Abstract
Novel compounds with antidepressant activity via monoamine oxidase inhibition are being sought. Among these, derivatives of 3-n-butylphthalide, a neuroprotective lactone from Apiaceae plants, may be prominent candidates. This study aimed to obtain the oxidation products of 3-n-butylphthalide and screen them regarding their activity against the monoamine oxidase A (MAO-A) isoform. Such activity of these compounds has not been previously tested. To obtain the metabolites, we used fungi as biocatalysts because of their high oxidative capacity. Overall, 37 strains were used, among which Penicillium and Botrytis spp. were the most efficient, leading to the obtaining of three main products: 3-n-butyl-10-hydroxyphthalide, 3-n-butylphthalide-11-oic acid, and 3-n-butyl-11-hydroxyphthalide, with a total yield of 0.38–0.82 g per g of the substrate, depending on the biocatalyst used. The precursor–3-n-butylphthalide and abovementioned metabolites inhibited the MAO-A enzyme; the most active was the carboxylic acid derivative of the lactone with inhibitory constant (Ki) < 0.001 µmol/L. The in silico prediction of the drug-likeness of the metabolites matches the assumptions of Lipinski, Ghose, Veber, Egan, and Muegge. All the compounds are within the optimal range for the lipophilicity value, which is connected to adequate permeability and solubility.
1. Introduction
Depression is a disease with many faces that affects people of all ages. There are several hypotheses regarding its etiology [1]. The most described and valid theory in the literature is the monoamine hypothesis, which assumes that depression is caused by the dysfunction of serotonergic (5-HT) and noradrenergic pathways [2]. In addition to reduced levels of 5-HT, dopamine, and norepinephrine in various areas of the brain (e.g., hippocampus, amygdala), there may be a genetic mutation of the 5-HT transporter and the amino oxidase (MAO) enzyme, which is associated with gene polymorphism and passed from mother to child [2,3]. This disease can affect people with dementia and those on the autistic spectrum or with bipolar diseases [1,2,3,4,5]. Monoamine oxidase belongs to the family of flavoenzymes located in the mitochondrial membrane [6]. There are two isoforms, MAO-A and MAO-B, which differ in affinity to the substrate and susceptibility to inhibition [7]. Generally, the enzymes are responsible for the deamination of neurotransmitters [6,8]. MAO-A is the main enzyme that deaminates 5-HT in the brain, affecting behavioral disorders [9]. Almost all MAO-A inhibitors on the market act irreversibly and require dietary restrictions to avoid tyramine accumulation, leading to serotonin toxicity syndrome [7]. The only reversible commercially available drug that regulates MAO is moclobemide [7,10]. Therefore, there is a necessity for novel, non-toxic compounds that would regulate the level of serotonin in the human body.
The compound 3-n-butylphthalide is a biologically active lactone found in plants from the celery family [11,12]. Originally an ingredient in traditional Chinese medicine, it was accepted in China as a drug for cerebral ischemic stroke that improves the neurological function of the patients [13,14,15]. The compound restores microcirculation and protects the mitochondria. It shows vasodilatory, antiplatelet, and anti-inflammatory activity [15,16,17]. The antidepressant properties of 3-n-butylphthalide have also been studied [18]. The available literature outlines the positive effect of 3-n-butylphthalide in lipopolysaccharide (LPS)-induced depression [19] among chronic-stressed rats by the alteration of the serotonergic system, BDNF-ERK-mTOR signaling [18], and other mechanisms [20].
According to the research conducted by Diao et al. [21], the lactone in the human body is rapidly metabolized to 23 compounds, and mainly hydroxyderivatives and glucosidation products are observed. Although 3-n-butylphthalide has been widely tested regarding its biological activity, information on the monoamine oxidase A (MAO-A)-inhibiting properties of its metabolites is lacking. Moreover, other biological activities of these phthalide metabolites remain undiscovered, as obtaining them in sufficient amounts for research often poses a challenge. Biotransformation using whole fungal cells may provide a solution to this issue. Filamentous fungi feature many CYP isoenzymes and are, therefore, particularly known for their oxidative capacity. As a result, these biocatalysts lead to the formation of aliphatic and aromatic hydroxy derivatives [22]. Fungal-mediated biotransformations have been exploited by Diao et al. [21] for 3-n-butylphthalide and by us for its derivative with an unsaturated bond in the side chain [23].
Thus, this study aimed to obtain human metabolites of 3-n-butylphthalide through biotransformation by whole cells of filamentous fungi. After obtaining the oxidation products, we tested them in terms of their MAO-A enzyme-inhibiting properties. In addition, selected pharmacokinetic parameters were investigated using bioinformatics tools to assess the suitability of the compounds as potential antidepressant drugs.
2. Results
2.1. Chemical Synthesis of 3-n-butylphthalide (1) and Biotransformations
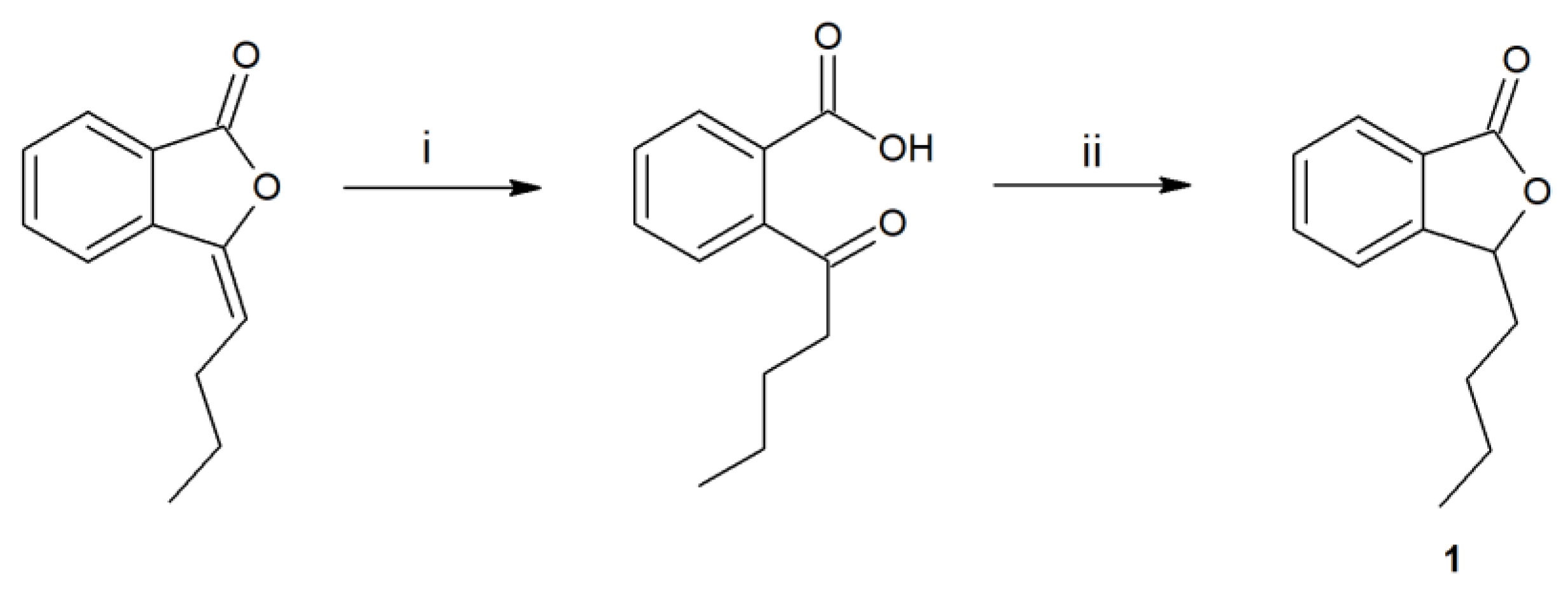
The first step of the research was the synthesis and purification of substrate 1, obtained in a two-step process (Figure 1).
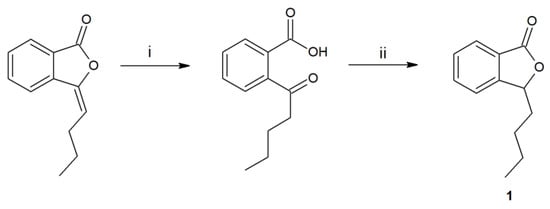
Figure 1.
Synthesis of 3-n-butylphthalide (1) by the hydrolysis of 3-n-butylidenephthalide to ketoacid (i-KOH in MeOH), its reduction by borohydride, and acidification (ii-THF, NaBH4, HCl).
After obtaining the 3-n-butylphthalide (1), the screening of whole fungal cells was carried out. We searched for biocatalysts capable of efficiently converting 3-n-butylphthalide (1) to oxidation products. During the biotransformation processes, we collected samples 4, 8, and 12 days after adding the substrate. After extraction with ethyl acetate, we performed high-performance liquid chromatography-diode array detector (HPLC-DAD) analyses.
