Genome-Wide Investigation of BAM Gene Family in Annona atemoya: Evolution and Expression Network Profiles during Fruit Ripening
Abstract
:1. Introduction
2. Results
2.1. Identification and Classification of AaBAMs Genes in Annona atemoya
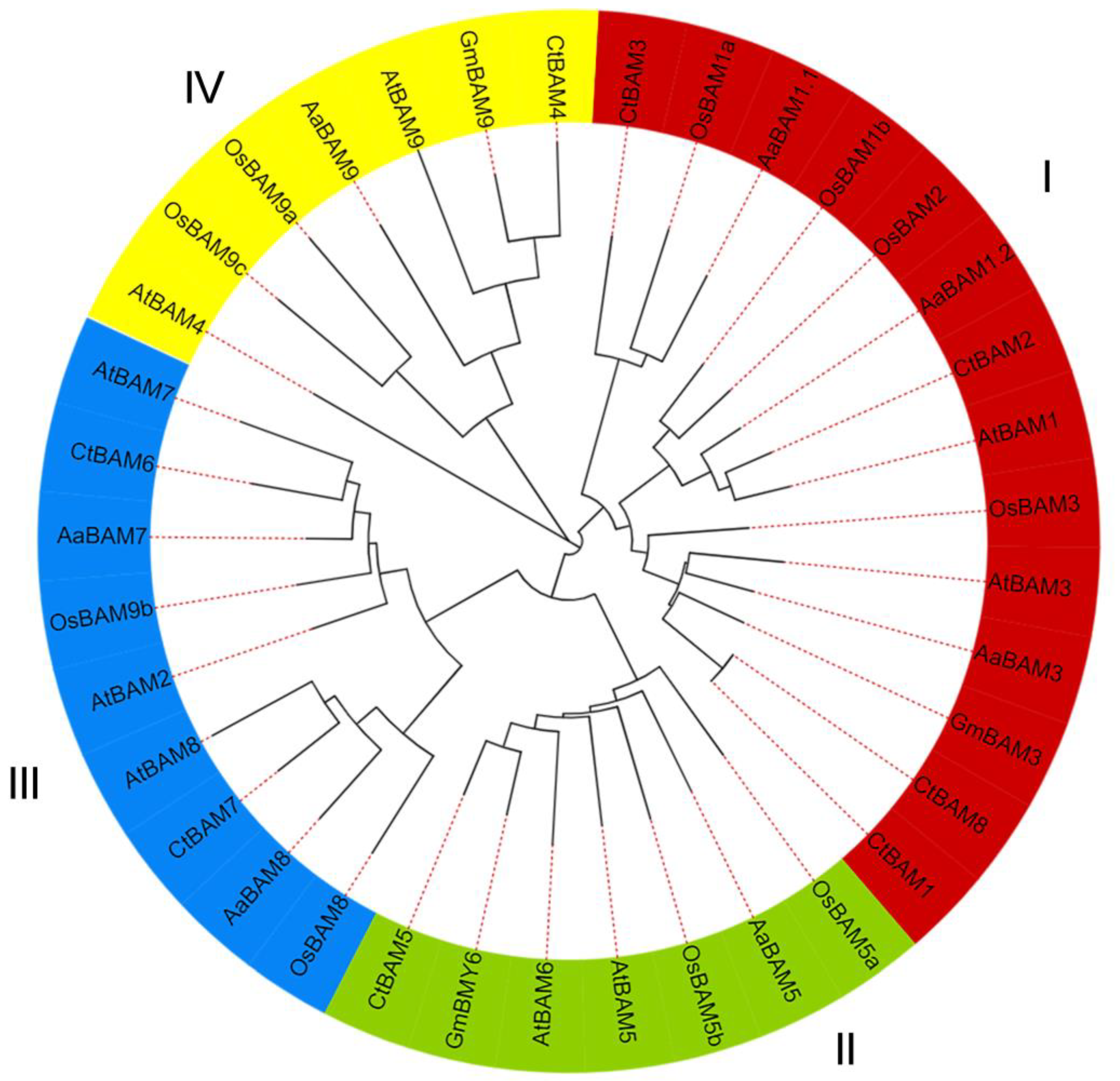
2.2. Multiple Sequence Alignment, Phylogenetic Analysis, and Classification of AaBAM Genes
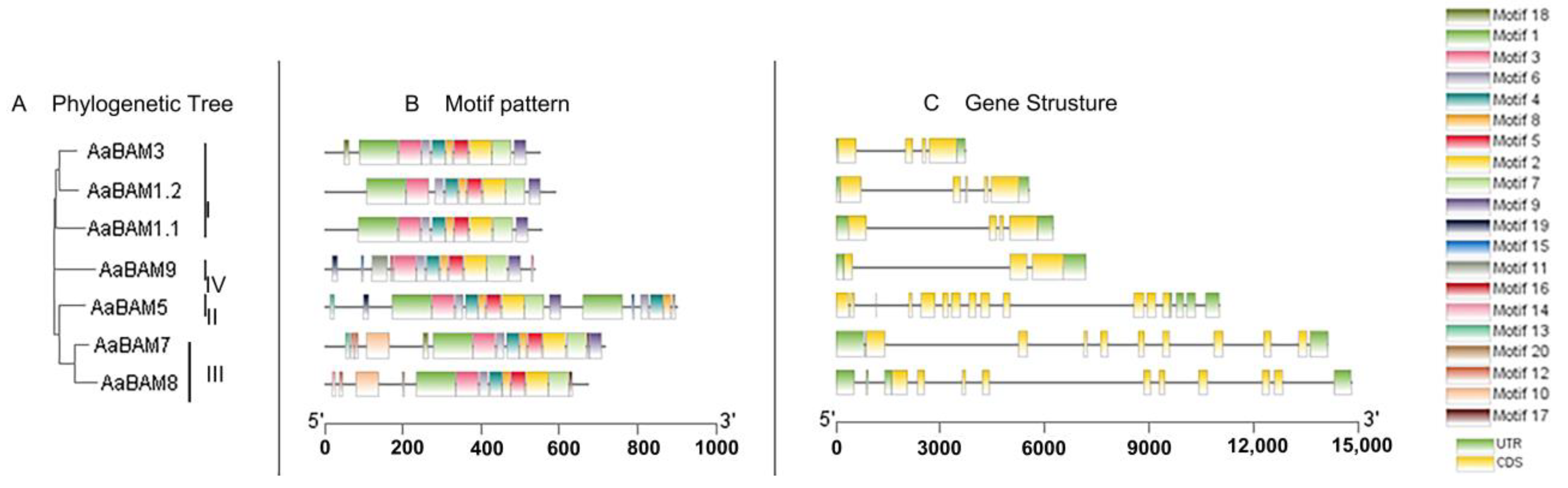
2.3. Gene Structure and Conserved Motif Analysis
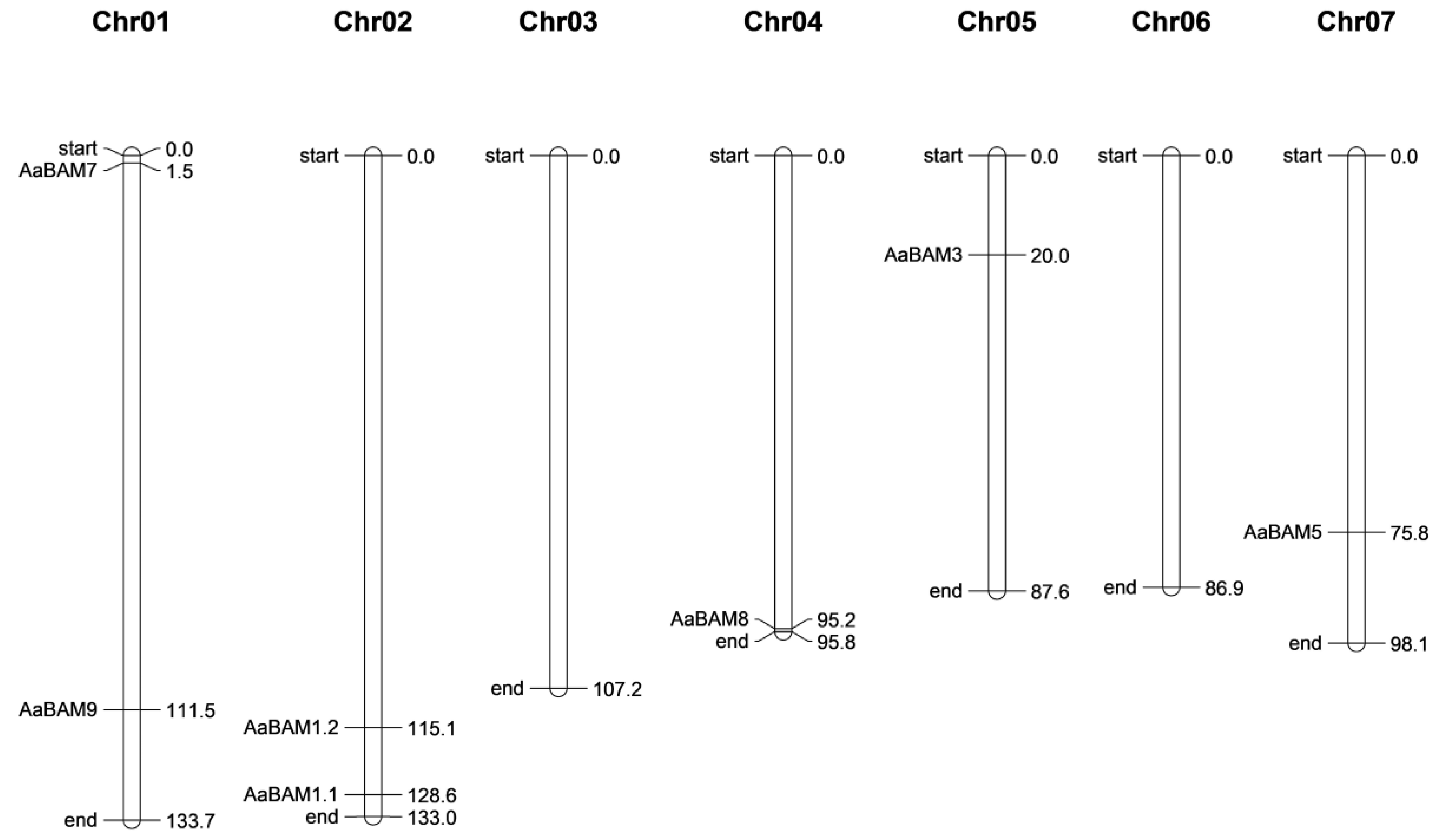
2.4. The Location and Synteny Relationship Analysis of BAM Genes
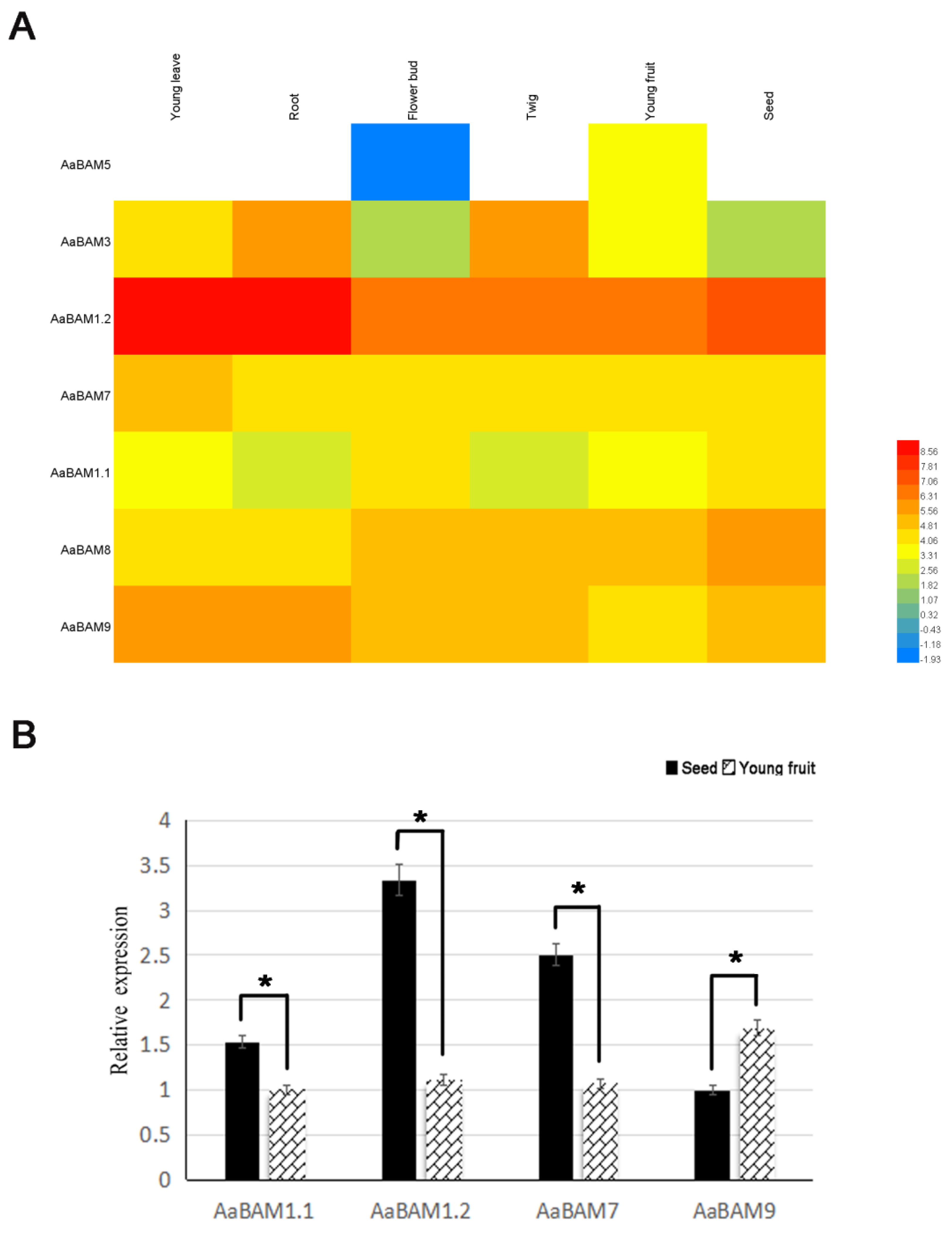
2.5. Expression Analysis of the AaBAM Genes with RNA-seq
2.6. Expression Patterns of Annona atemoya Genes in Response to Temperature Treaments
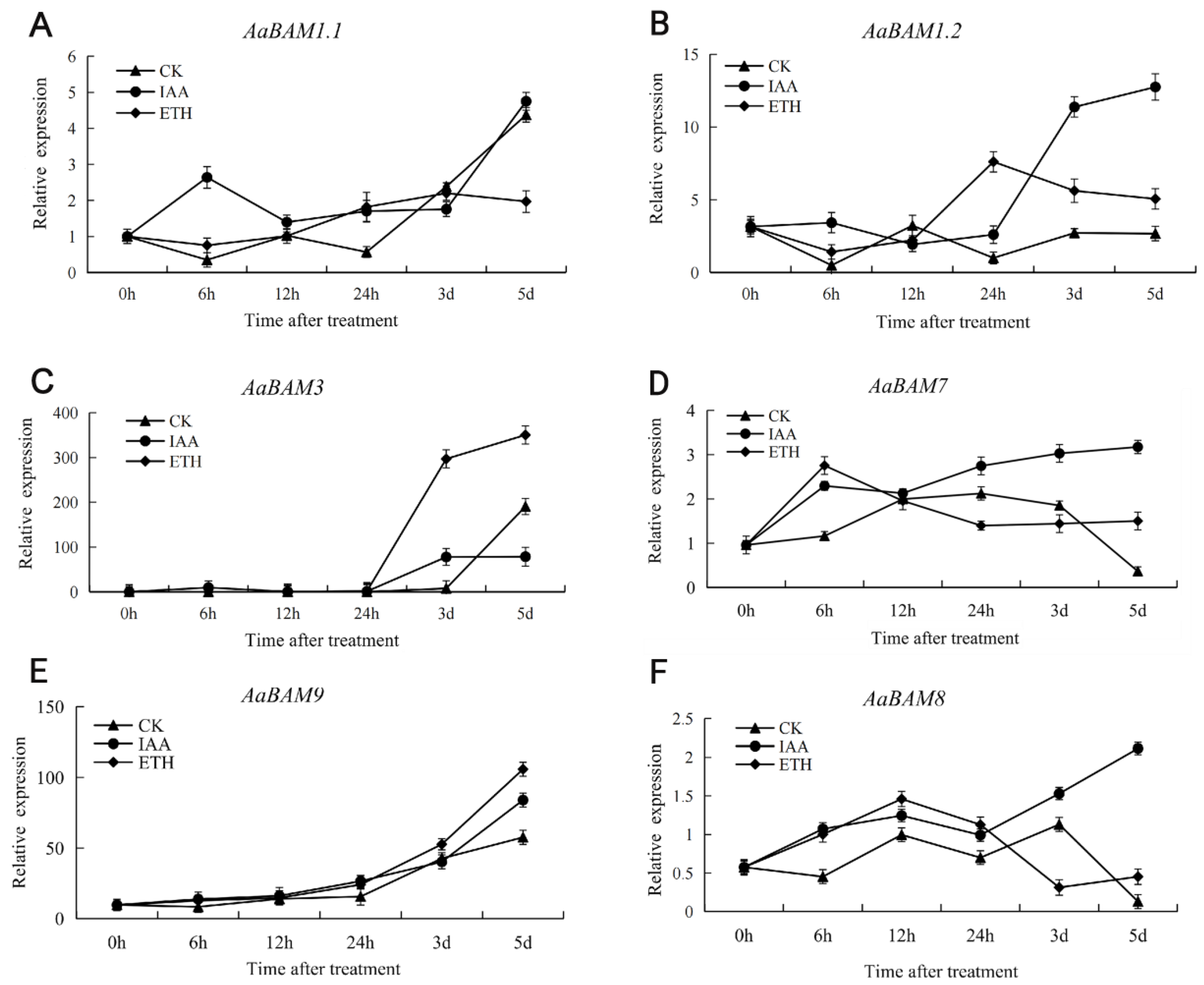
2.7. Expression Patterns of Annona atemoya Genes in Response to Ethylene and Auxin Treatments
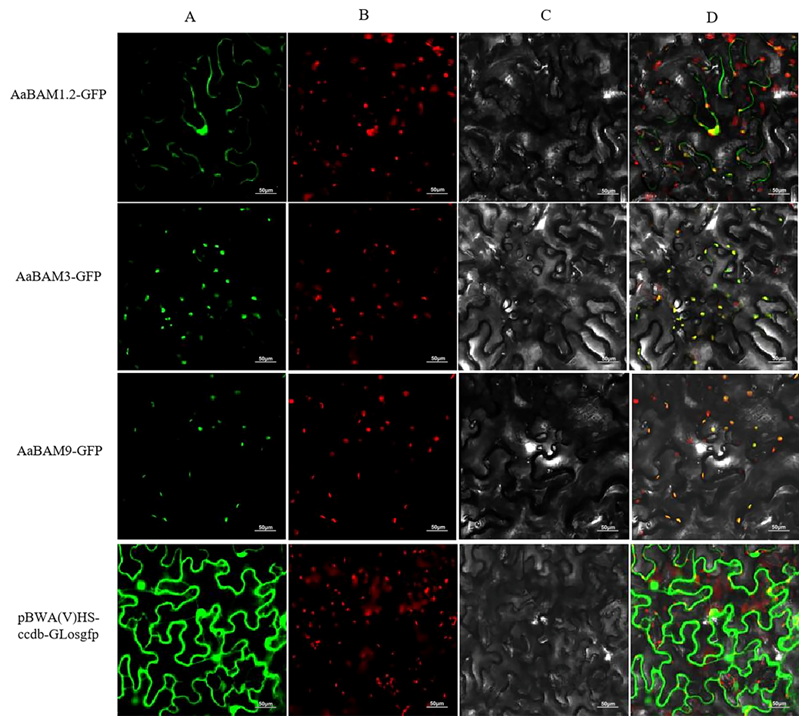
2.8. Subcellular Localization Analysis of AaBAM Protiens
3. Discussion
4. Materials and Methods
4.1. Gene Identification
4.2. Classification of AaBAM Genes
4.3. Gene Distribution, Structure, and Motif Analysis
4.4. Location of AaBAM Genes on Chromosomes
4.5. Expression Analysis of Annona atemoya BAM Genes in Four Tissues
