Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles
Abstract
1. Introduction
2. Results
2.1. Root Volatiles
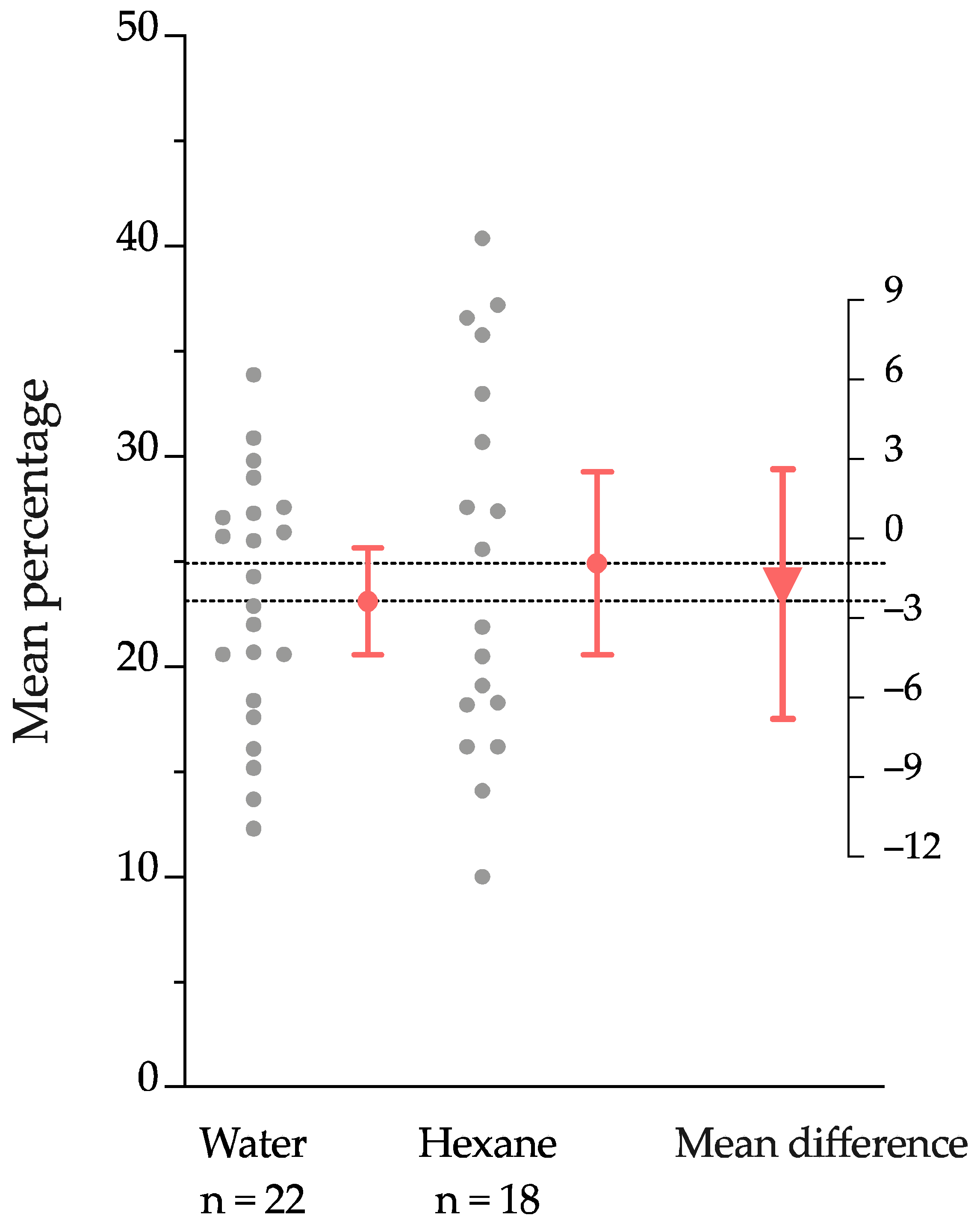
2.2. Nematode Response to Root Volatiles: Chemotaxis Assay
3. Discussion
4. Materials and Methods
4.1. Plants and Headspace Collection of Volatiles
4.2. Identification of the Volatile Organic Compounds (VOCs) Emitted by Roots
4.3. Culture of Entomopathogenic Nematodes (EPNs)
4.4. Odor Sources
4.5. Chemotaxis Assays Using Olfactometers
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Godfrey, A.J.R.; Potter, M.A.; Holopainen, J.K.; McCormick, A.C. Natural variation in volatile emissions of the invasive weed Calluna vulgaris in New Zealand. Plants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Köllner, T.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 2010, 36, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Erwin, A.C.; Halitschke, R.; Agrawal, A.A. Direct and indirect root defences of milkweed (Asclepias syriaca): Trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J. Ecol. 2011, 99, 16–25. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Bakhtiari, M.; Formenti, L.; Xiao, Z.; Defossez, E.; Rasmann, S. Biological control beneath the feet: A review of crop protection against insect root herbivores. Insects 2016, 7, 70–92. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Campos-Herrera, R.; Kaplan, F.; Duncan, L.W.; Rodriguez-Saona, C.; Koppenhöfer, A.M.; Stelinski, L.L. Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE 2012, 7, e38146. [Google Scholar] [CrossRef]
- Abraham, J.; Giacomuzzi, V.; Angeli, S. Root damage to apple plants by cockchafer larvae induces a change in volatile signals below- and above-ground. Entomol. Exp. Appl. 2015, 156, 279–289. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.G.; Erb, M. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef]
- Rasmann, S.; Turlings, T.C.J. First insights into specificity of belowground tritrophic interactions. Oikos 2008, 117, 362–369. [Google Scholar] [CrossRef]
- Gulati, S.; Ballhausen, M.B.; Kulkarni, P.; Grosch, R.; Garbeva, P. A non-invasive soil-based setup to study tomato root volatiles released by healthy and infected roots. Sci. Rep. 2020, 10, 12704. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Stelinski, L.L.; Willett, D.; Rivera, M.J.; Ali, J.G. ‘Tuning’ communication among four trophic levels of the root biome to facilitate biological control. Biol. Control 2019, 131, 49–53. [Google Scholar] [CrossRef]
- Rioja, T.; Ceballos, R.; Holuigue, L. Herbivore-induced plant volatiles emitted from avocado shoots infested by Oligonychus yothersi (Acari: Tetranychidae) increases the attraction of micro-coleopterans. Chil. J. Agric. Res. 2018, 78, 447–458. [Google Scholar] [CrossRef]
- Ceballos, R.; Fernandez, N.; Zuniga, S.; Zapata, N. Electrophysiological and behavioral responses of pea weevil Bruchus pisorum L. (Coleoptera: Bruchidae) to volatiles collected from its host Pisum sativum L. Chil. J. Agric. Res. 2015, 75, 202–209. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Hiltpold, I.; Rasmann, S. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 2012, 358, 51–60. [Google Scholar] [CrossRef]
