Atheroma-Relevant 7-Oxysterols Differentially Upregulate Cd14 Expression
Abstract
1. Introduction
2. Results
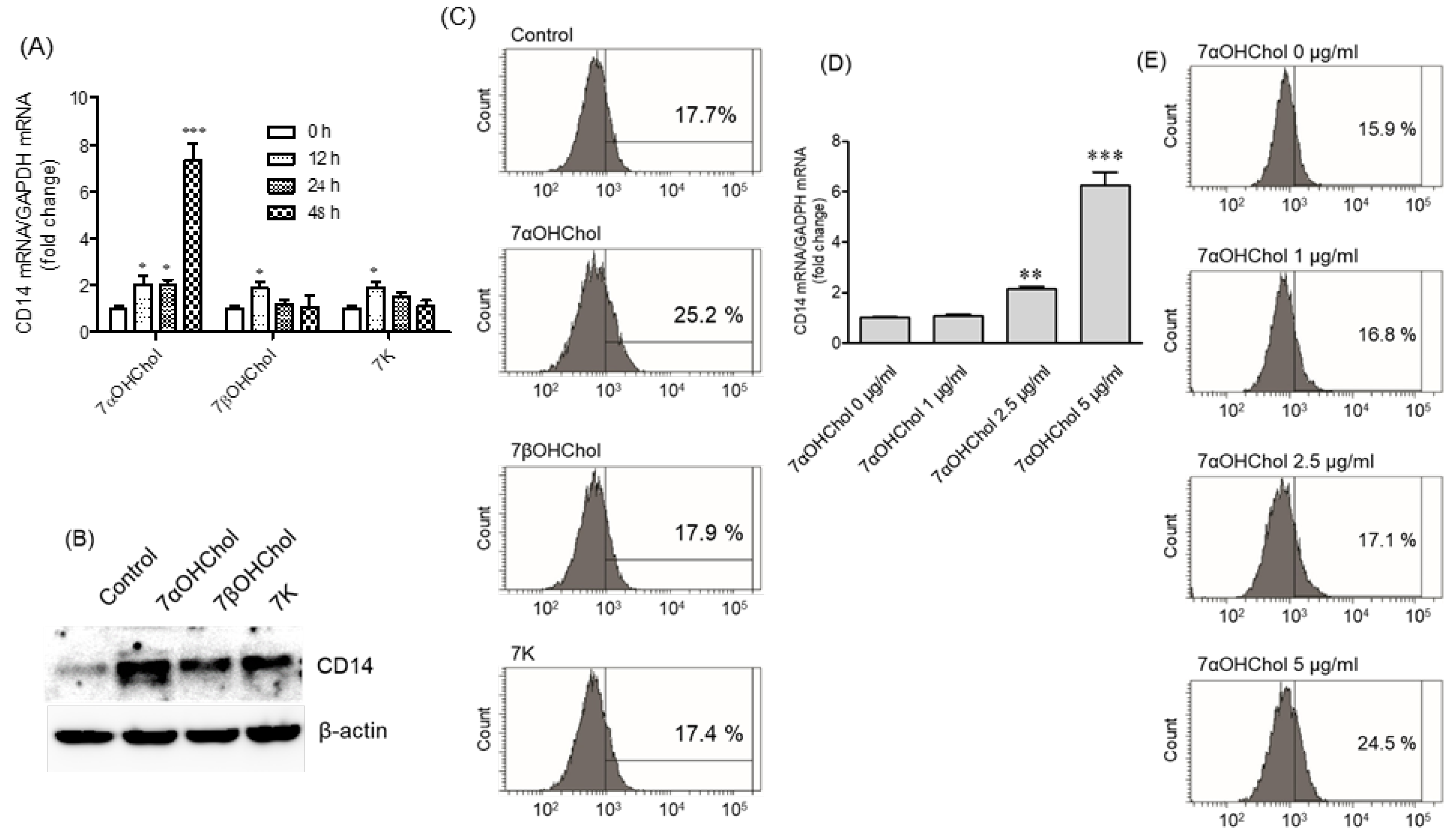
2.1. Differential Upregulation of CD14 in the Presence of 7-Oxysterols
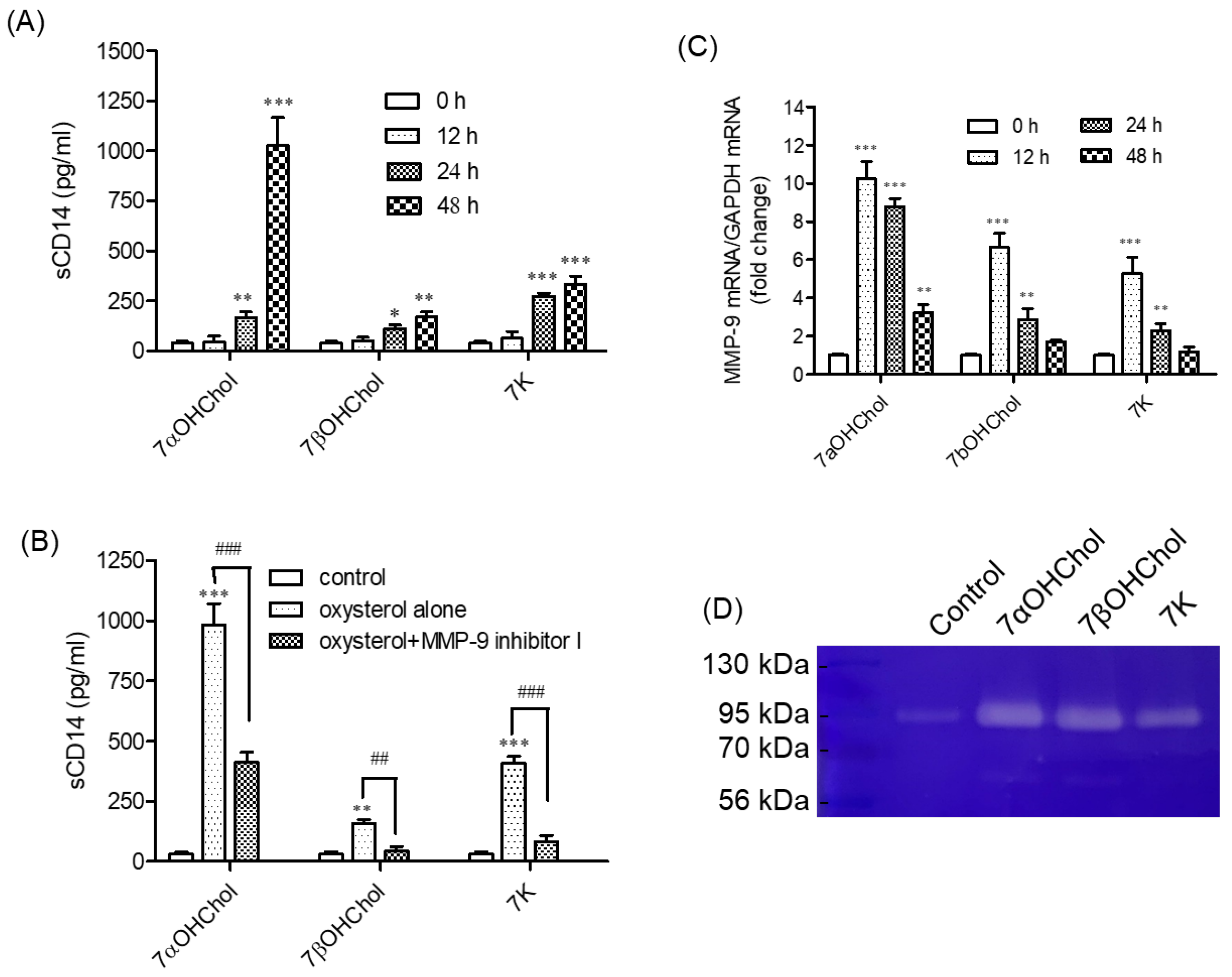
2.2. Involvement of MMP-9 in sCD14 Release following Incubation with 7-Oxysterols
2.3. Activation of MMP-9 Proteolytic Activity following Treatment with 7-Oxysterols
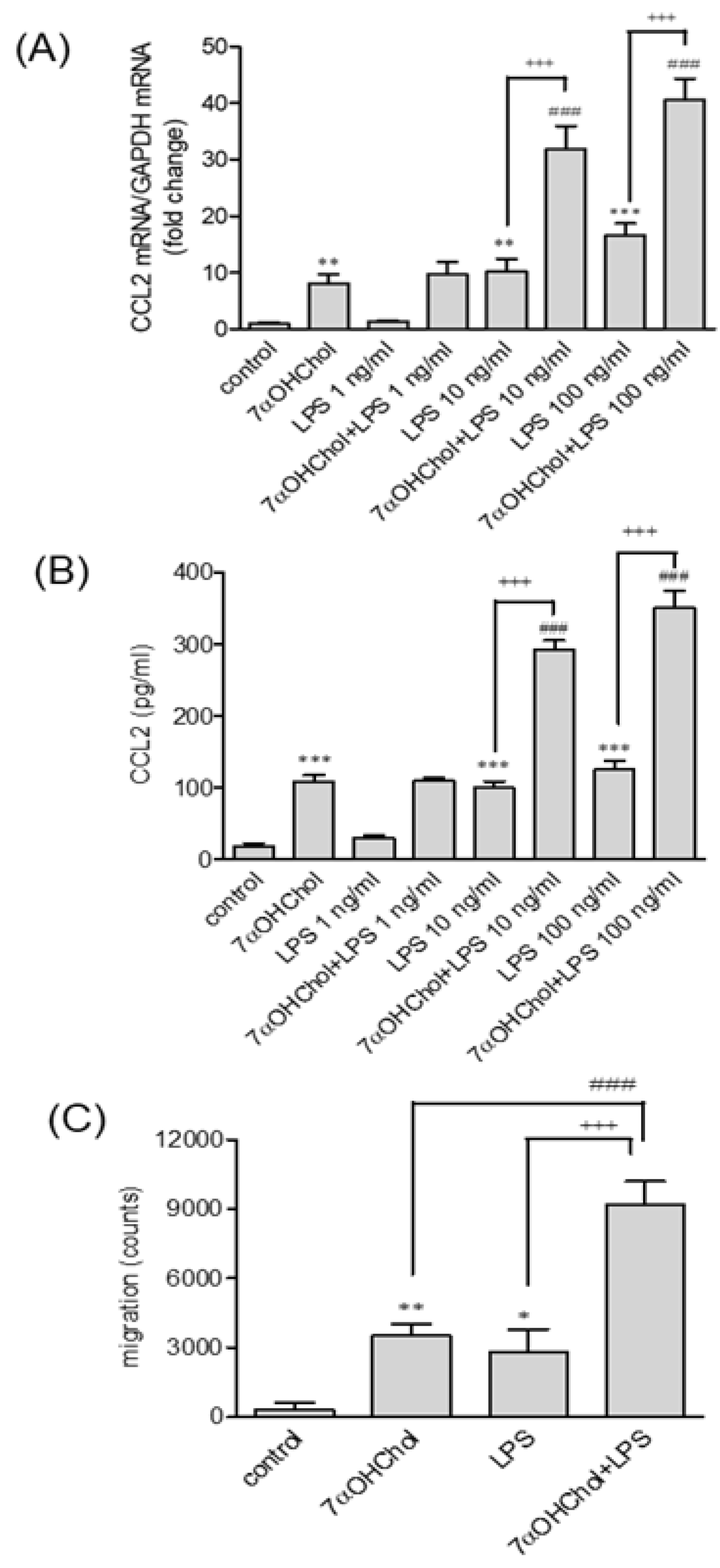
2.4. Enhanced Responses to LPS in the Presence of 7αOHChol
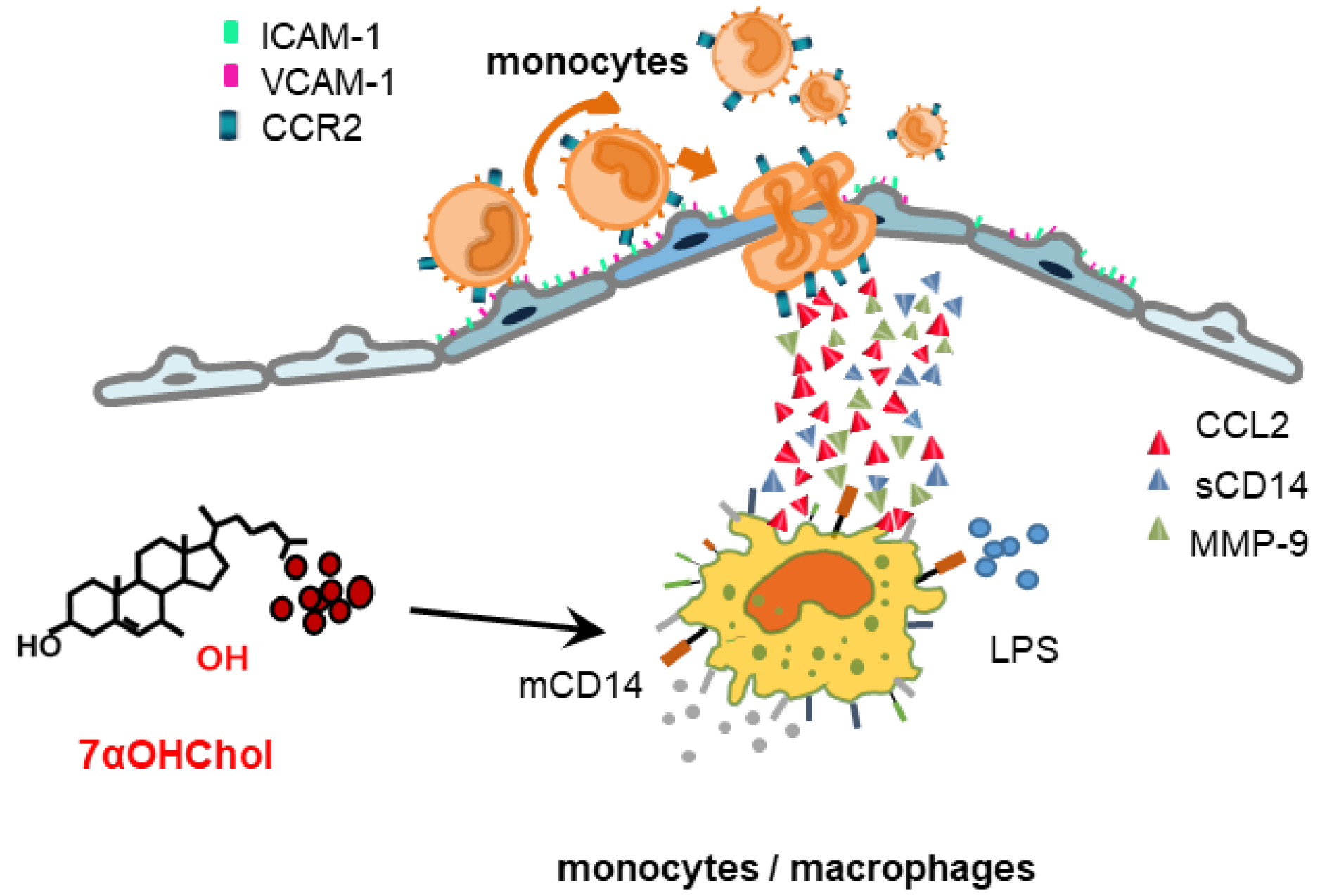
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Real-Time Polymerase Chain Reaction (qPCR)
4.3. Flow Cytometry
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Western Blot Analysis
4.6. Migration Assay
4.7. MMP-9 Gelatinolytic Activity in Cell Supernatants
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, G.J., Jr. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.J.; Ljunggren, S.A.; Karlsson, H.; Li, W.; Yuan, X.M. Exposure to atheroma-relevant 7-oxysterols causes proteomic alterations in cell death, cellular longevity, and lipid metabolism in THP-1 macrophages. PLoS ONE 2017, 12, e0174475. [Google Scholar] [CrossRef] [PubMed]
- Kandutsch, A.A.; Chen, H.W. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J. Biol. Chem. 1973, 248, 8408–8417. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.M.; Lee, S.A.; Eo, S.K.; Kim, K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem. Biophys. Res. Commun. 2013, 438, 161–168. [Google Scholar] [CrossRef]
