The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications
Abstract
1. Introduction
2. Subretinal Injection for Gene Therapy
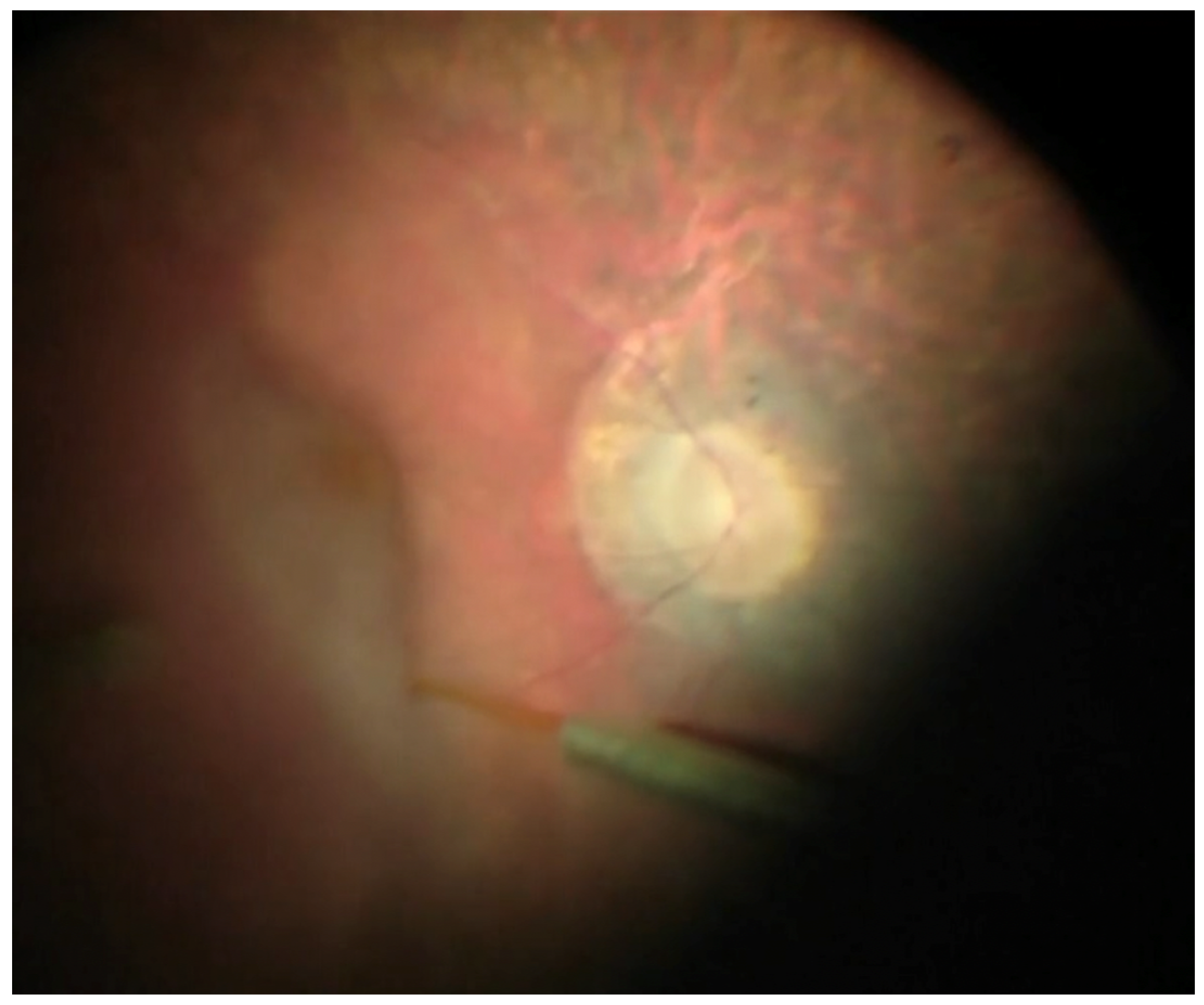
Surgical Technique
3. Subretinal Injection of tPA for Submacular Hemorrhage
Surgical Technique
4. Subretinal Injection in Full-Thickness Macular Hole Repair
Surgical Technique
5. Complications
Subretinal Injection for Gene Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Xue, K.; Jolly, J.K.; MacLaren, R.E. Structural and Functional Recovery Following Limited Iatrogenic Macular Detachment for Retinal Gene Therapy. JAMA Ophthalmol 2017, 135, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stanescu-Segall, D.; Balta, F.; Jackson, T.L. Submacular hemorrhage in neovascular age-related macular degeneration: A synthesis of the literature. Surv. Ophthalmol. 2016, 61, 18–32. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, J.B.; Kim, J.E.; Thordsen, J.; Dayani, P.; Ober, M.; Mahmoud, T.H. Pneumatic Displacement of Submacular Hemorrhage with Subretinal Air and Tissue Plasminogen Activator: Initial United States Experience. Ophthalmol Retin. 2018, 2, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.N.; Groenewald, C.; Wong, D. Treatment of retinal folds using a modified macula relocation technique with perfluorohexyloctane tamponade. Br. J. Ophthalmol. 2003, 87, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Rossi, T.; Borgia, A.; Catania, F.; Sorrentino, T.; Ferrara, M. Management of refractory and recurrent macular holes: A comprehensive review. Surv. Ophthalmol. 2022, 67, 908–931. [Google Scholar] [CrossRef]
- Fischer, M.D.; Hickey, D.G.; Singh, M.S.M.S.; MacLaren, R.R.E. Evaluation of an Optimized Injection System for Retinal Gene Therapy in Human Patients. Hum. Gene Ther. Methods 2016, 27, 150–158. [Google Scholar] [CrossRef]
- McClements, M.E.; MacLaren, R.E. Gene therapy for retinal disease. Transl. Res. 2013, 161, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.; Pennesi, M.E.; Birch, D.G.; Lam, B.L.; Tsang, S.H. Adeno-Associated Viral Gene Therapy for Inherited Retinal Disease. Pharm. Res. 2019, 36, 34. [Google Scholar] [CrossRef]
- Gupta, P.R.; Huckfeldt, R.M. Gene therapy for inherited retinal degenerations: Initial successes and future challenges. J. Neural Eng. 2017, 14, 051002. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003, 10, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ochakovski, G.A.; Bartz-Schmidt, K.U.; Fischer, M.D. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front. Neurosci. 2017, 11, 174. [Google Scholar] [CrossRef]
- Jalil, A.; Ivanova, T.; Moussa, G.; Parry, N.R.A.; Black, G.C.M. Retinal gene therapy in RPE-65 gene mediated inherited retinal dystrophy. Eye 2022, 37, 1874–1877. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of retinal gene therapy: Delivery of viral vector into the subretinal space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef]