- Rivera, M.J.; Rodriguez-Saona, C.; Alborn, H.T.; Koppenhöfer, A.M. Differential Response of a Local Population of Entomopathogenic Nematodes to Non-Native Herbivore Induced Plant Volatiles (HIPV) in the Laboratory and Field. J. Chem. Ecol. 2016, 42, 1259–1264. [Google Scholar] [CrossRef]
- Degenhardt, J.; Hiltpold, I.; Kollner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- De Lange, E.S.; Salamanca, J.; Polashock, J.; Rodriguez-Saona, C. Genotypic Variation and Phenotypic Plasticity in Gene Expression and Emissions of Herbivore-Induced Volatiles, and their Potential Tritrophic Implications, in Cranberries. J. Chem. Ecol. 2019, 45, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Polashock, J.; Malo, E.A. Jasmonate-mediated induced volatiles in the American cranberry, Vaccinium macrocarpon: From gene expression to organismal interactions. Front. Plant Sci. 2013, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, E. Insectos plaga de importancia económica asociados al arándano. In Boletín INIA N°263; Undurraga, P., Vargas, S., Eds.; Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2013; pp. 91–106. [Google Scholar]
- Luppichini, P.; France, A.; Urtubia, I.; Olivares, N.; Rodríguez, F. Manejo de Burrito de la vid, Naupactus xanthographus (Germar) y otros curculiónidos asociados a vides. In Boletín INIA N°260; Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2013; p. 81. [Google Scholar]
- France, A. Uso de nemátodos entomopatógenos para el control de insectos. In Boletín INIA N°260; Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2013; pp. 35–47. [Google Scholar]
- Navarro, P.D.; Palma-Millanao, R.; Ceballos, R.; Monje, A.J. Steinernema australe enhanced its efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) larvae in berry orchards after an artificial selection process. Agronomy 2022, 12, 1128. [Google Scholar] [CrossRef]
- Edgington, S.; Buddie, A.G.; Tymo, L.; Hunt, D.J.; Nguyen, K.B.; France, A.I.; Merino, L.M.; Moore, D. Steinernema australe n. sp. (panagrolaimomorpha: Steinernematidae), a new entomopathogenic nematode from isla magdalena, Chile. Nematology 2009, 11, 699–717. [Google Scholar] [CrossRef]
- Edgington, S.; Gowen, S.R. Ecological characterisation of Steinernema australe (Panagrolaimomorpha: Steinermatidae) an entomopathogenic nematode from Chile. Russ. J. Nematol. 2010, 18, 9–18. [Google Scholar]
- Forney, C.F.; Javorek, S.K.; Jordan, M.A.; Vander Kloet, S.P. Floral volatile composition of four species of Vaccinium. Botany 2012, 90, 365–371. [Google Scholar] [CrossRef]
- Rodriguez, M.; Gerding, M.; France, A.; Ceballos, R. Evaluation of Metarhizium anisopliae var. anisopliae Qu-M845 isolate to control Varroa destructor (Acari: Varroidae) in laboratory and field trials. Chil. J. Agric. Res. 2009, 69, 541–547. [Google Scholar]
- Rodriguez-Saona, C.; Parra, L.; Quiroz, A.; Isaacs, R. Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: Implications for flower visitation by bees. Ann. Bot. 2011, 107, 1377–1390. [Google Scholar] [CrossRef]
- Parra, L.; Mutis, A.; Ceballos, R.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Volatiles Released from Vaccinium corymbosum Were Attractive to Aegorhinus superciliosus (Coleoptera: Curculionidae) in an Olfactometric Bioassay. Environ. Entomol. 2009, 38, 781–789. [Google Scholar] [CrossRef]
