Upconversion Nanoparticles Intercalated in Large Polymer Micelles for Tumor Imaging and Chemo/Photothermal Therapy
Abstract
:1. Introduction
2. Results and Discussion
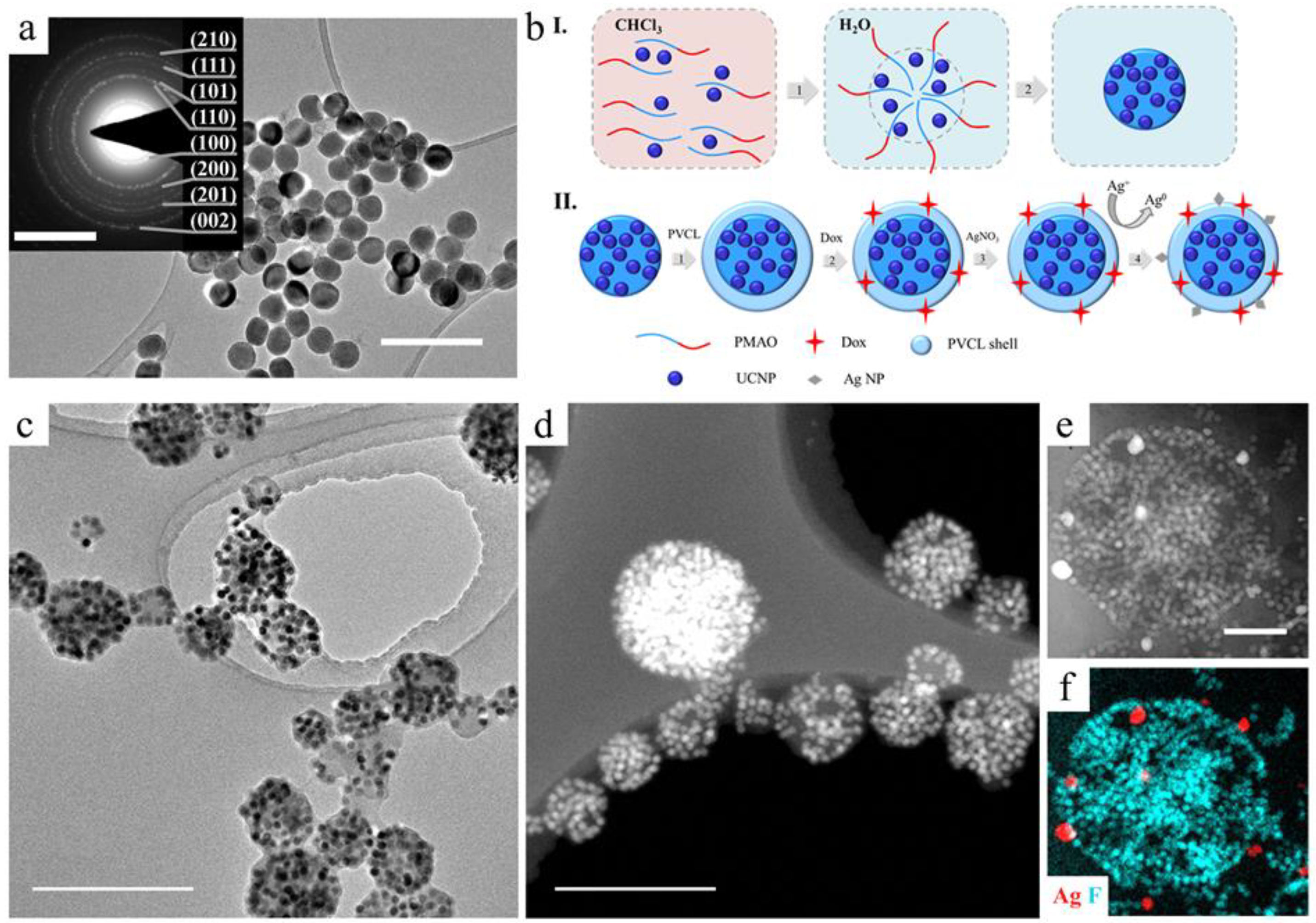
2.1. UCNP-Based Multifunctional Complex Formation
2.2. Complex Characterization
2.3. Cytotoxic Activity
2.4. Antitumor Effect on Xenografts
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. UCNP Intercalation in PMAO Micelles
3.2.2. UCNP–PMAO–PVCL Preparation
3.2.3. Doxorubicin Loading into UCNP–PMAO–PVCL
3.2.4. UCNP–PVCL Decoration with Ag NPs
3.2.5. Dynamic Light Scattering Measurement
3.2.6. Dox Release Study
3.2.7. Cytotoxicity Studies
3.2.8. Confocal Microscopy
3.2.9. Animals, Visualization, and Evaluation of Antitumor Activity
3.2.10. Histological Assays
3.2.11. Statistical Analysis
3.3. Key Insights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parodi, A.; Rudzinska, M.; Leporatti, S.; Anissimov, Y.; Zamyatnin, A.A. Smart nanotheranostics responsive to pathological stimuli. Front. Bioeng. Biotechnol. 2020, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for nanotheranostics: Tuning their properties according to disease needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, X.; Gu, Z.; Zhao, Y. Recent advances in upconversion nanoparticles-based multifunctional nanocomposites for combined cancer therapy. Adv. Mater. 2015, 27, 7692–7712. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. J. Control. Release 2010, 146, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G. Upconversion nanoparticles for cancer therapy. Adv. Nano Biomed. Res. 2022, 2, 2200092. [Google Scholar] [CrossRef]
- Lu, C.; Joulin, E.; Tang, H.; Pouri, H.; Zhang, J. Upconversion nanostructures applied in theranostic systems. Int. J. Mol. Sci. 2022, 23, 9003. [Google Scholar] [CrossRef]
- Generalova, A.N.; Chichkov, B.N.; Khaydukov, E. V Multicomponent nanocrystals with anti-Stokes luminescence as contrast agents for modern imaging techniques. Adv. Colloid. Interface Sci. 2017, 245, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Hemmer, E.; Marques-Hueso, J.; Rohani, S.; Lucchini, G.; Wang, M.; Zamani, R.R.; Roddatis, V.; Speghini, A.; Richards, B.S.; et al. Cubic versus hexagonal–phase size and morphology effects on the photoluminescence quantum yield of NaGdF4:Er3+/Yb3+ upconverting nanoparticles. Nanoscale 2022, 14, 1492–1504. [Google Scholar] [CrossRef]
- Rafique, R.; Kailasa, S.K.; Park, T.J. Recent advances of upconversion nanoparticles in theranostics and bioimaging applications. TrAC Trends Anal. Chem. 2019, 120, 115646. [Google Scholar] [CrossRef]
- Arnaoutakis, G.E.; Richards, B.S. Geometrical concentration for enhanced up-conversion: A review of recent results in energy and biomedical applications. Opt. Mater. 2018, 83, 47–54. [Google Scholar] [CrossRef]
- Mettenbrink, E.M.; Yang, W.; Wilhelm, S. Bioimaging with upconversion nanoparticles. Adv. Photonics Res. 2022, 3, 2200098. [Google Scholar] [CrossRef]
