The Sizes and Composition of HDL-Cholesterol Are Significantly Associated with Inflammation in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of RA Patients and HC Participants
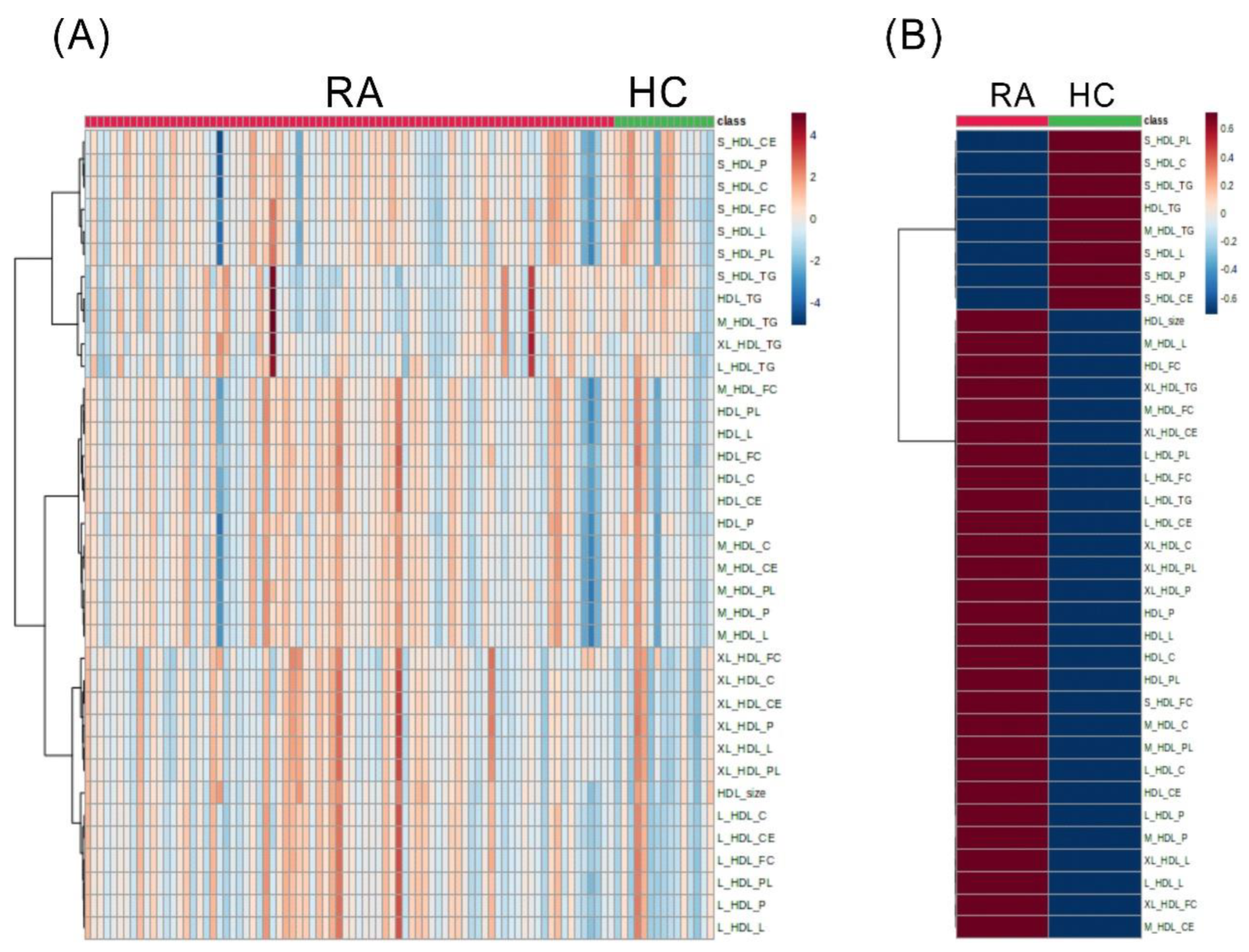
2.2. The Discriminant HDL-Related Lipid Metabolites between RA Patients and HC Subjects
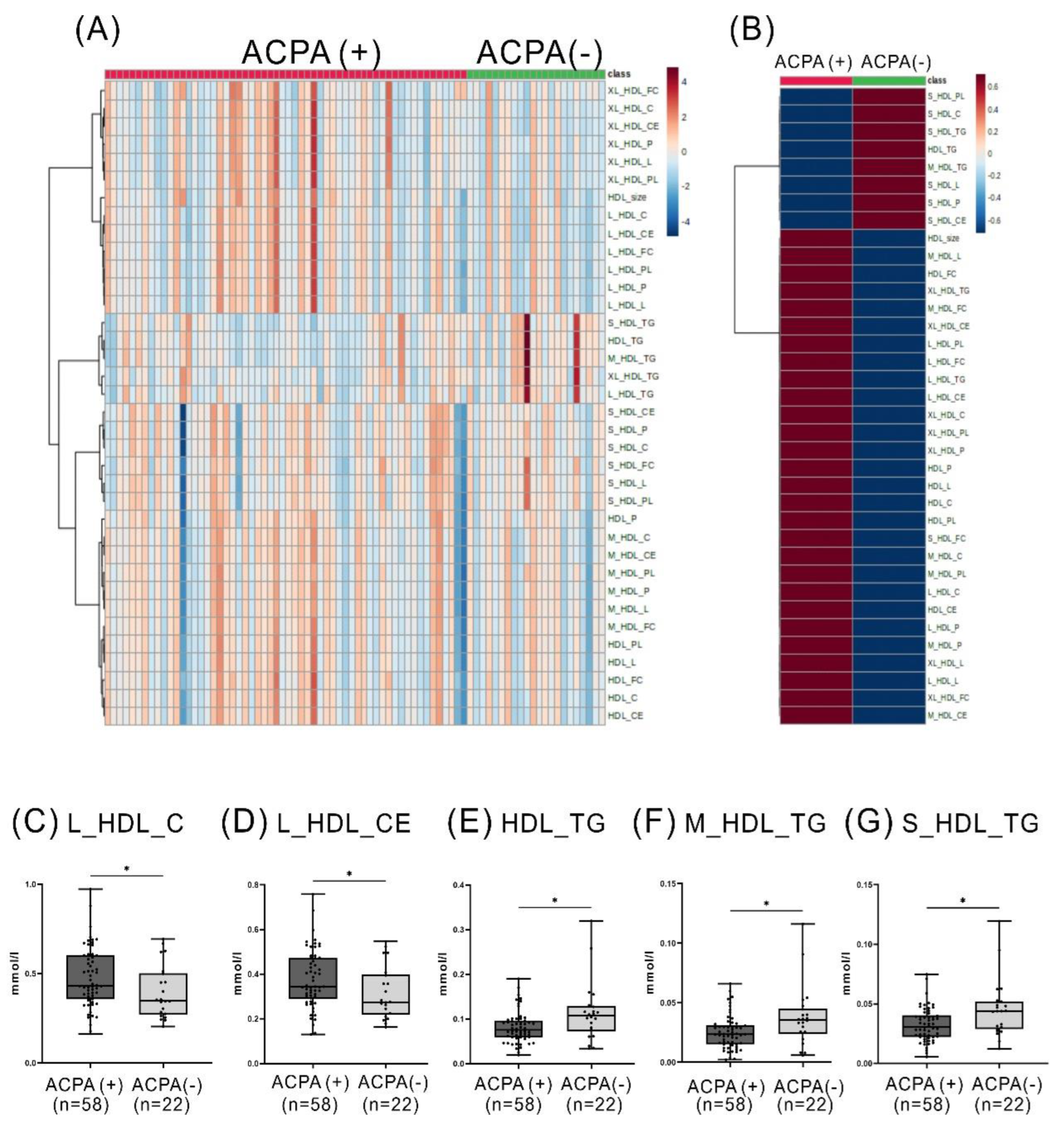
2.3. Comparison of the Levels of HDL-Related Metabolites between RA Patients with and without ACPA or between Patients with and without RF
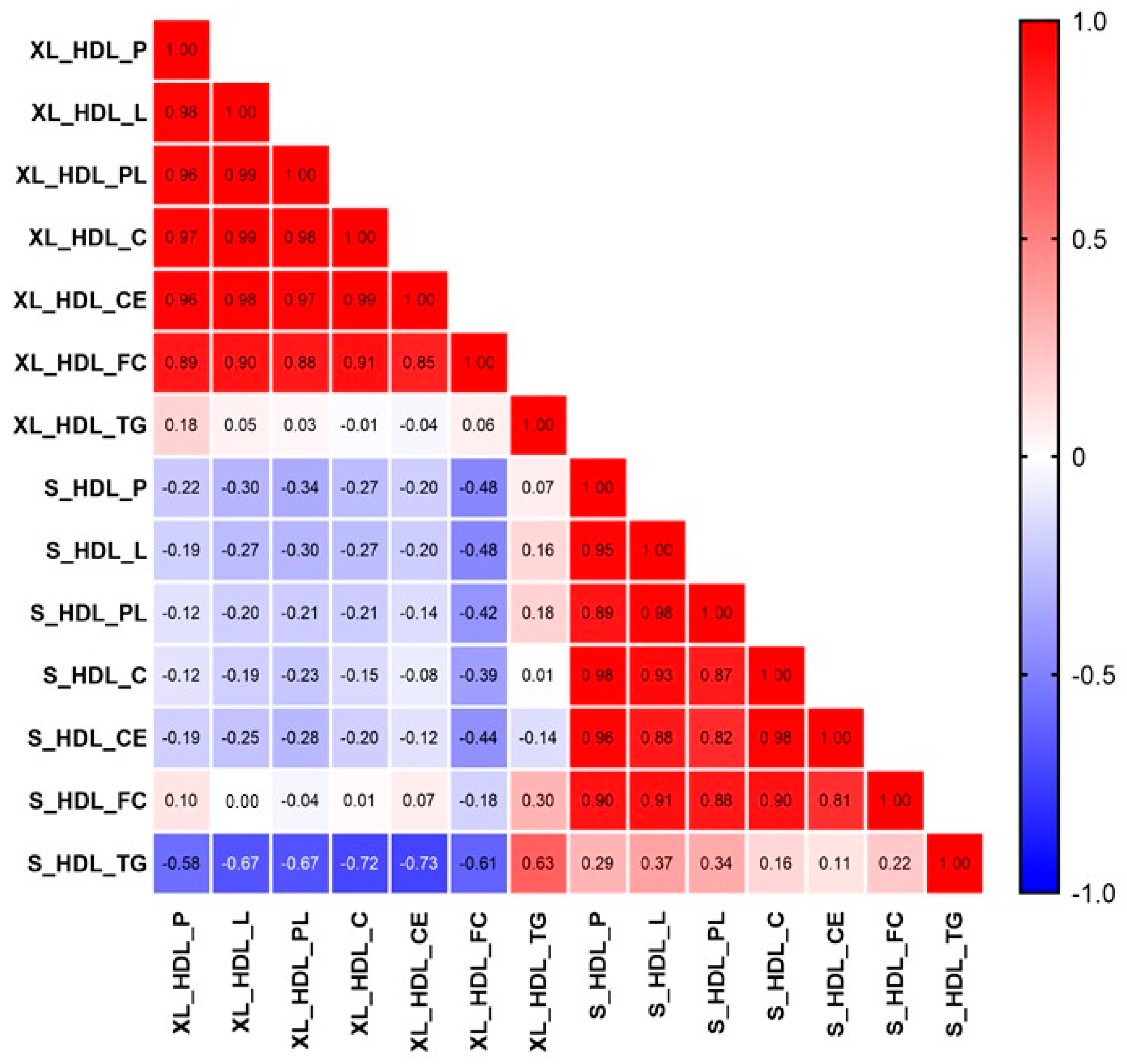
2.4. The Relationship among the Discriminant HDL-Related Metabolites in RA Patients
2.5. The Correlation between Inflammatory Parameter, Represented by CRP Levels, and HDL-Related Metabolites in RA Patients
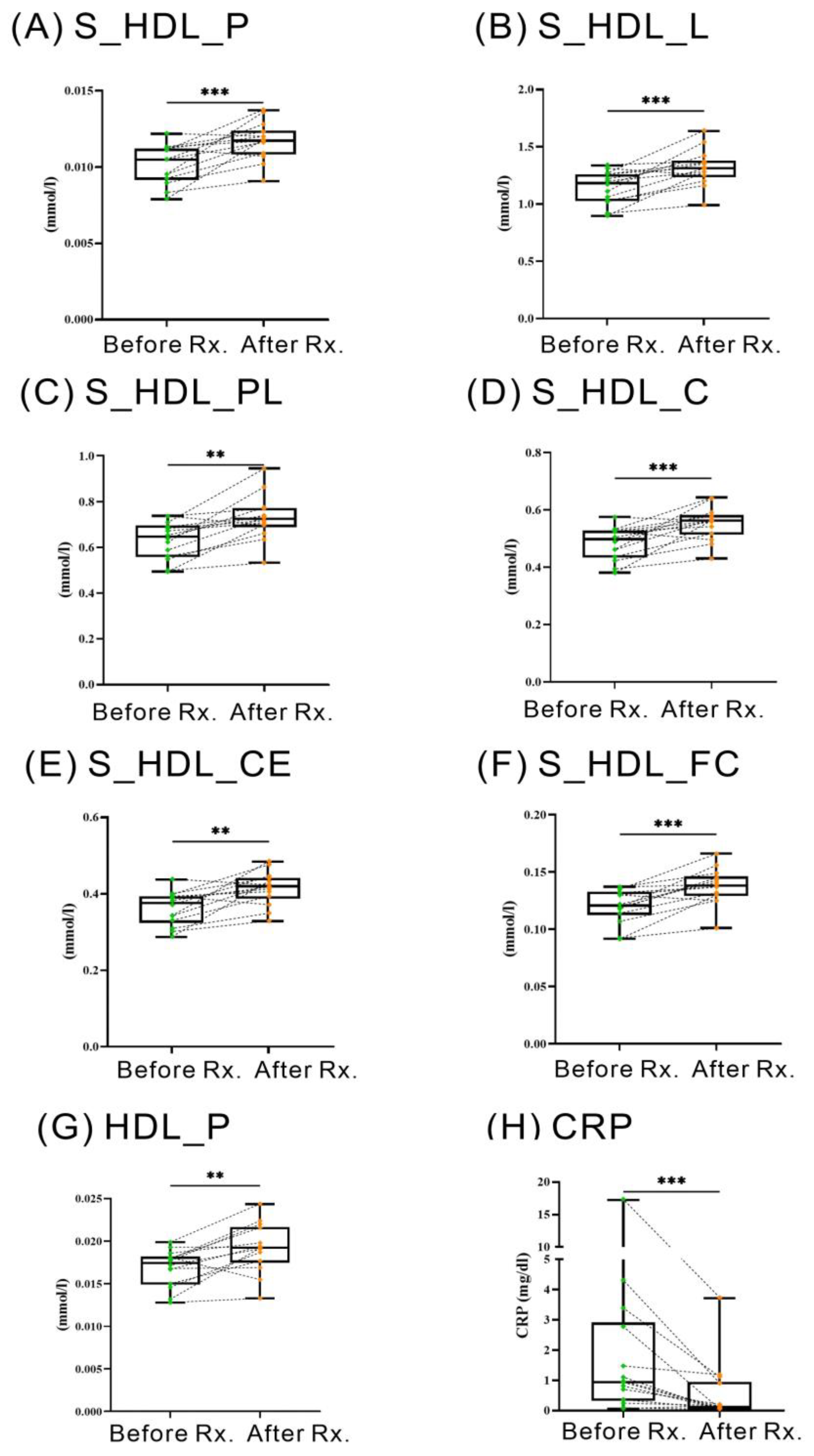
2.6. Changes of HDL-Related Metabolites in RA Patients Treated with JAKi
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Lipid Profile Assessment and Atherogenic Index Calculation
4.3. Employment of 1H-NMR Lipid/Metabolomics for the Determination of Serum Lipid Metabolites
4.4. Data Processing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACPA | Anti-citrullinated peptide antibodies |
| ASCVD | Atherosclerotic cardiovascular diseases |
| bDMARDs | Biologic DMARDs |
| C | Cholesterol |
| CE | Cholesterol ester |
| CETP | Cholesteryl ester transfer protein |
| CRP | C-reactive protein |
| csDMARDs | Conventional synthetic DMARDs |
| DAS28 | 28-joint disease activity score |
| FC | Free cholesterol |
| HC | Healthy control |
| HDL | High-density lipoprotein |
| HDL-c | High-density lipoprotein cholesterol |
| IQR | Interquartile range |
| JAKi | Janus kinase inhibitors |
| L | Total lipids |
| LC/MS | Liquid chromatography/mass spectrometry |
| LDL-c | Low-density lipoprotein cholesterol |
| L-HDL | Large-sized HDLs |
| P | Particle number |
| PL | Phospholipids |
| PLTP | Phospholipid transfer protein |
| RA | Rheumatoid arthritis |
| RF | Rheumatoid factor |
| XL-HDL | Very large-sized HDLs |
References