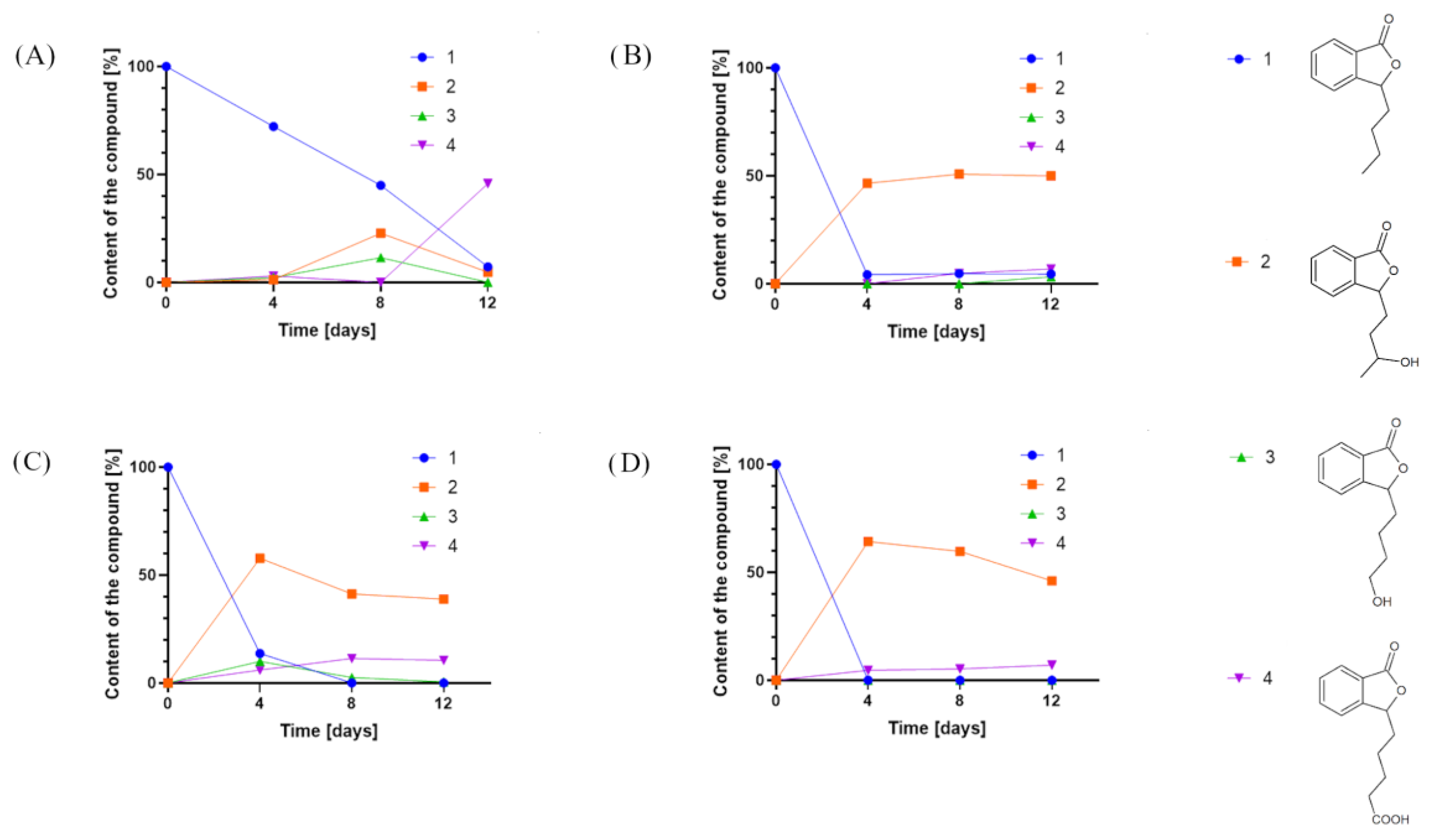
Overall, we tested 37 fungal strains, and 12 (Fusarium culmorum AM7, Sclerophoma pythiopila AM55, Spicaria fusispora AM136, Beauveria bassiana AM278, Pleurotus ostreatus AM482, Aspergillus niger strains KKP45, KKP423, KKP424, Aspergillus flavus KKP686, KKP689, Phanerochaete chrysosporium KKP784, and Trichoderma lignorum KKP786) did not transform the substrate. In the other biotransformation processes, we observed products formed from 3-n-butylphthalide (1) (Figure 2).
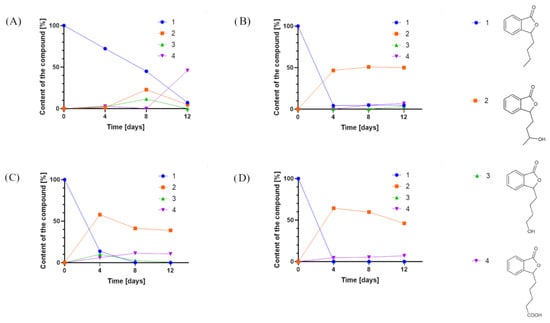
Figure 2.
Product content of 3-n-butylphthalide (1) biotransformations catalyzed by (A) Penicillium dierckxii AM32, (B) Penicillium sp. AM91, (C) Botrytis cinerea AM235, and (D) Botrytis sp. KKP3292, determined by an HPLC-DAD at a wavelength of 274 nm.
In most cases, the application of whole cells of fungi resulted in product 2 as the predominant product. Interestingly, during the biotransformations catalyzed by Penicillium dierckxii AM32, Botrytis sp. KKP3292, and Botrytis cinerea AM235, we observed a decrease in content during the biotransformation. Product 3 was observed using Penicillium dierckxii AM32, Penicillium sp. AM91, and Botrytis cinerea AM235. An almost total conversion of the starting substrate (1) was noted using both Botrytis strains as the catalysts.
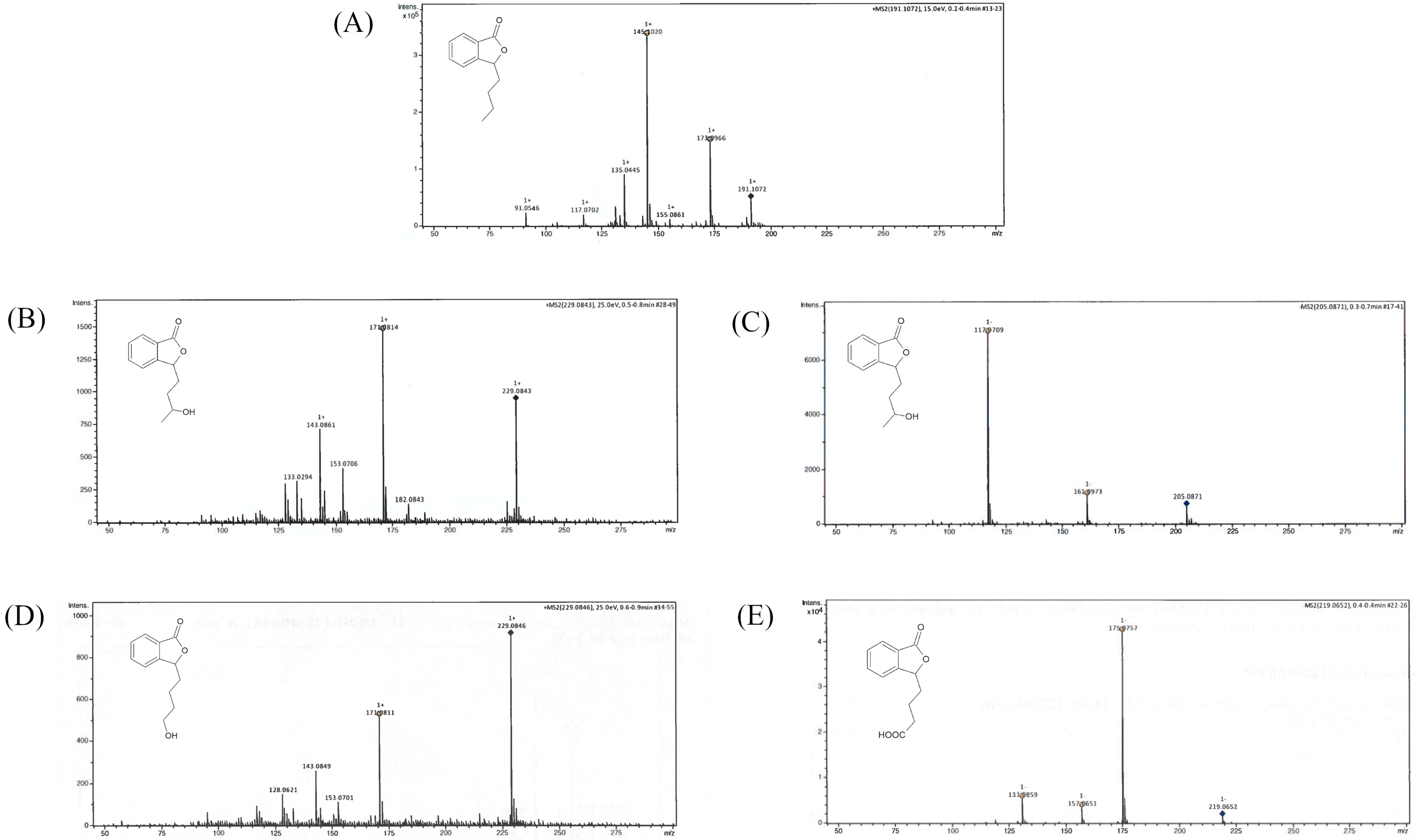
We conducted the upscale processes to determine the structure of products 2–4. The biotransformations with biocatalysts AM91 and AM235 were conducted in the bioreactor at a concentration of substrate (1) equaling 0.4 g/L, while that with AM32 was conducted in a 2 L flask. After extraction with ethyl acetate and evaporation, the mixtures were purified by column chromatography on silica gel using gradient elution, gradually increasing the concentration of polar solvents. The structures of compounds 2–4 were determined based on nuclear magnetic resonance (NMR) spectroscopy and confirmed by high-resolution electrospray ionization mass spectrometry (HRMS). Figure 3 shows the LC/ESI–MS/MS chromatograms, while Table 1 presents information about the quasi-molecular ions, the proposed elemental formula, and fragment ions of compounds 1–4, both from the experiments and from the literature. The NMR spectra for compounds 1–4 are presented in the Supplementary Materials (Figures S1–S8).