- Krylov, I.V.; Akasov, R.A.; Rocheva, V.V.; Sholina, N.V.; Khochenkov, D.A.; Nechaev, A.V.; Melnikova, N.V.; Dmitriev, A.A.; Ivanov, A.V.; Generalova, A.N.; et al. Local overheating of biotissue labeled with upconversion nanoparticles under Yb3+ resonance excitation. Front. Chem. 2020, 8, 295. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef]
- Fischer, S.; Kumar, D.; Hallermann, F.; von Plessen, G.; Goldschmidt, J.C. Enhanced upconversion quantum yield near spherical gold nanoparticles; A comprehensive simulation based analysis. Opt. Express 2016, 24, A460–A475. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Jin, R.; Su, Q. Preparation and applications of polymer-modified lanthanide-doped upconversion nanoparticles. Giant 2022, 12, 100130. [Google Scholar] [CrossRef]
- Generalova, A.N.; Oleinikov, V.A.; Khaydukov, E. V One-dimensional necklace-like assemblies of inorganic nanoparticles: Recent advances in design, preparation and applications. Adv. Colloid Interface Sci. 2021, 297, 102543. [Google Scholar] [CrossRef] [PubMed]
- Demina, P.A.; Sholina, N.V.; Akasov, R.A.; Khochenkov, D.A.; Arkharova, N.A.; Nechaev, A.V.; Khaydukov, E.V.; Generalova, A.N. A versatile platform for bioimaging based on colominic acid-decorated upconversion nanoparticles. Biomater. Sci. 2020, 8, 4570–4580. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Yang, C.-H.; Lee, R.-H.; Wang, T.-L. Dual stimuli-responsive block copolymers for controlled release triggered by upconversion luminescence or temperature variation. ACS Omega 2019, 4, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent progress and advances in stimuli-responsive polymers for cancer therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Liras, M.; González-Béjar, M.; Peinado, E.; Francés-Soriano, L.; Pérez-Prieto, J.; Quijada-Garrido, I.; García, O. Thin amphiphilic polymer-capped upconversion nanoparticles: Enhanced emission and thermoresponsive properties. Chem. Mater. 2014, 26, 4014–4022. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.; Wang, G. Micellar assembly of a photo- and temperature-responsive amphiphilic block copolymer for controlled release. Polym. Chem. 2015, 6, 7995–8002. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Kumar, D.V.; Kim, H.J.; Seok, Y.J.; Han, S.S. Dual responsive poly(vinyl caprolactam)-based nanogels for tunable intracellular doxorubicin delivery in cancer cells. Pharmaceutics 2022, 14, 852. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. The trojan horse goes wild: The effect of drug loading on the behavior of nanoparticles. Angew. Chem. Int. Ed. Engl. 2021, 60, 2202–2206. [Google Scholar] [CrossRef]
