CC Chemokine 2 Promotes Ovarian Cancer Progression through the MEK/ERK/MAP3K19 Signaling Pathway
Abstract
:1. Introduction
2. Results
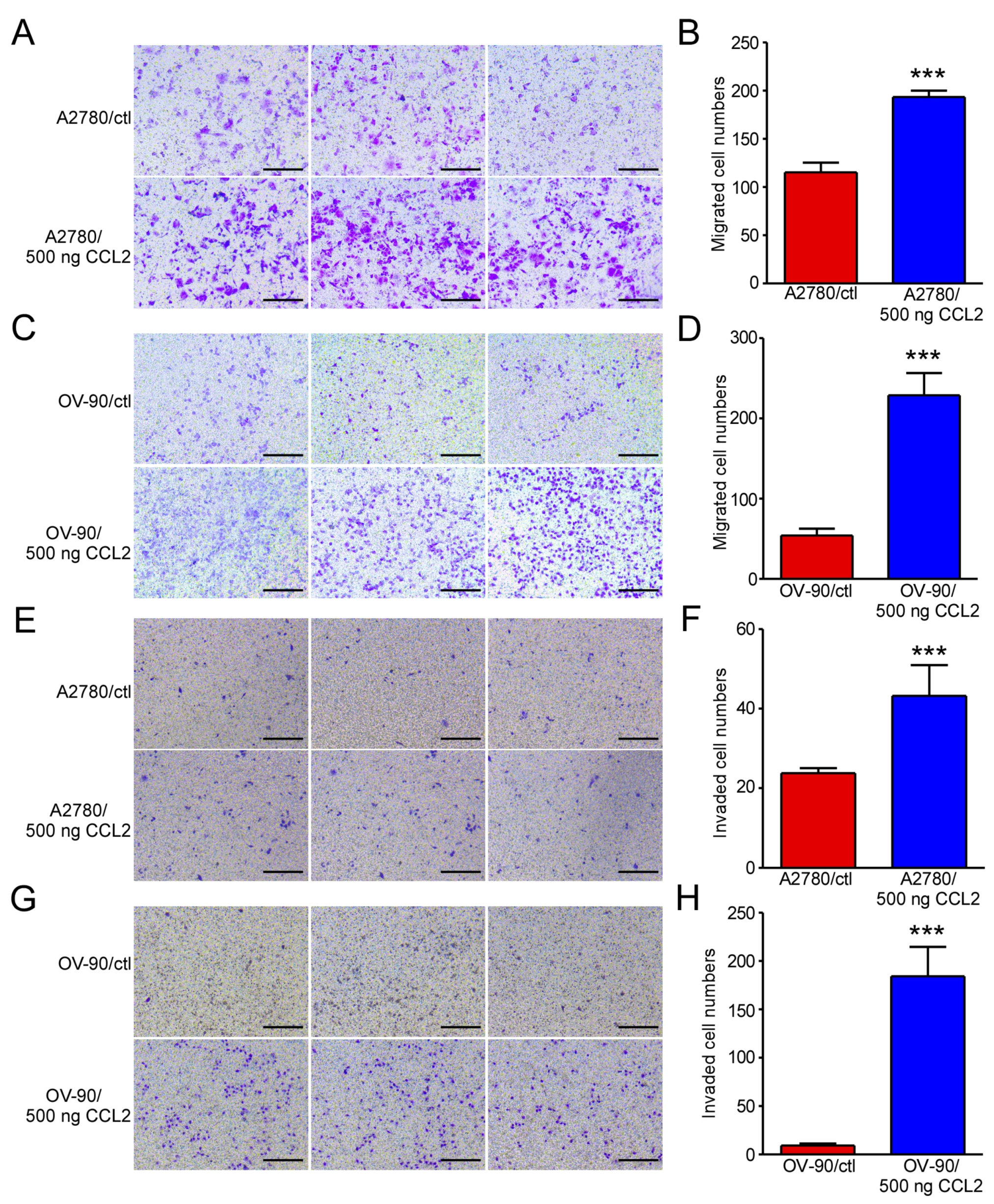
2.1. Exogenous CCL2 Promoted the Migration and Invasion of Ovarian Cancer Cells
2.2. CCL2 Overexpression Promoted Ovarian Cancer Cell Proliferation, Migration, and Invasion
2.3. CCL2 Knockout Inhibited Ovarian Cancer Cell Proliferation, Migration, and Invasion
2.4. MAP3K19 Was the Key Target for CCL2 in Regulating Ovarian Cancer Progression
2.5. MAP3K19 Knockout Inhibited Ovarian Cancer Cell Proliferation, Migration, and Invasion
2.6. CCL2 Promoted MAP3K19 Expression by Activating the MEK/ERK Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Generation of CCL2-Overexpressing Cell Lines
4.3. Generation of KO Cell Lines Using CRISPR/Cas9
4.4. Transcriptome Sequencing and Analysis
4.5. MTT Assays
4.6. Cell Migration and Invasion Assays
4.7. Inhibitor Treatment Assays
4.8. Quantitative Real-Time PCR
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, M.; Bennett, S.; Wang, Z.; Pfleger, K.D.G.; Xu, J. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J. Cell. Physiol. 2021, 236, 7211–7222. [Google Scholar] [CrossRef]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, L.L.; Feng, Y.; Wen, Y.; Wu, W.J.; Ni, H.F.; Li, Z.L.; Zhou, L.T.; Wang, B.; Zhang, J.D.; Crowley, S.D.; et al. Exosomal CCL2 from tubular epithelial cells is critical for Albumin-induced tubulointerstitial inflammation. J. Am. Soc. Nephrol. 2018, 29, 919–935. [Google Scholar] [CrossRef] [Green Version]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Pituitary tumour fibroblast-derived cytokines influence tumour aggressiveness. Endocr. Relat. Cancer 2019, 26, 853–865. [Google Scholar] [CrossRef]
- Yang, J.; Agarwal, M.; Ling, S.; Teitz-Tennenbaum, S.; Zemans, R.L.; Osterholzer, J.J.; Sisson, T.H.; Kim, K.K. Diverse injury pathways induce alveolar epithelial cell CCL2/12, which promotes lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 622–632. [Google Scholar] [CrossRef]
- Yumimoto, K.; Sugiyama, S.; Mimori, K.; Nakayama, K.I. Potentials of C-C motif chemokine 2-C-C chemokine receptor type 2 blockers including propagermanium as anticancer agents. Cancer Sci. 2019, 110, 2090–2099. [Google Scholar] [CrossRef]
- Shono, K.; Yamaguchi, I.; Mizobuchi, Y.; Kagusa, H.; Sumi, A.; Fujihara, T.; Nakajima, K.; Kitazato, K.T.; Matsuzaki, K.; Saya, H.; et al. Downregulation of the CCL2/CCR2 and CXCL10/CXCR3 axes contributes to antitumor effects in a mouse model of malignant glioma. Sci. Rep. 2020, 10, 15286. [Google Scholar] [CrossRef] [PubMed]
- Senarath, S.; Ades, A.; Nanayakkara, P. Ovarian cysts in pregnancy: A narrative review. J. Obstet. Gynaecol. 2021, 41, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nesbitt, K.M.; Bakkum-Gamez, J.N. Quality improvement in gynecologic oncology: Current successes and future promise. Gynecol. Oncol. 2019, 152, 486–491. [Google Scholar] [CrossRef]