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Aikawa, M. High-density lipoproteins: From function to therapy. J. Am. Coll. Cardiol. 2012, 60, 2380–2383. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Millan, I.; Yang, S.; DuVall, S.L.; Chen, L.; Baddley, J.; Cannon, G.W.; Delzell, E.S.; Zhang, J.; Safford, M.M.; Patkar, N.M.; et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: Data from the National Veterans Health Administration. Ann. Rheum. Dis. 2016, 75, 341–347. [Google Scholar] [CrossRef]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Negre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arter. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R.; Grunfeld, C. Effect of inflammation on HDL structure and function. Curr. Opin. Lipidol. 2016, 27, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014, 53, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Im, C.H.; Kim, N.R.; Kang, J.W.; Kim, J.H.; Kang, J.Y.; Bae, G.B.; Nam, E.J.; Kang, Y.M. Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology 2015, 54, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Gomez Rosso, L.; Lhomme, M.; Merono, T.; Sorroche, P.; Catoggio, L.; Soriano, E.; Saucedo, C.; Malah, V.; Dauteuille, C.; Boero, L.; et al. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: Relevance of inflammation. Atherosclerosis 2014, 237, 652–660. [Google Scholar] [CrossRef]
- Giraud, C.; Tournadre, A.; Pereira, B.; Dutheil, F.; Soubrier, M.; Lhomme, M.; Kontush, A.; Sebedio, J.L.; Capel, F. Alterations of HDL particle phospholipid composition and role of inflammation in rheumatoid arthritis. J. Physiol. Biochem. 2019, 75, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Watanabe, J.; Lee, Y.Y.; Furst, D.E.; Amjadi, S.; Elashoff, D.; Park, G.; McMahon, M.; Paulus, H.E.; Fogelman, A.M.; et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009, 60, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, E.; Fransen, J.; Lemmers, H.; Stalenhoef, A.; Joosten, L.; van Riel, P.; Popa, C.D. High-density lipoprotein cholesterol subfractions HDL2 and HDL3 are reduced in women with rheumatoid arthritis and may augment the cardiovascular risk of women with RA: A cross-sectional study. Arthritis Res. Ther. 2012, 14, R116. