin, V.Z.; Konovalova, G.G.; Domogatsky, S.P.; Tikhaze, A.K.; Klots, I.N.; Ezhov, M.V. Clearance and Utilization of Dicarbonyl-Modified LDL in Monkeys and Humans. Int. J. Mol. Sci. 2023, 24, 10471. https://doi.org/10.3390/ijms241310471
Lankin VZ, Konovalova GG, Domogatsky SP, Tikhaze AK, Klots IN, Ezhov MV. Clearance and Utilization of Dicarbonyl-Modified LDL in Monkeys and Humans. International Journal of Molecular Sciences. 2023; 24(13):10471. https://doi.org/10.3390/ijms241310471
Chicago/Turabian StyleLankin, Vadim Z., Galina G. Konovalova, Sergey P. Domogatsky, Alla K. Tikhaze, Igor N. Klots, and Marat V. Ezhov. 2023. "Clearance and Utilization of Dicarbonyl-Modified LDL in Monkeys and Humans" International Journal of Molecular Sciences 24, no. 13: 10471. https://doi.org/10.3390/ijms241310471
APA StyleLankin, V. Z., Konovalova, G. G., Domogatsky, S. P., Tikhaze, A. K., Klots, I. N., & Ezhov, M. V. (2023). Clearance and Utilization of Dicarbonyl-Modified LDL in Monkeys and Humans. International Journal of Molecular Sciences, 24(13), 10471. https://doi.org/10.3390/ijms241310471