- Davis, J.L.; Gregori, N.Z.; MacLaren, R.E.; Lam, B.L. Surgical Technique for Subretinal Gene Therapy in Humans with Inherited Retinal Degeneration. Retina 2019, 39, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.C.; Yannuzzi, N.A.; Patel, N.A.; Negron, C.I.; Sisk, R.A.; Nagiel, A.; Berrocal, A.M. Surgical Techniques for the Subretinal Delivery of Pediatric Gene Therapy. Ophthalmol. Retin. 2020, 4, 644–645. [Google Scholar] [CrossRef]
- Campa, C.; Gallenga, C.E.; Bolletta, E.; Perri, P. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr. Gene Ther. 2017, 17, 194–213. [Google Scholar] [CrossRef]
- Irigoyen, C.; Alonso, A.A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Vasconcelos, H.M.; Lujan, B.J.; Pennesi, M.E.; Yang, P.; Lauer, A.K. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int. J. Retin. Vitr. 2020, 6, 13. [Google Scholar] [CrossRef]
- Scruggs, B.A.; Vasconcelos, H.M.; da Palma, M.M.; Kogachi, K.; Pennesi, M.E.; Yang, P.; Bailey, S.T.; Lauer, A.K. Injection pressure levels for creating blebs during subretinal gene therapy. Gene Ther. 2021, 29, 601–607. [Google Scholar] [CrossRef]
- Olufsen, M.E.; Spindler, L.; Sørensen, N.B.; Christiansen, A.T.; Alberti, M.; Heegaard, S.; Kiilgaard, J.F. Controlled Subretinal Injection Pressure Prevents Damage in Pigs. Ophthalmologica 2022, 245, 285–294. [Google Scholar] [CrossRef]
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.-S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605. [Google Scholar] [CrossRef]
- Ducloyer, J.B.; Le Meur, G.; Lebranchu, P.; Billaud, F.; Weber, M. Macular Fold Complicating a Subretinal Injection of Voretigene Neparvovec. Ophthalmol. Retin. 2019, 4, 456–458. [Google Scholar] [CrossRef]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 846782. [Google Scholar] [CrossRef]
- Ladha, R.; Meenink, T.; Smit, J.; de Smet, M.D. Advantages of robotic assistance over a manual approach in simulated subretinal injections and its relevance for gene therapy. Gene Ther. 2021, 30, 264–270. [Google Scholar] [CrossRef]
- Yang, K.; Jin, X.; Wang, Z.; Fang, Y.; Li, Z.; Yang, Z.; Cong, J.; Yang, Y.; Huang, Y.; Wang, L. Robot-assisted subretinal injection system: Development and preliminary verification. BMC Ophthalmol. 2022, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.P.; Sato, Y.; Meyer, T.; Stoner, K.; Beltran, W.A. Surgical Procedure and Applicability of the Orbit Subretinal Delivery System (SDS)TM in the Normal Adult Canine Eye. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4118-F0355. [Google Scholar]
- Avery, R.L.; Fekrat, S.; Hawkins, B.S.; Bressler, N.M. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina 1996, 122, 763–764. [Google Scholar] [CrossRef]
- Lewis, H.; Resnick, S.C.; Flannery, J.G.; Straatsma, B.R. Tissue Plasminogen Activator Treatment of Experimental Subretinal Hemorrhage. Am. J. Ophthalmol. 1991, 111, 197–204. [Google Scholar] [CrossRef]
- Johnson, M.W.; Olsen, K.R.; Hernandez, E. Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Retina 1991, 11, 250–258. [Google Scholar] [CrossRef]
- Chew, G.W.M.; Ivanova, T.; Patton, N.; Dhawahir-Scala, F.; Jasani, K.M.; Turner, G.; Stephen, C.; Assad, J. Step-wise approach to the management of submacular hemorrhage using pneumatic displacement and vitrectomy the Manchester Protocol. Retina 2022, 42, 11–18. [Google Scholar] [PubMed]