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.H.; Mishra, P.P.; Mononen, N.; Hilvo, M.; Sievanen, H.; Juonala, M.; Laaksonen, M.; Hutri-Kahonen, N.; Viikari, J.; Kahonen, M.; et al. Lipidomic architecture shared by subclinical markers of osteoporosis and atherosclerosis: The Cardiovascular Risk in Young Finns Study. Bone 2020, 131, 115160. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chang, K.H.; Cheng, W.C.; Chen, P.K.; Chiang, E.I.; Chang, S.H.; Li, Y.C.; Chen, C.H.; Chen, D.Y. Lipid metabolomic signature might predict subclinical atherosclerosis in patients with active rheumatoid arthritis. Clin. Exp. Rheumatol. 2022, 41, 1120–11280. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Wurtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [Green Version]
- van Boheemen, L.; van Beers-Tas, M.H.; Kroese, J.M.; van de Stadt, L.A.; van Schaardenburg, D.; Nurmohamed, M.T. Cardiovascular risk in persons at risk of developing rheumatoid arthritis. PLoS ONE 2020, 15, e0237072. [Google Scholar] [CrossRef]
- Geraldino-Pardilla, L.; Giles, J.T.; Sokolove, J.; Zartoshti, A.; Robinson, W.H.; Budoff, M.; Detrano, R.; Bokhari, S.; Bathon, J.M. Association of Anti-Citrullinated Peptide Antibodies with Coronary Artery Calcification in Rheumatoid Arthritis. Arthritis Care Res. 2017, 69, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Torp-Pedersen, C.; Hansen, P.R. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: A Danish nationwide cohort study. Ann. Rheum. Dis. 2011, 70, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Audo, R.; Deckert, V.; Daien, C.I.; Che, H.; Elhmioui, J.; Lemaire, S.; Pais de Barros, J.P.; Desrumaux, C.; Combe, B.; Hahne, M.; et al. PhosphoLipid transfer protein (PLTP) exerts a direct pro-inflammatory effect on rheumatoid arthritis (RA) fibroblasts-like-synoviocytes (FLS) independently of its lipid transfer activity. PLoS ONE 2018, 13, e0193815. [Google Scholar] [CrossRef] [Green Version]