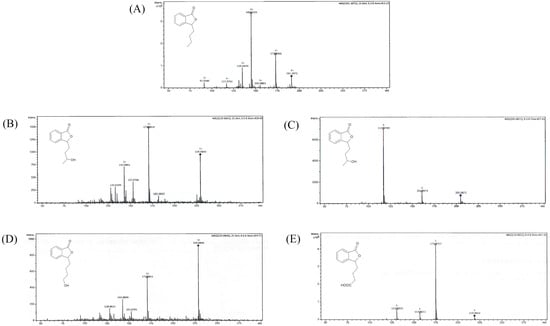
Figure 3.
LC/ESI–MS/MS chromatograms of (A) 3-n-butylphthalide (1)—positive ion mode, (B) 3-n-butyl-10-hydroxy-phthalide (2)—positive ion mode, (C) 3-n-butyl-10-hydroxy-phthalide (2)—negative ion mode, (D) 3-n-butyl-11-hydroxy-phthalide (3)—positive ion mode, and (E) 3-n-butylphthalide-11-oic acid (4)—negative ion mode.

Table 1.
Pseudo-molecular ions and fragment ions in ESI-HR-MS analysis of compounds 1–4.
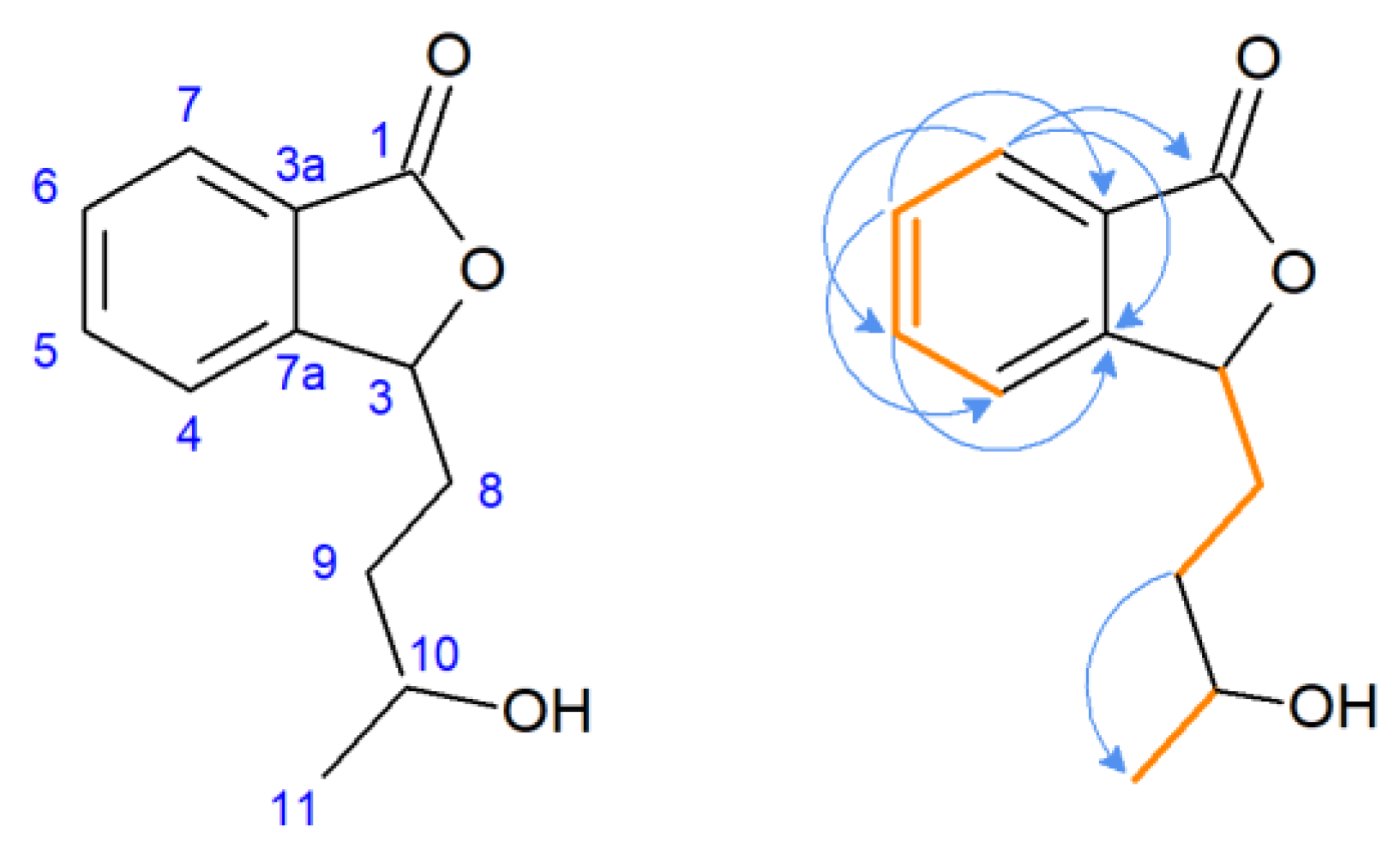
The 1H NMR spectra of compound 2 revealed duplicated peaks in the area of 1.40–5.60 ppm corresponding to protons in the side chain of the lactone, which suggested the presence of two diastereoisomers. The characteristic signal of substrate 1 for proton H-3 (δ = 5.47 ppm) was present, excluding the possibility of hydroxylation at the C-3 position, the predominant direction of biotransformation of 3-n-butylidenophthalide found in our previous study [23]. Correlation spectroscopy (COSY) revealed the coupling of H-5 with H-6 and H-7, and H-6 with H-7 in the aromatic region (Figure 4). Other key couplings between the protons in the side chain were H-8 with H-3 and H-9 and H-10 with H-11. The most characteristic signal of the carbon spectrum was a downfield shift of C-10 carbon in relation to the substrate (22.6 ppm) corresponding signal. Instead, signals appeared at δ = 81.18 and δ = 81.68 ppm (two signals of the isomers).
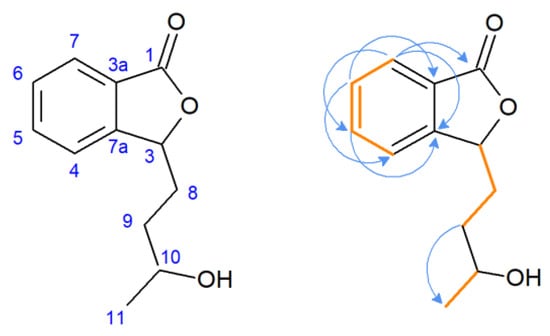
Figure 4.
Pivotal couplings for compound 2—correlation spectroscopy (orange) and nuclear multiple bond coherence (blue arrows).
Compound 2 ionized in negative and positive modes, resulting in the formation of [M + H]− 205.0863 and the adduct [M + Na]+ 229.0844, respectively. Fragment ion 171.0804 was formed after the loss of two water molecules. Product 2 was confirmed as 3-n-butyl-10-hydroxyphthalide (2).