- Haupert, C.L.; Mccuen, B.W.; Jaffe, G.J.; Steuer, E.R.; Cox, T.A.; Toth, C.A.; Fekrat, S.; Postel, E.A. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am. J. Ophthalmol. 2001, 131, 208–215. [Google Scholar] [CrossRef]
- Olivier, S.; Chow, D.R.; Packo, K.H.; MacCumber, M.W.; Awh, C.C. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degeneration. Ophthalmology 2004, 111, 1201–1208. [Google Scholar] [CrossRef]
- Peyman, G.A.; Blinder, K.J.; Paris, C.L.; Alturki, W.; Nelson, N.C.; Desai, U. A Technique for Retinal Pigment Epithelium Transplantation for Age-Related Macular Degeneration Secondary to Extensive Subfoveal Scarring. Ophthalmic Surg. 1991, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Drews-Botsch, C.; Sternberg, P.; Capone, A.; Aaberg, T.M. Submacular Hemorrhage Removal. Ophthalmology 1995, 102, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, A.P.; McAllister, I.L.; Constable, I.J. Initial clinical experience with tissue plasminogen activator (tPA) assisted removal of submacular haemorrhage. Eye 1995, 9, 582–588. [Google Scholar] [CrossRef]
- Martel, J.N.; Mahmoud, T.H. Subretinal Pneumatic Displacement of Subretinal Hemorrhage. JAMA Ophthalmol 2013, 131, 1632–1635. [Google Scholar] [CrossRef]
- Saito-Uchida, S.; Inoue, M.; Koto, T.; Kato, Y.; Hirakata, A. Vitrectomy combined with subretinal injection of tissue plasminogen activator for successful treatment of massive subretinal hemorrhage. Eur. J. Ophthalmol. 2021, 31, 2588–2595. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Manvikar, S.; Steel, D.H.W. Displacement of submacular hemorrhage associated with age-related macular degeneration using vitrectomy and submacular tPA injection followed by intravitreal ranibizumab. Clin. Ophthalmol. 2010, 4, 637–642. [Google Scholar] [CrossRef]
- Rickmann, A.; Paez, L.R.; Waizel, M.D.V.; Bisorca-Gassendorf, L.; Schulz, A.; Vandebroek, A.C.; Szurman, P.; Januschowski, K. Functional and structural outcome after vitrectomy combined with subretinal rtPA Injection with or without additional intravitreal Bevacizumab injection for submacular hemorrhages. PLoS ONE 2021, 16, e0250587. [Google Scholar] [CrossRef] [PubMed]
- Okanouchi, T.; Toshima, S.; Kimura, S.; Morizane, Y.; Shiraga, F. Novel Technique for Subretinal Injection Using Local Removal of the Internal Limiting Membrane. Retina 2016, 36, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Steel, D.H.; Geraghty, B.; Kearns, V.; Stanzel, B.; Wong, D. Subretinal injection under perfluorocarbon liquids to avoid foveal dehiscence. Retina 2021. [Google Scholar] [CrossRef]
- Wilkins, C.S.; Mehta, N.; Wu, C.Y.; Barash, A.; Deobhakta, A.A.; Rosen, R.B. Outcomes of pars plana vitrectomy with subretinal tissue plasminogen activator injection and pneumatic displacement of fovea-involving submacular haemorrhage. BMJ Open Ophthalmol. 2020, 5, e000394. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Edwards, T.L.; Meenink, T.C.; Beelen, M.J.; Naus, G.J.; de Smet, M.D.; MacLaren, R.E. First-in-Human Robot-Assisted Subretinal Drug Delivery Under Local Anesthesia. Am. J. Ophthalmol. 