- Yazdanyar, A.; Yeang, C.; Jiang, X.C. Role of phospholipid transfer protein in high-density lipoprotein- mediated reverse cholesterol transport. Curr. Atheroscler. Rep. 2011, 13, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Yazdanyar, A.; Lou, B.; Chen, Y.; Zhao, X.; Li, R.; Hoang Bui, H.; Kuo, M.S.; Navab, M.; Qin, S.; et al. Adipocyte phospholipid transfer protein and lipoprotein metabolism. Arter. Thromb. Vasc. Biol. 2015, 35, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz-Amaro, I.; Gonzalez-Gay, M.A.; Garcia-Dopico, J.A.; Diaz-Gonzalez, F. Cholesteryl ester transfer protein in patients with rheumatoid arthritis. J. Rheumatol. 2013, 40, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Klerkx, A.H.; El Harchaoui, K.; van der Steeg, W.A.; Boekholdt, S.M.; Stroes, E.S.; Kastelein, J.J.; Kuivenhoven, J.A. Cholesteryl ester transfer protein (CETP) inhibition beyond raising high-density lipoprotein cholesterol levels: Pathways by which modulation of CETP activity may alter atherogenesis. Arter. Thromb. Vasc. Biol. 2006, 26, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, M.; Yamashita, S.; Sakai, N.; Arai, T.; Hirano, K.; Hiraoka, H.; Kameda-Takemura, K.; Matsuzawa, Y. Large and cholesteryl ester-rich high-density lipoproteins in cholesteryl ester transfer protein (CETP) deficiency can not protect macrophages from cholesterol accumulation induced by acetylated low-density lipoproteins. J. Biochem. 1994, 116, 257–262. [Google Scholar] [CrossRef]
- Lee, R.G.; Kelley, K.L.; Sawyer, J.K.; Farese, R.V., Jr.; Parks, J.S.; Rudel, L.L. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 2004, 95, 998–1004. [Google Scholar] [CrossRef]
- Okada, T.; Ohama, T.; Okazaki, M.; Kanno, K.; Matsuda, H.; Sairyo, M.; Zhu, Y.; Saga, A.; Kobayashi, T.; Masuda, D.; et al. Particle number analysis of lipoprotein subclasses by gel permeation HPLC in patients with cholesteryl ester transfer protein deficiency. PLoS ONE 2018, 13, e0190875. [Google Scholar] [CrossRef]
- van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: Significance for cardiovascular risk: The IDEAL and EPIC-Norfolk studies. J. Am. Coll. Cardiol. 2008, 51, 634–642. [Google Scholar] [CrossRef] [Green Version]