- Horvat, R.J.; Schlotzhauer, W.S.; Chortyk, O.T.; Payne, J.A. Comparison of volatile compounds from rabbiteye blueberry (Vaccinium ashei) and deerberry (V. Stamineum) during maturation. J. Essent Oil Res. 1996, 8, 645–648. [Google Scholar] [CrossRef]
- Hall, I.V.; Forsyth, F.R.; Lightfoot, H.J. Volatiles from Developing Fruit of Vaccinium angustifolium* Contribution No. 1361. Can. Inst. Food Technol. J. 1970, 3, 1–3. [Google Scholar] [CrossRef]
- van Doan, C.; Züst, T.; Maurer, C.; Zhang, X.; Machado, R.A.R.; Mateo, P.; Ye, M.; Schimmel, B.C.J.; Glauser, G.; Robert, C.A.M. Volatile-mediated defence regulation occurs in maize leaves but not in maize root. Plant Cell Environ. 2020, 1–14. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Osbourn, A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019, 75, 2368–2377. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Willett, D.S.; Moino, A.; Pareja, M.; El Borai, F.; Dickson, D.W.; Stelinski, L.L.; Duncan, L.W. Stimulation of the salicylic acid pathway aboveground recruits entomopathogenic nematodes belowground. PLoS ONE 2016, 11, e0154712. [Google Scholar] [CrossRef]
- Filgueiras, C.C.; Willett, D.S.; Pereira, R.V.; Sabino, P.H.d.S.; Junior, A.M.; Pareja, M.; Dickson, D.W. Parameters affecting plant defense pathway mediated recruitment of entomopathogenic nematodes. Biocontrol Sci. Technol. 2017, 27, 833–843. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef]
- Xu, P.; Hooper, A.M.; Pickett, J.A.; Leal, W.S. Specificity Determinants of the Silkworm Moth Sex Pheromone. PLoS ONE 2012, 7, e44190. [Google Scholar] [CrossRef]
- Som, S.; Willett, D.S.; Alborn, H.T. Dynamics of belowground volatile diffusion and degradation. Rhizosphere 2017, 4, 70–74. [Google Scholar] [CrossRef]
- Rivera, M.J.; Martini, X.; Khrimian, A.; Stelinski, L. A weevil sex pheromone serves as an attractant for its entomopathogenic nematode predators. Chemoecology 2017, 27, 199–206. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Go, I.; Ul, O.; Ia, I.; Mu, E. Volatile Organic Constituents of Two Fractions of Leaves of Ficus vogelii. Nat. Prod. Chem. Res. 2018, 6, 1000344. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Hayata, Y. Optimum extraction method for volatile attractant compounds in cabbage to Pieris rapae. Biochem. Syst. Ecol. 2012, 40, 201–207. [Google Scholar] [CrossRef]
- Yasuda, T. Chemical cues from Spodoptera litura larvae elicit prey-locating behavior by the predatory stink bug, Eocanthecona furcellata. Entomol. Exp. Appl. 1997, 82, 349–354. [Google Scholar] [CrossRef]
- Fung, A.G.; Yamaguchi, M.S.; McCartney, M.M.; Aksenov, A.A.; Pasamontes, A.; Davis, C.E. SPME-based mobile field device for active sampling of volatiles. Microchem. J. 2019, 146, 407–413. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Eichholz, I.; Huyskens-keil, S.; Keller, A.; Ulrich, D.; Kroh, L.W.; Rohn, S. UV-B-induced changes of volatile metabolites and phenolic compounds in blueberries (Vaccinium corymbosum L.). Food Chem. 2011, 126, 60–64. [Google Scholar] [CrossRef]