- Hayden, J.M.; Brachova, L.; Higgins, K.; Obermiller, L.; Sevanian, A.; Khandrika, S.; Reaven, P.D. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid Res. 2002, 43, 26–35. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, B.Y.; Lee, S.A.; Eo, S.K.; Yun, Y.; Kim, C.D.; Kim, K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol. Appl. Pharmacol. 2014, 274, 462–470. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, B.-Y.; Son, Y.; Jung, Y.-S.; Eo, S.-K.; Park, Y.C.; Kim, K. 7alpha-Hydroxycholesterol induces inflammation by enhancing production of chemokine (C-C motif) ligand 2. Biochem. Biophys. Res. Commun. 2015, 467, 879–884. [Google Scholar] [CrossRef]
- Seo, H.C.; Kim, S.-M.; Eo, S.-K.; Rhim, B.-Y.; Kim, K. 7alpha-Hydroxycholesterol Elicits TLR6-Mediated Expression of IL-23 in Monocytic Cells. Biomol. Ther. 2015, 23, 84–89. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, H.; Ulevitch, R. CD14: Cell surface receptor and differentiation marker. Immunol. Today 1993, 14, 121–125. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Viriyakosol, S. Structure-function analysis of soluble and membrane-bound CD14. Prog. Clin. Biol. Res. 1998, 397, 79–87. [Google Scholar] [PubMed]