- McLaren, D.G.; Previs, S.F.; Phair, R.D.; Stout, S.J.; Xie, D.; Chen, Y.; Salituro, G.M.; Xu, S.S.; Castro-Perez, J.M.; Opiteck, G.J.; et al. Evaluation of CETP activity in vivo under non-steady-state conditions: Influence of anacetrapib on HDL-TG flux. J. Lipid Res. 2016, 57, 398–409. [Google Scholar] [CrossRef] [Green Version]
- Hardardottir, I.; Moser, A.H.; Fuller, J.; Fielding, C.; Feingold, K.; Grunfeld, C. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J. Clin. Investig. 1996, 97, 2585–2592. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessie, G.; Tadesse, Y.; Demelash, B.; Genet, S. Assessment of Serum Lipid Profiles and High-sensitivity C-reactive Protein Among Patients Suffering from Rheumatoid Arthritis at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: A Cross-Sectional Study. Open Access Rheumatol. 2020, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; He, Y.; Liu, L.; Ou, Q.; Lin, J. Association of serum lipids with autoantibodies and inflammatory markers in rheumatoid arthritis patients. Clin. Chim. Acta. 2018, 486, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.V.; Shrivastava, A.K.; Raizada, A.; Singh, S.K.; Pandey, A.; Singh, N.; Yadav, D.; Sharma, H. Atherogenic lipid profile and high sensitive C-reactive protein in patients with rheumatoid arthritis. Clin. Biochem. 2013, 46, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Oeser, A.; Raggi, P.; Sokka, T.; Pincus, T.; Solus, J.F.; Linton, M.F.; Fazio, S.; Stein, C.M. Lipoprotein subclasses determined by nuclear magnetic resonance spectroscopy and coronary atherosclerosis in patients with rheumatoid arthritis. J. Rheumatol. 2010, 37, 1633–1638. [Google Scholar] [CrossRef] [Green Version]
- Nash, P.; Kerschbaumer, A.; Dorner, T.; Dougados, M.; Fleischmann, R.M.; Geissler, K.; McInnes, I.; Pope, J.E.; van der Heijde, D.; Stoffer-Marx, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: A consensus statement. Ann. Rheum. Dis. 2021, 80, 71–87. [Google Scholar] [CrossRef]
- Dhillon, S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1987–2001. [Google Scholar] [CrossRef]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Fleischmann, R.; Davignon, J.; Schwartz, H.; Turner, S.M.; Beysen, C.; Milad, M.; Hellerstein, M.K.; Luo, Z.; Kaplan, I.V.; et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015, 67, 616–625. [Google Scholar] [CrossRef]