- Callari, M.; De Souza, P.L.; Rawal, A.; Stenzel, M.H. The effect of drug loading on micelle properties: Solid-state NMR as a tool to gain structural insight. Angew. Chem. Int. Ed. Engl. 2017, 56, 8441–8445. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, J.; Lu, M.; Garvey, C.J.; Stenzel, M.H. Correlation between drug loading content and biological activity: The complexity demonstrated in paclitaxel-loaded glycopolymer micelle system. Biomacromolecules 2019, 20, 1545–1554. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, release, and cytotoxicity of doxorubicin loaded in liposomes, micelles, and metal-organic frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.; Saboury, A.A.; Divsalar, A. The effects of silver nanoparticles and doxorubicin combination on DNA structure and its antiproliferative effect against T47D and MCF7 cell lines. J. Biomed. Nanotechnol. 2012, 8, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Alyatkin, S.; Asharchuk, I.; Khaydukov, K.; Nechaev, A.; Lebedev, O.; Vainer, Y.; Semchishen, V.; Khaydukov, E. The influence of energy migration on luminescence kinetics parameters in upconversion nanoparticles. Nanotechnology 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, M.V.; Doughan, S.; Han, Y.; Krull, U.J. Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: A review. Anal. Chim. Acta 2014, 832, 1–33. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhu, J. Encapsulation of inorganic nanoparticles into block copolymer micellar aggregates: Strategies and precise localization of nanoparticles. Polymer 2014, 55, 1079–1096. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Kisiel, A.; Kłucińska, K.; Głębicka, Z.; Gniadek, M.; Maksymiuk, K.; Michalska, A. Alternating polymer micelle nanospheres for optical sensing. Analyst 2014, 139, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Aleksandr B Zezin; Viktor A Kabanov A New class of complex water-soluble polyelectrolytes. Russ. Chem. Rev. 1982, 51, 833. [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L. V Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Fülöp, Z.; Gref, R.; Loftsson, T. A permeation method for detection of self-aggregation of doxorubicin in aqueous environment. Int. J. Pharm. 2013, 454, 559–561. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Lei, Z.; Liu, L.; Zhang, J.; You, H.; Zhang, H.; Wang, Z. Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv. Healthc. Mater. 2015, 4, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, M.; Chen, B.; Hu, B. Study on cytotoxicity, cellular uptake and elimination of rare-earth-doped upconversion nanoparticles in human hepatocellular carcinoma cells. Ecotoxicol. Environ. Saf. 2020, 203, 110951. [Google Scholar] [CrossRef] [PubMed]
- Guller, A.E.; Nadort, A.; Generalova, A.N.; Khaydukov, E.V.; Nechaev, A.V.; Kornienko, I.A.; Petersen, E.V.; Liang, L.; Shekhter, A.B.; Qian, Y.; et al. Rational Surface Design of Upconversion Nanoparticles with Polyethylenimine Coating for Biomedical Applications: Better Safe than Brighter? ACS Biomater. Sci. Eng. 2018, 4, 3143–3153. [Google Scholar] [CrossRef]
- Zhou, M.; Ge, X.; Ke, D.-M.; Tang, H.; Zhang, J.-Z.; Calvaresi, M.; Gao, B.; Sun, L.; Su, Q.; Wang, H. The Bioavailability, Biodistribution, and Toxic Effects of Silica-Coated Upconversion Nanoparticles in vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef]