- Tapping, R.I.; Tobias, P.S. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem. Immunol. 2000, 74, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, R.L. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem. Immunol. 2000, 74, 61–82. [Google Scholar] [CrossRef]
- Miller, Y.I.; Viriyakosol, S.; Binder, C.J.; Feramisco, J.R.; Kirkland, T.N.; Witztum, J.L. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 2003, 278, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Ranoa, D.R.; Kelley, S.L.; Tapping, R.I. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J. Biol. Chem. 2013, 288, 9729–9741. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Hermansson, C.; Lundqvist, A.; Magnusson, L.U.; Ullstrom, C.; Bergstrom, G.; Hulten, L.M. Macrophage CD14 expression in human carotid plaques is associated with complicated lesions, correlates with thrombosis, and is reduced by angiotensin receptor blocker treatment. Int. Immunopharmacol. 2014, 22, 318–323. [Google Scholar] [CrossRef]
- Bjorkhem, I. Do oxysterols control cholesterol homeostasis? J. Clin. Investig. 2002, 110, 725–730. [Google Scholar] [CrossRef]
- Beekhuizen, H.; Blokland, I.; Corsel-van Tilburg, A.J.; Koning, F.; van Furth, R. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J. Immunol. 1991, 147, 3761–3767. [Google Scholar] [CrossRef]
- Frey, E.A.; Finlay, B.B. Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb. Pathog. 1998, 24, 101–109. [Google Scholar] [CrossRef]