- Taylor, P.C.; Kremer, J.M.; Emery, P.; Zuckerman, S.H.; Ruotolo, G.; Zhong, J.; Chen, L.; Witt, S.; Saifan, C.; Kurzawa, M.; et al. Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann. Rheum. Dis. 2018, 77, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Wang, G.; Ran, N.; Lin, H.; Wang, Z.; Guan, X.; Yuan, Y.; Fang, K.; Liu, J.; Wang, F. Inhibitory Effect of Methotrexate on Rheumatoid Arthritis Inflammation and Comprehensive Metabolomics Analysis Using Ultra-Performance Liquid Chromatography-Quadrupole Time of Flight-Mass Spectrometry (UPLC-Q/TOF-MS). Int. J. Mol. Sci. 2018, 19, 2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naerr, G.W.; Rein, P.; Saely, C.H.; Drexel, H. Effects of synthetic and biological disease modifying antirheumatic drugs on lipid and lipoprotein parameters in patients with rheumatoid arthritis. Vascul. Pharmacol. 2016, 81, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yousri, N.A.; Bayoumy, K.; Elhaq, W.G.; Mohney, R.P.; Emadi, S.A.; Hammoudeh, M.; Halabi, H.; Masri, B.; Badsha, H.; Uthman, I.; et al. Large Scale Metabolic Profiling identifies Novel Steroids linked to Rheumatoid Arthritis. Sci. Rep. 2017, 7, 9137. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledingham, J.; Deighton, C.; British Society for Rheumatology Standards; Guidelines and Audit Working Group. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology 2005, 44, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Ramirez, D.; Herrington, W.G.; Alegre-Diaz, J.; Staplin, N.; Ramirez-Reyes, R.; Gnatiuc, L.F.; Hill, M.; Romer, F.; Trichia, E.; Bragg, F.; et al. Adiposity and NMR-measured lipid and metabolic biomarkers among 30,000 Mexican adults. Commun. Med. 2022, 2, 143. [Google Scholar] [CrossRef]
| RA (n = 80) | HC (n = 15) | p-Value | |
|---|---|---|---|
| Age at entry, years | 56.1 ± 9.5 | 48.5 ± 13.1 | 0.0525 |
| Gender (female), n (%) | 64 (80.0%) | 10 (66.7%) | 0.3098 |
| Body mass index, kg/m2 | 23.8 ± 4.0 | 23.0 ± 2.2 | 0.6646 |
| Disease duration (months) | 75.0 ± 29.3 | NA | |