2022, 237, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Szurman, P.; Haritoglou, C.; Maier, M.; Wolf, A.; Lytvynchuk, L.; Priglinger, S.; Hillenkamp, J.; Wachtlin, J.; Becker, M.; et al. Application of subretinal fluid to close refractory full thickness macular holes: Treatment strategies and primary outcome: APOSTEL study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2151–2161. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E. Biomechanical characteristics of retina. Retina 2004, 24, 967–970. [Google Scholar] [CrossRef]
- Ferrara, M.; Lugano, G.; Sandinha, M.T.; Kearns, V.R.; Geraghty, B.; Steel, D.H.W. Biomechanical properties of retina and choroid: A comprehensive review of techniques and translational relevance. Eye 2021, 35, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Szurman, P.; Wakili, P.; Stanzel, B.V.; Siegel, R.; Boden, K.T.; Rickmann, A. Persistent macular holes—What is the best strategy for revision? Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1781–1790. [Google Scholar] [CrossRef]
- Frisina, R.; Tozzi, L.; Sabella, P.; Cacciatori, M.; Midena, E. Surgically Induced Macular Detachment for Treatment of Refractory Full-Thickness Macular Hole: Anatomical and Functional Results. Ophthalmologica 2019, 242, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Felfeli, T.; Mandelcorn, E.D. Macular hole hydrodissection: Surgical Technique for the Treatment of Persistent, Chronic, and Large Macular Holes. Retina 2019, 39, 743–752. [Google Scholar] [CrossRef]
- Meyer, C.H.; Borny, R.; Horchi, N. Subretinal fluid application to close a refractory full thickness macular hole. Int. J. Retin. Vitr. 2017, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Fotis, K.; Alexander, P.; Sax, J.; Reddie, I.; Kang, C.Y.; Chandra, A. Macular Detachment for the Treatment of Persistent Full-Thickness Macular Holes. Retina 2019, 39, S104–S107. [Google Scholar] [CrossRef] [PubMed]
- Gurelik, G.; Sul, S.; Kılıç, G.; Özsaygılı, C. A Modified Foveal Advancement Technique in the Treatment of Persistent Large Macular Holes. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 793–798. [Google Scholar] [CrossRef]
- Inoue, M.; Shiraga, F.; Shirakata, Y.; Morizane, Y.; Kimura, S.; Hirakata, A. Subretinal injection of recombinant tissue plasminogen activator for submacular hemorrhage associated with ruptured retinal arterial macroaneurysm. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1663–1669. [Google Scholar] [CrossRef]
- Price, K.W.; Pennesi, M.E.; Lauer, A.K.; Bailey, S.T. Iatrogenic choroidal neovascularization associated with subretinal gene therapy surgery. Am. J. Ophthalmol. Case Rep. 2022, 27, 101677. [Google Scholar] [CrossRef]
- Le Meur, G.; Lebranchu, P.; Billaud, F.; Adjali, O.; Schmitt, S.; Bézieau, S.; Péréon, Y.; Valabregue, R.; Ivan, C.; Darmon, C.; et al. Safety and Long-Term Efficacy of AAV4 Gene Therapy in Patients with RPE65 Leber Congenital Amaurosis. Mol. Ther. 2018, 26, 256–268. [Google Scholar] [CrossRef]
- Mühlfriedel, R.; Michalakis, S.; Garrido, M.G.; Sothilingam, V.; Schön, C.; Biel, M.; Seeliger, M.W. Optimized Subretinal Injection Technique for Gene Therapy Approaches. Methods Mol. Biol. 2019, 834, 405–412. [Google Scholar] [CrossRef]