The characteristic of the 1H NMR spectrum of compound 3 is the presence of a triplet at δ = 3.64 ppm of the integration, indicating the presence of two protons. The 13C NMR spectrum of 3 is similar to that of the 10-hydroxylated derivative (2), with a slight shift upfield of the signals δ = 21.36, 32.39, and 62.58 ppm. Compound 3 ionized in positive mode, and the 229.0844 [M + Na]+ adduct was formed with a similar fragment ion 171.0804 as that for product 2. Product 3 was confirmed as 3-n-butyl-11-hydroxyphthalide.
In the 1H NMR spectrum of compound 4, we observed the shift of the proton signals concerning the side chain of lactone towards the lower field compared to that of the substrate (1) and the product with a hydroxyl group at the C-10 position. The total integration of multiplets in the areas 1.74–1.88, 2.10–2.20, and 2.37–2.50 ppm indicated the presence of six protons. In the carbon spectrum, there were only three signals in the area characteristic for the side chain (14–30 ppm), with a lack of signal characteristic for C-11 in the substrate 1 spectrum (at δ = 14.0 ppm). However, we observed a new strongly de-shielded signal at δ = 179.8 ppm. The product ionized in the negative mode, leading to the formation of [M + H]− 219.0650. The main fragment ions included 175.0765, formed after the loss of −CO2, 157.0659 (after the loss of −CO2 and −H2O), and 131.0866. Product 4 was identified as 3-n-butylphthalide-11-oic acid.
During the purification of the crude mixture after biotransformation, we isolated pure fractions of products 2–4 and mixtures of these compounds enriched with metabolites. As we had doubts concerning the assessment of the process efficiency, we conducted additional experiments to assess the percentage of substrate and products in the mixtures. For this purpose, biotransformations were performed with 3-n-butylphthalide (1) at a concentration of 260 mg per 1000 mL of culture. The cultures were extracted with ethyl acetate after 12 days for Penicillium sp. AM91, B. cinerea AM235, and Botrytis sp. KKP3292, and after 6 days for Penicillium dierckxii AM32. We prolonged the extraction time using new portions of solvent until no substrate or product remained in the extract. After the extraction, the amounts of the remaining substrate and the individual products 2–4 were assessed using gas chromatography coupled with a flame-ionization detector (GC-FID) using calibration curves from previously purified products. The obtained yields in relation to the biocatalyst are presented in Table 2.

Table 2.
Yields (g per g of the substrate; determined by GC) of compounds 2–4 in the biotransformation extracts in relation to the strains used.
Similar to the screening scale biotransformations, compound 2 was the most dominant metabolite for strains B. cinerea AM235 and Botrytis sp. KKP3292. Botrytis sp. KKP3292 led to an almost total conversion of substrate 1. However, Penicillium sp. AM91, applied as a biocatalyst, led to the obtainment of, predominantly, compound 3. AM32 led to the obtainment of mainly products 3 and 4, and their relative ratio changed with the prolonged biotransformation time in favor of 4 (from 2:1 to 18:1 in biotransformation after 6 and 12 days, respectively).
2.2. Inhibition of MAO-A Activity
Protein-ligand interaction analysis was performed using isothermal titration calorimetry (ITC). During the analysis, the heat of uptake or release, due to the interaction of MAO-A with the tested compounds, was recorded. In this way, raw data were obtained in the form of exothermic heat versus time graphs. Additionally, the ligand was injected into 1% DMSO with methanol to subtract the energetic effects of the dilution of the ligand solution. The obtained thermodynamic parameters are presented in Table 3. The highest negative change in enthalpy was characterized by the interaction of the enzyme with compounds 3 (−19.19 kJ/mol) and 4 (−13.37 kJ/mol), and the lowest value of ΔH was recorded during interactions with compound 1 (−4.51 kJ/mol). The paired compounds caused a decrease in enthalpy while maintaining the exothermic effect of the reaction; the enthalpy ranged from −3.80 kJ/mol (1 with 2) to −8.28 kJ/mol (3 with 4). Affinity (ΔG) values were more evenly distributed between compound 1, compounds 1 with 4, 2 with 4, and 2 with 3. The lowest affinities were characterized by 1 with 3 and 1 with 2, amounting to −20.69 and 22.69 kJ/mol, respectively, and the highest one was compounds 4, amounting to −28.42 kJ/mol. The ability of the ligands to inhibit BChE activity was described on the basis of the IC50 parameter. The lowest IC50 value was shown by compound 3 (1.29 μmol/L), and the highest by compound 1 with 2 (45.00 μmol/L).

Table 3.
Thermodynamic parameters of interactions between MAO-A and compounds 1–4.
2.3. Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) Profiling
Substrate 1 and products 2–4 were tested for their pharmacokinetic properties, using the bioinformatic SWISS ADME tool reported by Daina et al. [25], including lipophilicity, solubility, and inhibition of the selected isoenzymes and drug-likeness. Toxicity to rat cells after oral administration was determined using the Way2Drug platform described by Druzhilovskiy et al. [26] (Table 4).

Table 4.
In silico profiling, including lipophilicity (Log Po/w), solubility (Log S), and inhibition of the isoenzymes CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4 toxicity to rat cells, expressed as LD50 [mg/kg], and compliance with the objectives of Lipinski, Ghose, Webber, Egan, Muegge.
Lipophilicity is the distribution of the drug between the organic and water phases [27]. Biotransformation led to the obtaining of more polar and less lipophilic metabolites 2–4 compared to their precursor 1. Among these, the most soluble metabolite in water was 3-n-butylphthalide-11-oic acid (4).
There are several selection criteria for predicting the potential drug properties, including Lipinski, Ghose, Veber, Egan and Muegge [28,29,30,31,32], describing the optimal number of carbon atoms, heteroatoms, rings, hydrogen bond donors and acceptors, rotatable bonds, the range for lipophilicity, molar refractivity, total polar surface, and molecular weight [33]. According to the predictions, compounds 2–4 match the objectives described by these researchers [28,29,30,31,32]; however, their precursor, 3-n-butylphthalide (1) does not meet the criterion of Muegge.
Interestingly, metabolism led to an increase in toxicity as the half-lethal dose of compounds 2–4 declined. The half-lethal dose for precursor (1) was 4872 mg/kg. The least toxic of the metabolites was 3-n-butyl-10-hydroxyphthalide (2), while 3-n-butylphthalide-11-oic acid (4) was twice as toxic to rat cells than 3-n-butylphthalide (1).
The inhibition of CYP isoenzymes leads to drug interactions, causing side effects [34,35]. In silico tests were performed for the five isoforms, accounting for almost 90% of the metabolic reactions [35]. They revealed no inhibition of lactones 1–4 against CYP2C19, CYP2C9, CYP2D6, and CYP3A4. CYP1A2 was inhibited only by the precursor, 3-n-butylphthalide (1). This isoform is crucial for the inhibition of many drugs, including Alzheimer’s and Parkinson’s disease medications and analgesics [36].
2.4. Molecular Species Identification
The PROTAX-fungi tool results of the ITS fragment for the analyzed samples are shown in Figure S9. For the AM32 strain, the results indicate that the analyzed sample belongs to P. dierckxii (Figure S9, part A). The ITS results for the AM91 strain did not identify the systematic affiliation, as the results showed an 18% probability for unknown species and a 14% probability for P. spinulosum and P. thomii (Figure S10, part B). The AM235 strain was identified as Botrytis cinerea (Figure S9, part B). The ITS sequences for AM32 and AM235 were deposited in the National Center for Biotechnology Information (NCBI) database under accession number OQ875855 for AM32 and OQ875856 for AM235.
The genome sequence obtained for strain AM91 (after base-calling) had 1.64e + 09 bases with N50 = 1.23 kbp and median PHRED = 10.931. The Flye assembly results were 35,547,927 bp of total genome length with 91 fragments (N50 = 3,603,452 bp and longest fragment 7,742,766 bp) and a mean coverage of 28. Medaka analyses did not improve genome assembly in terms of length. Finally, the assembled genome was deposited in the NCBI database under accession number SAMN34128561.