- EI-Arabey, A.A.; Abdalla, M.; Abd-Allah, A.R. GATA3 and stemness of high-grade serous ovarian carcinoma: Novel hope for the deadliest type of ovarian cancer. Hum. Cell 2020, 33, 904–906. [Google Scholar] [CrossRef]
- EI-Arabey, A.A.; Abdalla, M. The role of GATA3 in the metastasis of triple-negative breast cancer and high-grade serous ovarian cancer. Hum. Cell 2022, 35, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, K.A.; Lisowska, K.M. Ovarian cancer—From biology to clinic. Postepy Hig Me.d Dosw. 2015, 69, 1275–1290. [Google Scholar] [CrossRef]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian cancer prevention and screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef] [Green Version]
- Sehouli, J.; Grabowski, J.P. Surgery in recurrent ovarian cancer. Cancer 2019, 125 (Suppl. S24), 4598–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Liu, L.; Li, C.; Luo, N.; Chen, R.; Li, L.; Yu, F.; Cheng, Z. TRIM52 plays an oncogenic role in ovarian cancer associated with NF-kB pathway. Cell Death Dis. 2018, 9, 908. [Google Scholar] [CrossRef] [Green Version]
- Bushley, A.W.; Ferrell, R.; McDuffie, K.; Terada, K.Y.; Carney, M.E.; Thompson, P.J.; Wilkens, L.R.; Tung, K.; Ness, R.B.; Goodman, M.T. Polymorphisms of interleukin (IL)-1alpha, IL-1beta, IL-6, IL-10, and IL-18 and the risk of ovarian cancer. Gynecol. Oncol. 2004, 95, 672–679. [Google Scholar] [CrossRef]
- Kampan, N.C.; Xiang, S.D.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Immunotherapeutic interleukin-6 or interleukin-6 receptor blockade in cancer: Challenges and opportunities. Curr. Med. Chem. 2018, 25, 4785–4806. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Turton, M.; Santillan, A.; Lu, D.; Bristow, R.E.; Chan, K.C.; Shih, I.M.; Roden, R.B.S. P53 autoantibodies, cytokine levels and ovarian carcinogenesis. Gynecol. Oncol. 2009, 114, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geller, M.A.; Bui-Nguyen, T.M.; Rogers, L.M.; Ramakrishnan, S. Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int. J. Gynecol. Cancer 2010, 20, 918–925. [Google Scholar] [PubMed]
- Liu, W.; Wang, L.; Zhang, J.; Qiao, L.; Liu, Y.; Yang, X.; Zhang, J.; Zheng, W.; Ma, Z. Purification of recombinant human chemokine CCL2 in E. coli and its function in ovarian cancer. 3 Biotech 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Soeda, S.; Kiko, Y.; Suzuki, O.; Hashimoto, Y.; Watanabe, T.; Nishiyama, H.; Tasaki, K.; Hojo, H.; Abe, M.; et al. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res. 2013, 33, 4785–4790. [Google Scholar]
- Yasui, H.; Kajiyama, H.; Tamauchi, S.; Suzuki, S.; Peng, Y.; Yoshikawa, N.; Sugiyama, M.; Nakamura, K.; Kikkawa, F. CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clin. Exp. Metastasis 2020, 37, 145–158. [Google Scholar] [CrossRef]
- Li, S.; Lv, M.; Qiu, S.; Meng, J.; Liu, W.; Zuo, J.; Yang, L. NF-κB p65 promotes ovarian cancer cell proliferation and migration via regulating mortalin. J. Cell. Mol. Med. 2019, 23, 4338–4348. [Google Scholar] [CrossRef] [Green Version]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Wu, Y.; Wan, P.; Yang, G. Rap1A promotes ovarian cancer metastasis via activation of ERK/p38 and notch signaling. Cancer Med. 2016, 5, 3544–3554. [Google Scholar] [CrossRef]