- Heidenreich, S.; Schmidt, M.; August, C.; Cullen, P.; Rademaekers, A.; Pauels, H.G. Regulation of human monocyte apoptosis by the CD14 molecule. J. Immunol. 1997, 159, 3178–3188. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, B.Y.; Eo, S.K.; Kim, C.D.; Kim, K. 27-Hydroxycholesterol up-regulates CD14 and predisposes monocytic cells to superproduction of CCL2 in response to lipopolysaccharide. Biochim. Biophys. Acta 2015, 1852, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Orso, E. CD14 signalling in lipid rafts: New ligands and co-receptors. Curr. Opin. Lipidol. 2002, 13, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, M.; Jeune, K.S.-L.; Jouneau, S.; Moulis, S.; Desrues, B.; Belleguic, C.; Brinchault, G.; Le Trionnaire, S.; Gangneux, J.-P.; Dimanche-Boitrel, M.-T.; et al. Soluble CD14 acts as a DAMP in human macrophages: Origin and involvement in inflammatory cytokine/chemokine production. FASEB J. 2017, 31, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Vila, C.; Baroja-Mazo, A.; de Torre-Minguela, C.; Martínez, C.M.; Martínez-García, J.J.; Martínez-Banaclocha, H.; García-Palenciano, C.; Pelegrin, P. CD14 release induced by P2X7 receptor restricts inflammation and increases survival during sepsis. eLife 2020, 9, e60849. [Google Scholar] [CrossRef]
- Senft, A.P.; Korfhagen, T.R.; Whitsett, J.A.; Shapiro, S.D.; LeVine, A.M. Surfactant protein-D regulates soluble CD14 through matrix metalloproteinase-12. J. Immunol. 2005, 174, 4953–4959. [Google Scholar] [CrossRef]