4.6. Plant Materials and Treatments, RNA Extraction and Quantitative qRT-PCR Analysis
4.7. Vector Construction Subcellular Localization Analysis of AaBAM Genes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nag, M.; Lahiri, D.; Garai, S.; Mukherjee, D.; Ray, R.R. Regulation of beta-amylase synthesis: A brief overview. Mol. Biol. Rep. 2021, 48, 6503–6511. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.J. Enzymic Synthesis and Degradation of Starch and Glycogen. Adv. Carbohydr. Chem. 1963, 17, 371–430. [Google Scholar]
- Lombard, V.; Golaconda, R.H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrissat, B.; Callebaut, I.; Fabrega, S.; Lehn, P.; Mornon, J.P.; Davies, G. Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc. Natl. Acad. Sci. USA 1995, 92, 7090–7094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef]
- Thoma, J.A.; Koshland, D.E., Jr. Three amino acids at the active site of beta amylase. J. Mol. Biol. 1960, 2, 125–170. [Google Scholar] [CrossRef]
- Monroe, J.D.; Breault, J.S.; Pope, L.E.; Torres, C.E.; Gebrejesus, T.B.; Berndsen, C.E.; Storm, A.R. Arabidopsis β-Amylase2 Is a K+-Requiring, Catalytic Tetramer with Sigmoidal Kinetics. Plant Physiol. 2017, 175, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Fulton, D.C.; Stettler, M.; Mettler, T.; Vaughan, C.K.; Li, J.; Francisco, P.; Gil, M.; Reinhold, H.; Eicke, S.; Messerli, G.; et al. Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell 2008, 20, 1040–1058. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Storm, A.R. Review: The Arabidopsis beta-amylase (BAM) gene family: Diversity of form and function. Plant Sci. 2018, 276, 163–170. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Miao, Y.; Liu, J.; Zhang, J.; Jia, C.; Wang, J.; Wang, Z.; Jin, Z.; Xu, B. Genome-wide identification and expression analysis of the β-amylase genes strongly associated with fruit development, ripening, and abiotic stress response in two banana cultivars. Front. Agric. Sci. Eng. 2016, 3, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Scheidig, A.; Frohlich, A.; Schulze, S.; Lloyd, J.R.; Kossmann, J. Downregulation of a chloroplast-targeted beta-amylase leads to a starch-excess phenotype in leaves. Plant J. 2002, 30, 581–591. [Google Scholar] [CrossRef]
- Valerio, C.; Costa, A.; Marri, L.; Issakidis-Bourguet, E.; Pupillo, P.; Trost, P.; Sparla, F. Thioredoxin-regulated β-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J. Exp. Bot. 2011, 62, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. β-Amylase1 and β-Amylase3 Are Plastidic Starch Hydrolases in Arabidopsis that Seem to Be Adapted for Different Thermal, pH, and Stress Conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Huang, S.; Dong, T.; Yang, Q.; Yi, G. Analysis of resistant starch degradation in postharvest ripening of two banana cultivars: Focus on starch structure and amylases. Postharvest Biol. Technol. 2016, 119, 1–8. [Google Scholar] [CrossRef]
- Zhang, A.D.; Wang, W.Q.; Tong, Y.; Li, M.J.; Grierson, D.; Ferguson, I.; Chen, K.S.; Yin, X.R. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178, 850–863. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D. Involvement of five catalytically active Arabidopsis beta-amylases in leaf starch metabolism and plant growth. Plant Direct. 2020, 4, e00199. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005, 44, 730–743. [Google Scholar] [CrossRef]
- Flutsch, S.; Wang, Y.; Takemiya, A.; Vialet-Chabrand, S.; Klejchova, M.; Nigro, A.; Hills, A.; Lawson, T.; Blatt, M.R.; Santelia, D. Guard Cell Starch Degradation Yields Glucose for Rapid Stomatal Opening in Arabidopsis. Plant Cell 2020, 32, 2325–2344. [Google Scholar] [CrossRef]
- Fabio, Y.; Silva, M.L.H.D.; Azevedo, M.L.D. Effects of packaging and temperature on postharvest of atemoya. Rev. Bras. Frutic. 2002, 24, 658–660. [Google Scholar]