3. Discussion
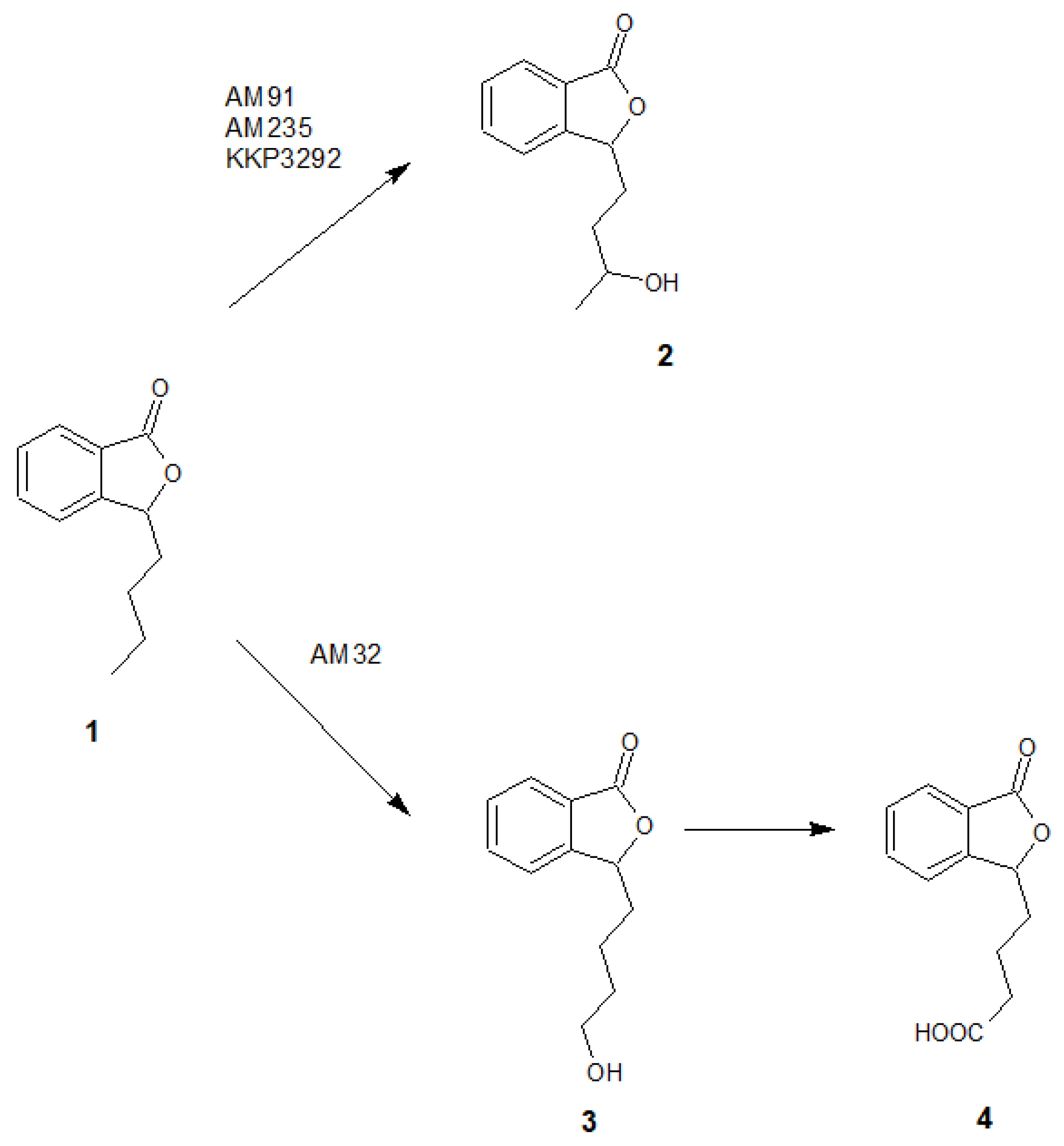
Based on the results of chromatographic analysis, we selected four fungal strains that allowed high substrate (1) conversion, including Penicillium dierckxii AM32, Penicillium sp. AM91, Botrytis cinerea AM235, and Botrytis sp. KKP3292, and we observed three main products. Notably, several Penicillium spp. have been used previously to convert one of the phthalides, mycophenolic acid, to the corresponding hydroxyderivative [11]. The hydroxylation of piperitone occurred in Botrytis AM235 [37]. The structures of the obtained main products depending on the biocatalyst are presented in Figure 5.
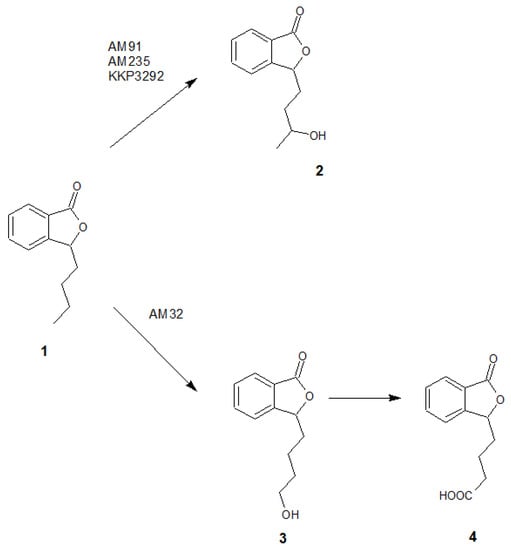
Figure 5.
The structures of the main products formed during fungal-mediated biotransformations of 3-n-butylphthalide (1)—3-n-butyl-10-hydroxyphthalide (2), 3-n-butyl-11-hydroxyphthalide (3) and 3-n-butylphthalide-11-oic acid (4).
Diao et al. reported that the main direction of 3-n-butylphthalide (1) metabolism using human liver microsomes was the hydroxylation of the side chain. In vivo studies showed that 3-n-butyl-10-hydroxyphthalide (2) was the dominant circulating human metabolite, followed by the carboxylic acid derivative 4, the hydroxylation product in the C-3 position, and the keto derivative. The biocatalysis of 3-n-butylphthalide (1) by whole cells of the Cunninghamella blakesleana ATCC9244 strain led to the formation of the products mentioned above; however, their ratio was not stated [21]. Moreover, the research conducted by Lin et al. confirmed that 3-n-butyl-10-hydroxyphthalide (2) was the main metabolite in the rat plasma and liver microsomes [38].
In our research, whole-cell biotransformations, similar to the in vivo tests, resulted in the obtaining of, primarily, the derivative in the C-10 position. Interestingly, there are no articles on the formation of two diastereoisomers of compound 2 during biotransformation; however, their presence was confirmed in the extract of Ligusticum chuanxiong [12]. Based on the decreasing content of C-11 hydroxyderivative (3) in favor of the 3-n-butylphthalide-11-oic acid (4) and the research conducted by Diao et al. [21], it can be confirmed that 3-n-butyl-11-hydroxyphthalide (3) is the mediatory product and is further oxidized to compound 4.
Compounds 1–4 were tested for their inhibition of MAO-A activity by the ITC method. In the first stage, we assumed interactions between compounds 1–4 and the MAO-A enzyme to be potential ligands protecting against 5-HT degradation. Considering that a mixture of compounds may act differently than a single substance, with possible synergistic, additive, and antagonistic effects, we also tested compounds 1–4 in combinations [39,40]. The interactions between 1–4 and the MAO-A enzyme showed an exothermic effect, evidenced by negative enthalpy changes. An analysis of the stoichiometry of the resulting complexes showed a single 1:1 reaction-binding model [41]. During the analysis, there was a decrease in heat emitted after subsequent injections, which reflects the observed negatively charged compounds or mixtures in compounds, while interactions 1 and 2 with MAO-A were so weak that ITC could detect them. The change in the free enthalpy (affinity ΔG) indicated negative values, which characterizes the spontaneous nature of interactions. The values of individual compounds ranged from −28.42 to −23.49 kJ/mol for 4 and 3 associated with MAO-A complexation and blocking. The pairing of the compounds resulted in the stabilization of affinity at approximately −27 kJ/mol for pairs 1 with 4, 2 with 3, and 2 with 4. However, for the remaining pairs, the affinity decreased, which may be related to the change in the energetic effect resulting from the rearrangement of the protein structure.
The compounds with the most significant exothermic effects were 3 and 4, suggesting the most significant conformational changes of MAO-A. In addition to complexation with active sites, exothermic changes were observed; therefore, injections of these compounds may limit the ability to deviate 5-HT. According to Prah, the calculated 5-HT degradation barrier is 62 kJ/mol, while the reaction catalyzed by the enzyme is reduced to −35.42 kJ/mol at pH 7.5. However, the same reaction in an aquatic environment shows a lower free energy of −22.06 kJ/mol, which means that the compounds are less stable in the aquatic environment than in pH 7.5 [42]. Our research confirms that the most stable bonds with the enzyme are characterized by compound 4 and the pairs of compounds 2 with 4, 1 with 4, and 2 with 3. The values of the binding constant of the resulting complexes ranged from 3.12 × 103 to 50 × 103 L/mol. The significant differences in the height of KA may be because some of the compounds have deprotonated, and the resulting electrostatic interaction affects the binding constant of these compounds with the enzyme. The highest MAO-A bond constant was characterized by the pair of compounds 2 and 4, amounting to 50 × 103 L/mol. In all cases, the values of free enthalpy and enthalpy of interactions were negative, and the entropy values were positive, indicating the formation of a ligand–protein complex through non-covalent hydrophobic interactions [43].