- Sugita, S.; Mandai, M.; Kamao, H.; Takahashi, M. Immunological aspects of RPE cell transplantation. Prog. Retin. Eye Res. 2021, 84, 100950. [Google Scholar] [CrossRef] [PubMed]
- Gange, W.S.; Sisk, R.A.; Besirli, C.G.; Lee, T.C.; Havunjian, M.; Schwartz, H.; Borchert, M.; Sengillo, J.D.; Mendoza, C.; Berrocal, A.M.; et al. Perifoveal Chorioretinal Atrophy after Subretinal Voretigene Neparvovec-rzyl for RPE65-Mediated Leber Congenital Amaurosis. Ophthalmol. Retin. 2022, 6, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, R.; Zollet, P.; De Rosa, F.P.; Tsoutsanis, P.; Stravalaci, M.; Paulis, M.; Inforzato, A.; Romano, M.R. Where Are We with RPE Replacement Therapy? A Translational Review from the Ophthalmologist Perspective. Int. J. Mol. Sci. 2022, 23, 682. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell–Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Opthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef] [PubMed]



| Inherited Retinal Disease | Gene Therapy | Targeted Gene | National Clinical Trial Number | Trial Phase |
|---|---|---|---|---|
| Achromatopsia | AAV2-mediated | CNGA3 | NCT02935517 | I/II |
| AAV8-mediated | CNGA3 | NCT02610582 | I/II | |
| AAV2/8-mediated | CNGB3 or CNGA3 | NCT03278873 | I/II | |
| LCA type 2 | rAAV2-mediated | RPE 65 | NCT00481546 | I |
| AAV2-mediated (voretigene neparvovec-rzyl) * | RPE 65 | NCT01208389 | I/II | |
| NCT00999609 | III | |||
| NCT04516369 | III | |||
| NCT03602820 | IV | |||
| LCA type 10 | AA5-mediated | CEP290 | NCT03872479 | I/II |
| Retinitis Pigmentosa | AA2-mediated | RPGR | NCT03316560 | I/II |
| AA2/5-mediated | hPDE6B | NCT03328130 | I/II | |
| rAAV-mediated | hPDE6A | NCT04611503 | I/II | |
| AAV8-mediated | RLBP1 | NCT03374657 | I/II | |
| AAV2-mediated (voretigene neparvovec-rzyl) * | RPE65 | NCT00999609 | III | |
| NCT04516369 | III | |||
| NCT03602820 | IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripepi, D.; Jalil, A.; Ally, N.; Buzzi, M.; Moussa, G.; Rothschild, P.-R.; Rossi, T.; Ferrara, M.; Romano, M.R. The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications. Int. J. Mol. Sci. 2023, 24, 10535. https://doi.org/10.3390/ijms241310535
Tripepi D, Jalil A, Ally N, Buzzi M, Moussa G, Rothschild P-R, Rossi T, Ferrara M, Romano MR. The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications. International Journal of Molecular Sciences. 2023; 24(13):10535. https://doi.org/10.3390/ijms241310535
Chicago/Turabian StyleTripepi, Domenico, Assad Jalil, Naseer Ally, Matilde Buzzi, George Moussa, Pierre-Raphaël Rothschild, Tommaso Rossi, Mariantonia Ferrara, and Mario R. Romano. 2023. "The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications" International Journal of Molecular Sciences 24, no. 13: 10535. https://doi.org/10.3390/ijms241310535
APA StyleTripepi, D., Jalil, A., Ally, N., Buzzi, M., Moussa, G., Rothschild, P.-R., Rossi, T., Ferrara, M., & Romano, M. R. (2023). The Role of Subretinal Injection in Ophthalmic Surgery: Therapeutic Agent Delivery and Other Indications. International Journal of Molecular Sciences, 24(13), 10535. https://doi.org/10.3390/ijms241310535