- Gutiérrez, M.; Del Mar Sola, M.; Pascual, L.; Vargas, A.M. Postharvest Changes of Sugar Concentrations in Chilled-Injured Cherimoya (Annona cherimola Mill.). J. Plant Physiol. 1994, 143, 27–32. [Google Scholar] [CrossRef]
- Rose, J.K.; Cosgrove, D.J.; Albersheim, P.; Darvill, A.G.; Bennett, A.B. Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol. 2000, 123, 1583–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannoni, J. Molecular Biology of Fruit Maturation and Ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Xiang, X.; Tang, J.; Wang, X. Nutritional functions of the funiculus in Brassica napus seed maturation revealed by transcriptome and dynamic metabolite profile analyses. Plant Mol. Biol. 2016, 92, 539–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niittyla, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A previously unknown maltose transporter essential for starch degradation in leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Horrer, D.; Flutsch, S.; Pazmino, D.; Matthews, J.S.; Thalmann, M.; Nigro, A.; Leonhardt, N.; Lawson, T.; Santelia, D. Blue Light Induces a Distinct Starch Degradation Pathway in Guard Cells for Stomatal Opening. Curr. Biol. 2016, 26, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Lling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Nelson, C.J.; Trosch, J.; Castleden, I.; Huang, S.; Millar, A.H. Protein