The tested compounds demonstrated the ability to reduce the rate of 5-HT deamination by the MAO-A enzyme. The IC50 was lowest for compound 3, and the pair of compounds 3 and 4, at 1.29 μmol/μmol of enzyme and 2.02 μmol/μmol of enzyme, respectively. Compounds 4 and 4 combined with 2 were at a similar level of about 5 μmol/μmol of the enzyme. Substrate 1, tested with lactones 2 and 3, turned out to be the least beneficial compounds, with a high concentration inhibiting the activity of the enzyme by 50%, and had the least favorable affinity. This effect can be explained by the binding of several compounds in the mixture to different MAO-A residues at the active site and in other regions. Molecular docking can be used in future research to confirm this assumption [44,45].
The Ki of the tested compounds indicates that they bound to MAO-A as competitive inhibitors and were characterized by a fairly similar and low Ki value for compounds 3 and 4 combined with 2, amounting to <0.001 μmol/L. Various phthalide analogs have been tested regarding their MAO-inhibiting activity. According to Strydom et al., the Ki values for 6-phenyl-propoxyphthalide and CF3-substituted benzyloxyphthalide, the most active phthalide derivatives regarding MAO-A and MAO-B inhibition, were 0.062 µM and 0.0007 µM, respectively [46]. Qiang et al. tested the 3-n-butylphthalide–Edaravone complexes, that showed an inhibitory effect at IC50, in the ranges of 4.40–19.32 µM [47].
Absorption, distribution, metabolism, excretion, and toxicity are key in drug development [48]. The increase in lipophilicity is related to higher permeability through membranes and low solubility [49]. The values of this parameter for both butylphthalide and its metabolite were within the optimal range of logP, described as logP 1–3, ensuring their sufficient permeability and solubility [50]. The drug-likeness prediction for metabolites 2–4 revealed their obedience to the most common set of guidelines described by Lipinski, Ghose, Webber, Egan, and Muegge. However, 3-n-butylphthalide (1) showed incompatibility with the drug-likeness concept, according to Muegge, for the molecular weight, which is under 200.
The predictions indicated the increased toxicity of the metabolites 2–4 compared to that of their precursor 1. According to the research of Xue et al., the compounds phthalide 1 and their metabolites 2–4 were not toxic for primary rat hepatocytes and primary human hepatocytes tested at a concentration of 0–500 µM. However, the repetitive administration of 1 caused a reduction in cell viability [51]. Considering that toxicity is the second cause for clinical failures of drug development, it is crucial to conduct further preclinical studies on the metabolites 2–4 [52].
4. Materials and Methods
4.1. General
The content of the products was monitored using an HPLC—Dionex UltiMate 3000 instrument with a diode array detector (Thermo Fisher Scientific, Waltham, MA, USA) with a column Agilent Zorbax Bonus-RP 3.5 µm 150 × 3 mm. The mobile phase was composed of water acidified with 0.5% formic acid (A) and acetonitrile (B) using gradient elution conditions: 0–3 min, 65% A/35% B; 3–12 min, 35% A/65% B, 12–13 min, 10% A/90% B; 13–14 min, 0% A/100% B; 14–16 min 0% A/100% B; 16–19 min, 65% A/35% B; and 19–23 min, 65% A/35% B. The following parameters were selected: flow rate, 0.4 mL/min; column incubation temperature, 30 °C; and detection wavelength, 275 nm. The compounds were purified by column chromatography using silica gel Kieselgel 60, 230–400 mesh, 40–63 µm (Merck, Darmstadt, Germany). The purity of the substrate and the yield of the products were assessed by GC using Agilent Technologies 8860 (GC System, Santa Clara, USA) with a flame-ionization detector and carrier gas H2 using HP-5 column 30 m × 0.32 mm × 0.25 µm (Agilent, Santa Clara, CA, USA) with the following temperature program: 80 °C (1 min), 320° (30 °C/min) (1 min).
The structures of the compounds were evaluated by NMR techniques. 1H NMR, 13C NMR, COSY, HSQC, and HMBC spectra were recorded in CDCl3 on a Bruker Avance 500 (500 MHz, Billerica, MA, USA) spectrometer.
HR-ESI–MS/MS analyses of the compounds were performed with RSLC UltiMate 3000 (Dionex, Sunnyvale, CA, USA) coupled with the ESI-Q-TOF, maXis impact mass spectrometer (Bruker Daltonics, MA, USA), and the operating parameters were: flow rate of the sample of 180 µL/min, nebulizer pressure 0.4 bar, the heating gas flow of 3.0 L/min, heating gas temperature of 180/200 °C, data acquisition range of m/z 50–1300/1400 m/z, ionization mode: positive and negative, and ion source energy of 5 eV.
4.2. Microorganisms
The strains used in this study were obtained from the Department of Food Chemistry and Biocatalysis collection at the Wrocław University of Environmental and Life Sciences and from the Institute of Agricultural and Food Biotechnology Collection of Industrial Microbial Cultures (full list available in the Supplementary Materials). The strains were stored on Sabouraud or Czapek agar slants at 4 °C.
Molecular Species Identification
We performed DNA analyses for strains with high substrate conversion to confirm proper species identification (based on morphology). Strain KKP3292 was not included in this step, as it was obtained from commercial culture collections and was already identified. DNA from samples AM32, AM91, and AM235 was isolated with a Bead-Beat Micro AX Gravity isolation kit from A&A Biotechnology (Poland, Gdańsk) according to the manufacturer’s instructions. Next, isolated cellular DNA was assessed for its quality (with agarose gel electrophoresis) and quantity (with Qubit 4 fluorometer). An internal transcribed spacer (ITS) fragment was used as a genetic marker in fungal species identification. PCR conditions and sequencing procedures were similar to those described by Hernik et al. [53]. ITS sequences from all analyzed samples were input into the online PROTAX-fungi tool [54] to identify the species.
As we could not determine the identification of the species level for strain AM32 with ITS, we sequenced the whole genome of the strain to aid in additional identification in the future. We used the Oxford Nanopore MinION platform with SQK-LSK110 chemistry and R10.3.1 flow cells and obtained approximately 2 Gb of data for the sample. After sequencing, we used Guppy 6.4.2 (https://community.nanoporetech.com/ (accessed on 4 April 2023) and dna_r10.3_450bps_sup model for base-calling. Basecalled fastq files were then screened for quality with PycoQC [55]. To assemble the genome, we first used Canu v2.2 [56] and then Flye [57,58] and polished assembly with Medaka (https://github.com/nanoporetech/medaka (accessed on 4 April 2023)) to enhance assembly length. Finally, before submission to the NCBI, the obtained data were screened for contaminants using the Foreign Contamination Screen (FCS) tool (https://github.com/ncbi/fcs (accessed on 4 April 2023)) and submitted to the NCBI genome database.
4.3. Chemical Synthesis
For this step, 4.3 g (0.023 M) of 3-n-butylidenephthalide (Sigma–Aldrich; Saint Louis, MO, USA) and 110 mL of 0.5 M KOH solution in methanol were added to a two-neck round-bottom flask mounted under a reflux condenser. The reaction mixture turned orange. The hydrolysis reaction was carried out at boiling point for 1.5 h. The mixture was cooled, and 10% hydrochloric acid was added until a yellow color appeared. Then, methanol and water were evaporated; 100 mL of THF, 5 mL of 0.1 M KOH aqueous solution, and 0.95 g (0.025 M) of sodium borohydride (Sigma–Aldrich) were added to the residue. The solution again turned orange. The reaction was stirred at 22 °C for 12 h. An aqueous solution of 10% hydrochloric acid was added until a yellow color appeared, and stirring was continued for another 4 h. The solvents were evaporated, ethyl acetate was added, and the reaction mixture was filtered through a 30 cm layer of silica gel placed in a 3 cm diameter column. After complete elution of the product and evaporation of the solvents, 4.0 g of product was obtained (84% yield) with 92% purity according to GC. Compound 1 was purified on silica gel using a mixture of petroleum ether: acetone: ethyl acetate: isopropanol 150:5:5:15 v/v as the eluent.
4.4. Biotransformations
4.4.1. Screening Scale Biotransformations
The screening scale biotransformations were performed in 300 mL Erlenmeyer flasks with sterile Sabouraud medium [23], acidified by HCl to pH 5.6. After inoculation, the cultures were incubated for eight days at 25 °C on a rotary shaker. Next, 20 mg of 3-n-butylphthalide (1) dissolved in 0.5 mL of acetone was added. The samples were collected after 4, 8, and 12 days, extracted using ethyl acetate, and dried with MgSO4. After evaporation, they were dissolved in acetonitrile, filtered through a 0.45 µm PTFE filter, and subjected to HPLC analysis.