- Pugin, J.; Heumann, D.; Tomasz, A.; Kravchenko, V.V.; Akamatsu, Y.; Nishijima, M.; Glauser, M.P.; Tobias, P.S.; Ulevitch, R.J. CD14 is a pattern recognition receptor. Immunity 1994, 1, 509–516. [Google Scholar] [CrossRef]
- Yu, B.; Hailman, E.; Wright, S.D. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J. Clin. Investig. 1997, 99, 315–324. [Google Scholar] [CrossRef]
- Ichise, Y.; Saegusa, J.; Tanaka-Natsui, S.; Naka, I.; Hayashi, S.; Kuroda, R.; Morinobu, A. Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4. Cells 2020, 9, 1689. [Google Scholar] [CrossRef]
- Choudhary, S.; Higgins, C.L.; Chen, I.Y.; Reardon, M.; Lawrie, G.; Vick, G.W., III; Karmonik, C.; Via, D.P.; Morrisett, J.D. Quantitation and localization of matrix metalloproteinases and their inhibitors in human carotid endarterectomy tissues. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2351–2358. [Google Scholar] [CrossRef]
- Sluijter, J.P.; Pulskens, W.P.; Schoneveld, A.H.; Velema, E.; Strijder, C.F.; Moll, F.; de Vries, J.-P.; Verheijen, J.; Hanemaaijer, R.; de Kleijn, D.P.; et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: A study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke 2006, 37, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.J.; Gomez, I.G.; Wille, P.T.; Raines, E.W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Investig. 2006, 116, 59–69. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Lijnen, R.; Baes, M.; Lemaître, V.; Tipping, P.; Drew, A.; Eeckhout, Y.; Shapiro, S.; Lupu, F.; et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 1997, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-Y.; Son, Y.; Lee, J.; Choi, J.; Kim, C.D.; Bae, S.S.; Eo, S.-K.; Kim, K. Dexamethasone inhibits activation of monocytes/macrophages in a milieu rich in 27-oxygenated cholesterol. PLoS ONE 2017, 12, e0189643. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-Y.; Son, Y.; Kim, B.J.; Chung, S.W.; Lee, D.; Eo, S.-K.; Kim, K. Atheroma-Relevant 7-Oxysterols Differentially Upregulate Cd14 Expression. Int. J. Mol. Sci. 2023, 24, 10542. https://doi.org/10.3390/ijms241310542
Kim B-Y, Son Y, Kim BJ, Chung SW, Lee D, Eo S-K, Kim K. Atheroma-Relevant 7-Oxysterols Differentially Upregulate Cd14 Expression. International Journal of Molecular Sciences. 2023; 24(13):10542. https://doi.org/10.3390/ijms241310542
Chicago/Turabian StyleKim, Bo-Young, Yonghae Son, Byoung Joon Kim, Sung Woon Chung, Dongjun Lee, Seong-Kug Eo, and Koanhoi Kim. 2023. "Atheroma-Relevant 7-Oxysterols Differentially Upregulate Cd14 Expression" International Journal of Molecular Sciences 24, no. 13: 10542. https://doi.org/10.3390/ijms241310542
APA StyleKim, B.-Y., Son, Y., Kim, B. J., Chung, S. W., Lee, D., Eo, S.-K., & Kim, K. (2023). Atheroma-Relevant 7-Oxysterols Differentially Upregulate Cd14 Expression. International Journal of Molecular Sciences, 24(13), 10542. https://doi.org/10.3390/ijms241310542