| RF positive, n (%) | 60 (75.0%) | NA | |
| ACPA positive, n (%) | 58 (72.5%) | NA | |
| ESR, mm/h | 23.5 (13.0–35.5) | NA | |
| C reactive protein, mg/dL | 0.83 (0.12–1.81) | NA | |
| TC, mg/dL | 201.0 (168.0–228.0) | 182.0 (148.0–225.0) | 0.4466 |
| TG, mg/dL | 89.5 (64.3–131.8) | 81.5 (42.5–147.5) | 0.4469 |
| HDL-C, mg/dL | 61.5 (49.9–72.9) | 51.3 (44.5–75.5) | 0.4438 |
| LDL-C, mg/dL | 120.2 (87.6–143.4) | 121.7 (90.4–151.4) | 0.9279 |
| Atherogenic index | 3.23 (2.62–4.25) | 3.69 (2.59–4.20) | 0.6999 |
| Stains treatment | 14 (17.5%) | 1 (6.7%) | 0.4519 |
| Concomitant csDMARDs | |||
| Methotrexate, n (%) | 64 (80.0%) | NA | |
| Hydroxychloroquine, n (%) | 51 (63.8%) | NA | |
| Sulfasalazine, n (%) | 48 (60.0%) | NA | |
| The use of bDMARDs | |||
| TNFi plus csDMARDs, n (%) | 17 (21.3%) | NA | |
| Tocilizumab plus csDMARDs, n (%) | 11 (13.8%) | NA | |
| Tocilizumab monotherapy, n (%) | 5 (6.3%) | NA | |
| Abatacept plus csDMARDs, n (%) | 6 (7.5%) | NA | |
| Rituximab plus csDMARDs, n (%) | 1 (1.3%) | NA | |
| The use of tsDMARDs | |||
| Tofacitinib plus csDMARDs, n (%) | 26 (32.5%) | NA | |
| Tofacitinib monotherapy, n (%) | 4 (5.0%) | NA | |
| Baricitinib plus csDMARDs, n (%) | 3 (3.8%) | NA | |
| Baricitinib monotherapy, n (%) | 1 (1.3%) | NA | |
| Upatacitinib plus csDMARDs, n (%) | 2 (2.5%) | NA | |
| Comorbidities, n (%) | |||
| Hypertension, n (%) | 15 (18.8%) | 1 (6.7%) | 0.4532 |
| Diabetes mellitus, n (%) | 3 (3.8%) | 0 (0%) | >0.9999 |
| Current smoker, n (%) | 11 (13.8%) | 1 (6.7%) | 0.6837 |
| CRP Levels | CRP Levels | ||||
|---|---|---|---|---|---|
| Small-Sized HDL Composition | r Value | p-Value | Medium-Sized HDL Composition | r Value | p-Value |
| Particle number (P) | −0.411 | <0.001 | Particle number (P) | −0.225 | 0.045 |
| Total lipids (L) | −0.38 | <0.001 | Total lipids (L) | −0.206 | 0.067 |
| Phospholipids (PL) | −0.338 | 0.002 | Phospholipids (PL) | −0.190 | 0.091 |
| Cholesterol (C) | −0.403 | <0.001 | Cholesterol (C) | −0.191 | 0.090 |
| Cholesteryl ester (CE) | −0.382 | <0.001 | Cholesteryl ester (CE) | −0.183 | 0.104 |