Degradation Rate in Arabidopsis thaliana Leaf Growth and Development. Plant Cell 2017, 29, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Lao, N.T.; Schoneveld, O.; Mould, R.M.; Hibberd, J.M.; Gray, J.C.; Kavanagh, T.A. An Arabidopsis gene encoding a chloroplast-targeted beta-amylase. Plant J. 1999, 20, 519–527. [Google Scholar] [CrossRef]
- Caspar, T.; Lin, T.P.; Monroe, J.; Bernhard, W.; Spilatro, S.; Preiss, J.; Somerville, C. Altered regulation of beta-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc. Natl. Acad. Sci. USA 1989, 86, 5830–5833. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.P.; Spilatro, S.R.; Preiss, J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988, 86, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Mita, S.; Suzuki-Fujii, K.; Nakamura, K. Sugar-inducible expression of a gene for beta-amylase in Arabidopsis thaliana. Plant Physiol. 1995, 107, 895–904. [Google Scholar] [CrossRef]
- Nascimento, J.; Júnior, A.V.; Bassinello, P.Z.; Cordenunsi, B.R.; Mainardi, J.A.; Purgatto, E.; Lajolo, F.M. Beta-amylase expression and starch degradation during banana ripening. Postharvest Biol. Technol. 2006, 40, 41–47. [Google Scholar] [CrossRef]