- Bastos, V.; Oskoei, P.; Andresen, E.; Saleh, M.I.; Rühle, B.; Resch-Genger, U.; Oliveira, H. Stability, dissolution, and cytotoxicity of NaYF4-upconversion nanoparticles with different coatings. Sci. Rep. 2022, 12, 3770. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cai, Y.; Tu, Z.; Luo, J.; Qiao, X.; Chen, Q.; Zhang, W. Reducing the cytotoxicity while improving the anti-cancer activity of silver nanoparticles through α-tocopherol succinate modification. RSC Adv. 2015, 5, 82050–82055. [Google Scholar] [CrossRef]
- Devanesan, S.; Ponmurugan, K.; AlSalhi, M.S.; Al-Dhabi, N.A. Cytotoxic and antimicrobial efficacy of silver nanoparticles synthesized using a traditional phytoproduct, asafoetida gum. Int. J. Nanomed. 2020, 15, 4351–4362. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Cao, Y.; Li, D.; Ma, J.; Liu, P. DOX-loaded silver nanotriangles and photothermal therapy exert a synergistic antibreast cancer effect via ROS/ERK1/2 signaling pathway. Nanotechnology 2021, 33, ac378c. [Google Scholar] [CrossRef]
- Nicoletto, R.E.; Ofner, C.M. 3rd Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother. Pharmacol. 2022, 89, 285–311. [Google Scholar] [CrossRef]
- Generalova, A.N.; Kochneva, I.K.; Khaydukov, E.V.; Semchishen, V.A.; Guller, A.E.; Nechaev, A.V.; Shekhter, A.B.; Zubov, V.P.; Zvyagin, A.V.; Deyev, S.M. Submicron polyacrolein particles in situ embedded with upconversion nanoparticles for bioassay. Nanoscale 2015, 7, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, D.; Stenzel, M.H. Polymer-Functionalized Upconversion Nanoparticles for Light/Imaging-Guided Drug Delivery. Biomacromolecules 2021, 22, 3168–3201. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Fang, J.; Zhong, C.; Mu, R. The study of deposited silver particulate films by simple method for efficient SERS. Chem. Phys. Lett. 2005, 401, 271–275. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demina, P.A.; Khaydukov, K.V.; Babayeva, G.; Varaksa, P.O.; Atanova, A.V.; Stepanov, M.E.; Nikolaeva, M.E.; Krylov, I.V.; Evstratova, I.I.; Pokrovsky, V.S.; et al. Upconversion Nanoparticles Intercalated in Large Polymer Micelles for Tumor Imaging and Chemo/Photothermal Therapy. Int. J. Mol. Sci. 2023, 24, 10574. https://doi.org/10.3390/ijms241310574
Demina PA, Khaydukov KV, Babayeva G, Varaksa PO, Atanova AV, Stepanov ME, Nikolaeva ME, Krylov IV, Evstratova II, Pokrovsky VS, et al. Upconversion Nanoparticles Intercalated in Large Polymer Micelles for Tumor Imaging and Chemo/Photothermal Therapy. International Journal of Molecular Sciences. 2023; 24(13):10574. https://doi.org/10.3390/ijms241310574
Chicago/Turabian StyleDemina, Polina A., Kirill V. Khaydukov, Gulalek Babayeva, Pavel O. Varaksa, Alexandra V. Atanova, Maxim E. Stepanov, Maria E. Nikolaeva, Ivan V. Krylov, Irina I. Evstratova, Vadim S. Pokrovsky, and et al. 2023. "Upconversion Nanoparticles Intercalated in Large Polymer Micelles for Tumor Imaging and Chemo/Photothermal Therapy" International Journal of Molecular Sciences 24, no. 13: 10574. https://doi.org/10.3390/ijms241310574