- Nickavar, B.; Salehi-Sormagi, M.H.; Amin, G.; Daneshtalab, M. Steam Volatiles of Vaccinium arctostaphylos. Pharm. Biol. 2002, 40, 448–449. [Google Scholar] [CrossRef]
- Kelly, J.L.; Hagler, J.R.; Kaplan, I. Semiochemical lures reduce emigration and enhance pest control services in open-field predator augmentation. Biol. Control 2014, 71, 70–77. [Google Scholar] [CrossRef]
- Libbey, L.M.; Ryker, L.C.; Yandell, K.L. Laboratory and field studies of volatiles released by Dendroctonus ponderosae Hopkins (Coleoptera, Scolytidae). J. Appl. Entomol. 1985, 100, 381–392. [Google Scholar] [CrossRef]
- Bauske, E.M.; Rodriguez-Kabana, R.; Estaún, V.; Kloepper, J.W.; Robertson, D.G.; Weaver, C.F.; King, P.S. Management of Meloidogyne incognita on cotton by use of botanical aromatic compounds. Nematropica 1994, 24, 143–150. [Google Scholar]
- Weissteiner, S.; Huetteroth, W.; Kollmann, M.; Weißbecker, B.; Romani, R.; Schachtner, J.; Schütz, S. Cockchafer larvae smell host root scents in soil. PLoS ONE 2012, 7, e45827. [Google Scholar] [CrossRef] [PubMed]
- Lackus, N.D.; Lackner, S.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. The occurrence and formation of monoterpenes in herbivore-damaged poplar roots. Sci. Rep. 2018, 8, 17936. [Google Scholar] [CrossRef]
- Al-Nagar, N.M.A.; Abou-Taleb, H.K.; Shawir, M.S.; Abdelgaleil, S.A.M. Comparative toxicity, growth inhibitory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis (Boisd.). J. Asia-Pac. Entomol. 2020, 23, 67–75. [Google Scholar] [CrossRef]
- Farag, M.A.; Paré, P.W. C6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 2002, 61, 545–554. [Google Scholar] [CrossRef]
- De Backer, L.; Megido, R.C.; Fauconnier, M.L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-induced plant volatiles: Attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Morse, A.; Kevan, P.; Shipp, L.; Khosla, S.; McGarvey, B. The impact of greenhouse tomato (Solanales: Solanaceae) floral volatiles on bumble bee (Hymenoptera: Apidae) pollination. Environ. Entomol. 2012, 41, 855–864. [Google Scholar] [CrossRef]
- Bal, H.K.; Taylor, R.A.J.; Grewal, P.S. Ambush Foraging Entomopathogenic Nematodes Employ ‘Sprinters’ for Long-Distance Dispersal in the Absence of Hosts. J. Parasitol. 2014, 100, 422–432. [Google Scholar] [CrossRef]
- Willett, D.S.; Alborn, H.T.; Stelinski, L.L.; Shapiro-Ilan, D.I. Risk taking of educated nematodes. PLoS ONE 2018, 13, e0205804. [Google Scholar] [CrossRef]
- Willett, D.S.; Alborn, H.T.; Duncan, L.W.; Stelinski, L.L. Social Networks of Educated Nematodes. Sci. Rep. 2015, 5, 14388. [Google Scholar] [CrossRef] [PubMed]
- Willett, D.S.; Alborn, H.T.; Stelinski, L.L. Multitrophic effects of belowground parasitoid learning. Sci. Rep. 2017, 7, 2067. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, T.; Lee, G.; Choe, D.H.; Dillman, A.R. Host seeking parasitic nematodes use specific odors to assess host resources. Sci. Rep.-UK 2017, 7, 6270. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Zhang, Y.; Kang, W. Volatiles from Acer oliverianum leaves. Chem. Nat. Compd. 2014, 50, 931–932. [Google Scholar] [CrossRef]