4.4.2. Preparative Scale Biotransformations
- Method I
Biotransformations with AM91 and AM235 were carried out in the bioreactor–3 L New Brunswick Scientific BioFlo III (Brunswick, Ramsey, MN, USA). The bioreactor with Sabouraud medium was sterilized at 121 °C for 25 min. After sterilization, the temperature was maintained at 20–22 °C, and agitation was set to 75 rpm. A Broadley James D100 Series Oxyprobe was used to measure the level of dissolved oxygen. The air was passed through a sterile 0.2 μm PTFE filter at a 1 L/min flow rate. The previously pre-cultured inoculum (10% v/v) was placed into the bioreactor, resulting in a final volume of 1500 mL; 0.4 g of 3-n-butylphthalide (1) diluted in 5 mL of acetone was added during the exponential phase of growth. The 8-day biotransformation progress was monitored by HPLC. The reaction mixture was divided into three portions (3 × 500 mL), acidified by 0.1 M HCl, washed with brine, and extracted overnight with ethyl acetate (3 × 500 mL) on a laboratory shaker. After extraction, the mixture was centrifuged and evaporated. The crude product was purified by column chromatography using gradient elution—a mixture of petroleum ether: acetone: ethyl acetate: isopropanol 150:5:5:15 v/v was initially used and, after substrate 1 was washed, their proportion was changed to 50:5:5:15 v/v.
- Method II
In the second method for the preparative scale biotransformation, we used AM32, AM91, AM235, and KKP3292 as the biocatalysts. The microorganisms were grown in 2 L flasks with 500 mL of a sterile Sabouraud medium containing 10% pre-cultured inoculum. After five days of incubation at 25 °C on a rotary shaker, 130 mg of lactone 1 in 0.5 mL was added to the cultures.
The biotransformations were extracted by ethyl acetate after 12 days for Penicillium sp. AM91, B. cinerea AM235, and Botrytis sp. KKP3292 and, additionally, after 6 days for Penicillium dierckxii AM32. The prolonged extraction of the biotransformation mixtures on the laboratory shaker lasted 96 h. After the extraction, the isolation of the products proceeded as described above.
4.5. MAO-A Activity
A MicroCal PEAQ-ITC200 calorimeter (Malvern, Worcestershire, UK) was used to study the interactions of MAO-A with the compounds. The enzyme and compounds were prepared in 1% DMSO with methanol. The measurements were performed at 36.6 °C. The stirring speed was set to 307 rpm, and a total of 19 injections were performed within 50 min, with the reference power set to 10.00 μcal/s. All sample solutions were thoroughly degassed before use. The calorimetric cell was filled with a 20 μM (350 µL) MAO-A sample, and the syringe (2 µL of injection volume) was loaded with a 1 mM/L solution or/and 5-HT (1 mM/L). The ITC data were processed using MicroCal PEAQ-ITC analysis software with calorimetric routines. The following parameters were determined: the standard interaction Gibbs energy (ΔG), standard interaction enthalpy (ΔH), entropy (ΔS), equilibrium constant (KD), and stoichiometry of reaction (N) [59].
4.6. ADMET Profiling
The pharmacokinetic parameters involving lipophilicity, solubility, and inhibition of CYP isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 were predicted using the Swiss Institute of Bioinformatics tool at the University of Lausanne (http://www.swissadme.ch/index.php (accessed on 25 March 2023)). Toxicity to rat cells was assessed using the Way2Drug platform (http://www.way2drug.com/gusar/acutoxpredict.html (accessed on 25 March 2023)).
5. Conclusions
Biotransformations catalyzed by fungi are a useful method to obtain derivatives of 3-n-butylphthalide (1). Two strains of Botrytis and two isolates of Penicillium were the most effective in terms of substrate transformation. Both 3-n-butylphthalide (1) and its metabolites 2–4, obtained via fungal biotransformation, acted as MAO-A inhibitors. Two metabolites, 3 and 4, were more active than substrate 1. The lipophilicity for all products, assessed by in silico tests, was in the optimal range, correlating with good permeability and solubility. Contrary to its precursor 1, the most active 3-n-butyl-11-hydroxyphthalide (3) had no inhibitory activity on the CYP1A2 isoform; therefore, the possibility of interference with drugs metabolized by this isoform is lower. However, further evaluation of the toxicity of compound 3 is required, as the in silico prediction of this parameter revealed higher toxicity to rat cells than 3-n-butylphthalide (1).
Further research may involve tests regarding the reversibility of the process to determine if the usage of phthalide 1 analogs leads to tyramine accumulation, like most commercially available drugs, or has similar actions with moclobemide, a reversible inhibitor of MAO-A.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310605/s1.
Author Contributions
Conceptualization, T.O. and J.G (Joanna Gach).; methodology, T.O. and F.B.; software, J.G. (Joanna Gach); formal analysis, J.G. (Joanna Gach), T.O. and F.B.; investigation, J.G. (Joanna Gach), T.O., J.G. (Joanna Grzelczyk) and T.S.; resources, J.G. (Joanna Gach), T.O. and F.B.; writing—original draft preparation, J.G. (Joanna Gach), J.G. (Joanna Grzelczyk) and T.S.; writing—review and editing, T.O. and F.B.; visualization, J.G. (Joanna Gach), J.G. (Joanna Grzelczyk) and T.S.; supervision, T.O. and F.B.; funding acquisition, J.G. (Joanna Gach). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “UPWR 2.0: international and interdisciplinary program of development of Wrocław University of Environmental and Life Sciences” and co-financed by the European Social Fund under the Operational Program Knowledge Education Development, under contract No. POWR.03.05.00-00-Z062/18 of 4 June 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Devadoss, T.; Jha, N.K.; Baidya, M.; Gupta, G.; Chellappan, D.K.; Singh, S.K.; Dua, K. Targeting Inflammation: A Potential Approach for the Treatment of Depression. Metab. Brain Dis. 2023, 38, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Roets, M.; Brand, L.; Steyn, S.F. Increased Depressive-like Behaviour of Postpartum Flinders Sensitive and Resistant Line Rats Is Reversed by a Predictable Postpartum Stressor. Behav. Brain Res. 2023, 442, 114321. [Google Scholar] [CrossRef]
- Greener, M. New Antidepressants: Monoamines and Beyond. Prescriber 2023, 34, 17–20. [Google Scholar] [CrossRef]
- Kim, T.T.; Amsterdam, J.D. Effectiveness and Safety of Monoamine Oxidase Inhibitor Treatment for Bipolar Depression versus Unipolar Depression: An Exploratory Case Cohort Study. Acta Psychiatr. Scand. 2023, 147, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Merce, A.P.; Ionică, L.N.; Bînă, A.M.; Popescu, S.; Lighezan, R.; Petrescu, L.; Borza, C.; Sturza, A.; Muntean, D.M.; Creţu, O.M. Monoamine Oxidase Is a Source of Cardiac Oxidative Stress in Obese Rats: The Beneficial Role of Metformin. Mol. Cell. Biochem. 2023, 478, 59–67. [Google Scholar] [CrossRef]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef]
- Muellers, S.N.; Tararina, M.A.; Kuzmanovic, U.; Galagan, J.E.; Allen, K.N. Structural Insights into the Substrate Range of a Bacterial Monoamine Oxidase. Biochemistry 2023, 62, 851–862. [Google Scholar] [CrossRef]
- Syu, G.-D.; Sutandy, F.X.R.; Chen, K.; Cheng, Y.; Chen, C.-S.; Shih, J.C. Autoantibody Profiling of Monoamine Oxidase A Knockout Mice, an Autism Spectrum Disorder Model. Brain Behav. Immun. 2023, 107, 193–200. [Google Scholar] [CrossRef]
- Barrowman, J.; Wilson, M. Antidepressants and Antipsychotics. Anaesth. Intensive Care Med. 2023, 24, 228–234. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 104, pp. 127–246. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Z.; Yang, Y.; Jiang, J.; Zhang, P. Bioactive Butylphthalide Derivatives from Ligusticum Chuanxiong. Bioorganic Chem. 2019, 84, 505–510. [Google Scholar] [CrossRef]