| Free cholesterol (FC) | −0.433 | <0.001 | Free cholesterol (FC) | −0.225 | 0.045 |
| Triglycerides (TG) | −0.163 | 0.150 | Triglycerides (TG) | −0.254 | 0.023 |
| CRP Levels | CRP Levels | ||||
| Large-Sized HDL Composition | r Value | p-Value | Very Large-Sized HDL Composition | r Value | p-Value |
| Particle number (P) | −0.110 | 0.333 | Particle number (P) | −0.116 | 0.305 |
| Total lipids (L) | −0.032 | 0.776 | Total lipids (L) | −0.095 | 0.404 |
| Phospholipids (PL) | 0.006 | 0.960 | Phospholipids (PL) | −0.078 | 0.490 |
| Cholesterol (C) | −0.046 | 0.684 | Cholesterol (C) | −0.087 | 0.443 |
| Cholesteryl ester (CE) | −0.073 | 0.519 | Cholesteryl ester (CE) | −0.088 | 0.438 |
| Free cholesterol (FC) | 0.016 | 0.888 | Free cholesterol (FC) | −0.088 | 0.438 |
| Triglycerides (TG) | −0.223 | 0.047 | Triglycerides (TG) | −0.277 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Chiang, E.-P.I.; Chang, K.-H.; Tang, K.-T.; Chen, P.-K.; Yip, H.-T.; Chen, C.-H.; Chen, D.-Y. The Sizes and Composition of HDL-Cholesterol Are Significantly Associated with Inflammation in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2023, 24, 10645. https://doi.org/10.3390/ijms241310645
Chang C-K, Chiang E-PI, Chang K-H, Tang K-T, Chen P-K, Yip H-T, Chen C-H, Chen D-Y. The Sizes and Composition of HDL-Cholesterol Are Significantly Associated with Inflammation in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2023; 24(13):10645. https://doi.org/10.3390/ijms241310645
Chicago/Turabian StyleChang, Ching-Kun, En-Pei Isabel Chiang, Kuang-Hsi Chang, Kuo-Tung Tang, Po-Ku Chen, Hei-Tung Yip, Chu-Huang Chen, and Der-Yuan Chen. 2023. "The Sizes and Composition of HDL-Cholesterol Are Significantly Associated with Inflammation in Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 24, no. 13: 10645. https://doi.org/10.3390/ijms241310645
APA StyleChang, C.-K., Chiang, E.-P. I., Chang, K.-H., Tang, K.-T., Chen, P.-K., Yip, H.-T., Chen, C.-H., & Chen, D.-Y. (2023). The Sizes and Composition of HDL-Cholesterol Are Significantly Associated with Inflammation in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 24(13), 10645. https://doi.org/10.3390/ijms241310645