- Villavicencio, J.D.; Zoffoli, J.P.; Plotto, A.; Contreras, C. Aroma compounds are responsible for an herbaceous off-flavor in the sweet cherry (Prunus avium L.) cv. regina during fruit development. Agronomy 2021, 11, 2020. [Google Scholar]
- Navarro, P.D.; McMullen, J.G.; Stock, S.P. Interactions between the entomopathogenic nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae) and the saprobic fungus Fusarium oxysporum (Ascomycota: Hypocreales). J. Invertebr. Pathol. 2014, 115, 41–47. [Google Scholar] [CrossRef]
- Stock, S.P.; Goodrich-Blair, H. Nematode parasites, pathogens and associates of insects and invertebrates of economic importance. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 373–426. [Google Scholar]
- Calin-Jageman, R.J.; Cumming, G. Estimation for Better Inference in Neuroscience. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Claridge-Chang, A.; Assam, P.N. Estimation statistics should replace significance testing. Nat. Methods 2016, 13, 108–109. [Google Scholar] [CrossRef]
- Ernst, M.D. Permutation methods: A basis for exact inference. Stat. Sci. 2004, 19, 676–685. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]



| Compound | Relative Abundance in Roots (%) | |
|---|---|---|
| Uninfested | Infested | |
| Terpenoids | ||
| 4,8-Dimethyl-1,7-nonadien-4-ol | 0.17 ± 0.12 | 0.08 ± 0.03 |
| 2-Carene | 17.78 ± 7.03 | 24.94 ± 12.16 |
| Limonene | 3.60 ± 2.63 | 4.35 ± 1.45 |
| Eucalyptol | 4.02 ± 1.25 | 3.00 ± 1.40 |
| Linalool | 2.06 ± 1.04 | 2.98 ± 0.23 |
| Myrcenol | 4.91 ± 2.06 | 4.19 ± 1.1 |
| cis-Myrtanol | 1.63 ± 1.45 | 2.05 ± 1.61 |
| α-Terpineol | 29.21 ± 12.38 | 30.44 ± 8.57 |
| Esters | ||
| Isobutyl Isobutyrate | 0.66 ± 0.35 | 0.63 ± 0.12 |
| Methyl salicylate | 17.89 ± 9.37 | 11.44 ± 5.53 |
| Vinyl sorbate | 0.86 ± 0.54 | 0.93 ± 0.11 |
| Aliphatic Hydrocarbons | ||
| 1-nonyne | 4.15 ± 2.63 | 2.53 ± 0.66 |
| 3-Ethenyl-1,4-pentadiene | 0.06 ± 0.03 | 0.08 ± 0.03 |
| Alcohols | ||
| 3-Butynol | 0.24 ± 0.04 | 0.24 ± 0.05 |
| 10-undecyn-1-ol | 5.21 ± 3.84 | 4.27 ± 3.54 |
| Ketones | ||
| 3-Octanone | 6.81 ± 6.16 | 7.11 ± 5.08 |
| 3-Hexanone | 0.29 ± 0.04 | 0.28 ± 0.02 |
| 2-Hydroxy-2,4-dimethyl-3-pentanone | 0.43 ± 0.23 | 0.44 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos, R.; Palma-Millanao, R.; Navarro, P.D.; Urzúa, J.; Alveal, J. Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles. Int. J. Mol. Sci. 2023, 24, 10536. https://doi.org/10.3390/ijms241310536
Ceballos R, Palma-Millanao R, Navarro PD, Urzúa J, Alveal J. Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles. International Journal of Molecular Sciences. 2023; 24(13):10536. https://doi.org/10.3390/ijms241310536
Chicago/Turabian StyleCeballos, Ricardo, Rubén Palma-Millanao, Patricia D. Navarro, Julio Urzúa, and Juan Alveal. 2023. "Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles" International Journal of Molecular Sciences 24, no. 13: 10536. https://doi.org/10.3390/ijms241310536
APA StyleCeballos, R., Palma-Millanao, R., Navarro, P. D., Urzúa, J., & Alveal, J. (2023). Positive Chemotaxis of the Entomopathogenic Nematode Steinernema australe (Panagrolaimorpha: Steinenematidae) towards High-Bush Blueberry (Vaccinium corymbosum) Root Volatiles. International Journal of Molecular Sciences, 24(13), 10536. https://doi.org/10.3390/ijms241310536