- Bi, M.; Zhang, M.; Guo, D.; Bi, W.; Liu, B.; Zou, Y.; Li, Q. N-Butylphthalide Alleviates Blood–Brain Barrier Impairment in Rats Exposed to Carbon Monoxide. Front. Pharmacol. 2016, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Q.; Hua, W.; Huang, M.; Zhou, W.; Lou, K.; Peng, Y. Pharmacokinetics, Safety and Tolerability of L-3-n-Butylphthalide Tablet after Single and Multiple Oral Administrations in Healthy Chinese Volunteers. Braz. J. Pharm. Sci. 2015, 51, 525–531. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, J.; Xu, Y.; Xie, S.; Zhou, X.; Cheng, S. Butylphthalide Combined With Conventional Treatment Attenuates MMP-9 Levels and Increases VEGF Levels in Patients With Stroke: A Prospective Cohort Study. Front. Neurol. 2021, 12, 686199. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, D.; Zhao, G.; Xie, Z. Efficacy and Safety of Butylphthalide in Secondary Prevention of Stroke: Study Protocol for a Multicenter, Real World Trial Based on Internet. BMC Neurol. 2022, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, Y.; Yuan, Y.; Gui, L.; Wang, J.; Gao, P.; Qin, B.; Sima, D.; Wang, Q.; Pan, W. Use of L-3-n-Butylphthalide within 24 h after Intravenous Thrombolysis for Acute Cerebral Infarction. Complement. Ther. Med. 2020, 52, 102442. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, H.; Fu, Z. Antidepressant-like Effect of 3-n-Butylphthalide in Rats Exposed to Chronic Unpredictable Mild Stress: Modulation of Brain-Derived Neurotrophic Factor Level and MTOR Activation in Cortex. Neurochem. Res. 2021, 46, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Hao, G.; Yi, Q.; Guo, Y.; Chen, D.; Han, W.; Zhang, J.; Yang, M.; Jiang, P. The Impact of Dl-3-n-Butylphthalide on the Lipidomics of the Hippocampus in a Rat Model of Lipopolysaccharide-Induced Depression. Prostaglandins Other Lipid Mediat. 2020, 150, 106464. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; Wei, W. Dl-3-n-butylphthalide prevents chronic restraint stress-induced depression-like behaviors and cognitive impairment via regulating CaMKII/CREB/BDNF signaling pathway in hippocampus. Neuroreport 2022, 33, 597–603. [Google Scholar] [CrossRef]
- Diao, X.; Deng, P.; Xie, C.; Li, X.; Zhong, D.; Zhang, Y.; Chen, X. Metabolism and Pharmacokinetics of 3- n -Butylphthalide (NBP) in Humans: The Role of Cytochrome P450s and Alcohol Dehydrogenase in Biotransformation. Drug Metab. Dispos. 2013, 41, 430–444. [Google Scholar] [CrossRef]
- Shanu-Wilson, J.; Evans, L.; Wrigley, S.; Steele, J.; Atherton, J.; Boer, J. Biotransformation: Impact and Application of Metabolism in Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 2087–2107. [Google Scholar] [CrossRef]
- Gach, J.; Olejniczak, T.; Krężel, P.; Boratyński, F. Microbial Synthesis and Evaluation of Fungistatic Activity of 3-Butyl-3-Hydroxyphthalide, the Mammalian Metabolite of 3-n-Butylidenephthalide. Int. J. Mol. Sci. 2021, 22, 7600. [Google Scholar] [CrossRef]
- Tian, J.; Lei, P.; He, Y.; Zhang, N.; Ge, X.; Luo, L.; Yan, S.; Diao, X. Absorption, Distribution, Metabolism, and Excretion of [14C]NBP (3-n-Butylphthalide) in Rats. J. Chromatogr. B 2021, 1181, 122915. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Dmitriev, A.V.; Pogodin, P.V.; Dubovskaya, V.I.; Ivanov, S.M.; Tarasova, O.A.; et al. Computational Platform Way2Drug: From the Prediction of Biological Activity to Drug Repurposing. Russ. Chem. Bull. 2017, 66, 1832–1841. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Kil, H.; Pandya, D.N.; Tichacek, C.J.; Doligalski, M.L.; Budzevich, M.M.; Delva, N.C.; Langsen, M.L.; Vallas, J.A.; Boulware, D.C.; et al. Lipophilicity Determines Routes of Uptake and Clearance, and Toxicity of an Alpha-Particle-Emitting Peptide Receptor Radiotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 953–965. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Singh, P.K. Analyzing FDA-Approved Drugs for Compliance of Pharmacokinetic Principles: Should There Be a Critical Screening Parameter in Drug Designing Protocols? Expert Opin. Drug Metab. Toxicol. 2021, 17, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Sychev, D.; Ashraf, G.M.; Svistunov, A.; Maksimov, M.; Tarasov, V.; Chubarev, V.N.; Otdelenov, V.A.; Denisenko, N.P.; Barreto, G.E.; Aliev, G. The Cytochrome P450 Isoenzyme and Some New Opportunities for the Prediction of Negative Drug Interaction in Vivo. DDDT 2018, 12, 1147–1156. [Google Scholar] [CrossRef]
- Yim, S.-K.; Kim, K.; Chun, S.; Oh, T.; Jung, W.; Jung, K.; Yun, C.-H. Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro. Mar. Drugs 2020, 18, 603. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Dhiman, S.; Kumar, V.; Gour, A.; Manhas, D.; Sharma, K.; Ojha, P.K.; Nandi, U. Assessment of the CYP1A2 Inhibition-Mediated Drug Interaction Potential for Pinocembrin Using In Silico, In Vitro, and In Vivo Approaches. ACS Omega 2022, 7, 20321–20331. [Google Scholar] [CrossRef]
- Grudniewska, A.; Gniłka, R.; Wawrzeńczyk, C. Enantioselectivity of Hydroxylation of Racemic Piperitone by Fungi. Chirality 2010, 22, 929–935. [Google Scholar] [CrossRef]
- Lin, L.; Lin, L.; Lin, T.; Wu, Y. Simultaneous Determination of 3-n-butylphthalide and Its Metabolite 10-hydroxy-butylphthalide in Rat Plasma Using Liquid Chromatography–Tandem Mass Spectrometry and Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2021, 35, e5184. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy—Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef]
- Tallarida, R.J. Quantitative Methods for Assessing Drug Synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Prah, A.; Purg, M.; Stare, J.; Vianello, R.; Mavri, J. How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step. J. Phys. Chem. B 2020, 124, 8259–8265. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Escorial Camps, M.; Pérez-Sánchez, H.; García-Carmona, F. Physicochemical and Thermodynamic Characterization of the Encapsulation of Methyl Jasmonate by Natural and Modified Cyclodextrins Using Reversed-Phase High-Pressure Liquid Chromatography. J. Agric. Food Chem. 2013, 61, 11347–11354. [Google Scholar] [CrossRef]
- Konc, J.; Lešnik, S.; Janežič, D. Modeling Enzyme-Ligand Binding in Drug Discovery. J. Cheminform. 2015, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. CAD 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Strydom, B.; Bergh, J.J.; Petzer, J.P. Inhibition of Monoamine Oxidase by Phthalide Analogues. Bioorganic Med. Chem. Lett. 2013, 23, 1269–1273. [Google Scholar] [CrossRef]
- Qiang, X.; Li, Y.; Yang, X.; Luo, L.; Xu, R.; Zheng, Y.; Cao, Z.; Tan, Z.; Deng, Y. DL-3-n-Butylphthalide-Edaravone Hybrids as Novel Dual Inhibitors of Amyloid- β Aggregation and Monoamine Oxidases with High Antioxidant Potency for Alzheimer’s Therapy. Bioorganic Med. Chem. Lett. 2017, 27, 718–722. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score-a comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2018, 10, 148–157. [Google Scholar] [CrossRef]
- Miller, R.R.; Madeira, M.; Wood, H.B.; Geissler, W.M.; Raab, C.E.; Martin, I.J. Integrating the Impact of Lipophilicity on Potency and Pharmacokinetic Parameters Enables the Use of Diverse Chemical Space during Small Molecule Drug Optimization. J. Med. Chem. 2020, 63, 12156–12170. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Xue, Y.; Ren, X.; Zhu, Z.; Lei, P.; Liu, M.; Wan, M.; Zhong, D.; Huang, H.; Diao, X. Site-Specific Protein Modification by 3-n-Butylphthalide in Primary Hepatocytes: Covalent Protein Adducts Diminished by Glutathione and N-Acetylcysteine. Life Sci. 2021, 287, 120125. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Hernik, D.; Szczepańska, E.; Brenna, E.; Patejuk, K.; Olejniczak, T.; Strzała, T.; Boratyński, F. Trametes hirsuta as an Attractive Biocatalyst for the Preparative Scale Biotransformation of Isosafrole into Piperonal. Molecules 2023, 28, 3643. [Google Scholar] [CrossRef]
- Abarenkov, K.; Somervuo, P.; Nilsson, R.H.; Kirk, P.M.; Huotari, T.; Abrego, N.; Ovaskainen, O. Protax-fungi: A web-based tool for probabilistic taxonomic placement of fungal internal transcribed spacer sequences. New Phytol. 2018, 220, 517–525. [Google Scholar] [CrossRef]
- Leger, A.; Leonardi, T. pycoQC, interactive quality control for Oxford Nanopore Sequencing. J. Open Source Softw. 2019, 4, 1236. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive κ-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yuan, J.; Kolmogorov, M.; Shen, M.W.; Chaisson, M.; Pevzner, P.A. Assembly of long error-prone reads using de Bruijn graphs. Proc. Natl. Acad. Sci. USA 2016, 113, E8396–E8405. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the Inhibition of Monoamine Oxidase A by Bioactive Coffee Compounds Protecting Serotonin Degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).