- Purgatto, E.; Lajolo, F.M.; Nascimento, J.R.O.D.; Cordenunsi, B.R. Inhibition of beta-amylase activity, starch degradation and sucrose formation by indole-3-acetic acid during banana ripening. Planta 2001, 212, 823–828. [Google Scholar] [CrossRef]
- Wiberley-Bradford, A.E.; Busse, J.S.; Bethke, P.C. Temperature-dependent regulation of sugar metabolism in wild-type and low-invertase transgenic chipping potatoes during and after cooling for low-temperature storage. Postharvest Biol. Technol. 2016, 115, 60–71. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Liu, J.; Reid, S.; Liu, T.; Xu, S.; Tian, Z.; Sonnewald, U.; Song, B.; Xie, C. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot. 2017, 68, 2317–2331. [Google Scholar] [CrossRef] [Green Version]
- Broughton, W.J.; Tan, G. Storage conditions and ripening of the custard apple Annona squamosa L. Sci. Hortic-Amst. 1979, 10, 73–82. [Google Scholar] [CrossRef]
- Kim, T.W.; Wang, Z.Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Vafeados, D.; Tao, Y.; Yoshida, S.; Asami, T.; Chory, J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 2005, 120, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.; Wu, C.; Liu, H.; Hu, Z.; Sun, G. Cloning and Molecular Characterization of a SERK Gene Transcriptionally Induced During Somatic Embryogenesis in Ananas comosus cv. Shenwan. Plant Mol. Biol. Rep. 2012, 30, 195–203. [Google Scholar] [CrossRef]
- Wongs-Aree, C.; Noichinda, S. Sugar apple (Annona squamosa L.) and atemoya (A. cherimola Mill. × A. squamosa L.)—ScienceDirect. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 399–426. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jing, M.; Gu, S.; Li, D.; Dai, X.; Chen, Z.; Chen, J. Genome-Wide Investigation of BAM Gene Family in Annona atemoya: Evolution and Expression Network Profiles during Fruit Ripening. Int. J. Mol. Sci. 2023, 24, 10516. https://doi.org/10.3390/ijms241310516
Wang L, Jing M, Gu S, Li D, Dai X, Chen Z, Chen J. Genome-Wide Investigation of BAM Gene Family in Annona atemoya: Evolution and Expression Network Profiles during Fruit Ripening. International Journal of Molecular Sciences. 2023; 24(13):10516. https://doi.org/10.3390/ijms241310516
Chicago/Turabian StyleWang, Luli, Minmin Jing, Shuailei Gu, Dongliang Li, Xiaohong Dai, Zhihui Chen, and Jingjing Chen. 2023. "Genome-Wide Investigation of BAM Gene Family in Annona atemoya: Evolution and Expression Network Profiles during Fruit Ripening" International Journal of Molecular Sciences 24, no. 13: 10516. https://doi.org/10.3390/ijms241310516