- Deng, W.; Gu, X.; Lu, Y.; Gu, C.; Zheng, Y.; Zhang, Z.; Chen, L.; Yao, Z.; Li, L.Y. Down-modulation of TNFSF15 in ovarian cancer by VEGF and MCP-1 is a pre-requisite for tumor neovascularization. Angiogenesis 2012, 15, 71–85. [Google Scholar] [CrossRef]
- Pasquier, J.; Gosset, M.; Gey, C.; Hoarau-Véchot, J.; Chevrot, A.; Pocard, M.; Mirshahi, M.; Lis, R.; Rafii, A.; Touboul, C. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol. Cancer 2018, 17, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EI-Arabey, A.A.; Denizli, M.; Kanlikilicer, P.; Bayraktar, R.; Ivan, C.; Rashed, M.; Kabil, N.; Ozpolat, B.; Calin, G.A.; Salama, S.A.; et al. GATA3 as a master regulator for interactions of tumor-associated macrophages with high-grade serous ovarian carcinoma. Cell. Signal. 2020, 68, 109539. [Google Scholar] [CrossRef] [PubMed]
- EI-Arabey, A.A.; Abdalla, M.; Abd-Allah, A.R. SnapShot: TP53 status and macrophages infiltration in TCGA-analyzed tumors. Int. Immunopharmacol. 2020, 86, 106758. [Google Scholar] [CrossRef]
- Miyamoto, T.; Murakamo, R.; Hamanishi, J.; Tanigaki, K.; Hosoe, Y.; Mise, N.; Takamatsu, S.; Mise, Y.; Ukita, M.; Taki, M.; et al. B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol. Res. 2022, 10, 56–69. [Google Scholar] [CrossRef]
- Boehme, S.A.; Franz-Bacon, K.; DiTirro, D.N.; Ly, T.W.; Bacon, K.B. MAP3K19 is a novel regulator of TGF-β signaling that impacts Bleomycin-induced lung injury and pulmonary fibrosis. PLoS ONE 2016, 11, e0154874. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.C.; Espindola, M.S.; Narayanan, R.; Coelho, A.L.; Habiel, D.M.; Boehme, S.A.; Ly, T.W.; Bacon, K.B.; Hogaboam, C.M. Targeting MAP3K19 prevents human lung myofibroblast activation both in vitro and in a humanized SCID model of idiopathic pulmonary fibrosis. Sci. Rep. 2019, 9, 19796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehme, S.A.; Franz-Bacon, K.; Ludka, J.; DiTirro, D.N.; Ly, T.W.; Bacon, K.B. MAP3K19 is overexpressed in COPD and is a central mediator of cigarette smoke-induced pulmonary inflammation and lower airway destruction. PLoS ONE 2016, 11, e0167169. [Google Scholar] [CrossRef]
- Hoang, V.T.; Nyswaner, K.; Torres-Ayuso, P.; Brognard, J. The protein kinase MAP3K19 phosphorylates MAP2Ks and thereby activates ERK and JNK kinases and increases viability of KRAS-mutant lung cancer cells. J. Biol. Chem. 2020, 295, 8470–8479. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Huang, J.; Jia, X.; Deng, M.; Cui, D.; Du, Z.; Fu, G.; Ouyang, G.; Xiao, C. A critical role of periostin in B-cell acute lymphoblastic leukemia. Leukemia 2017, 31, 1835–1837. [Google Scholar] [CrossRef]
- Zaboli, K.A.; Rahimi, H.; Thekkiniath, J.; Taromchi, A.H.; Kaboli, S. Plasmid-based CRISPR-Cas9 system efficacy for introducing targeted mutations in CD81 gene of MDA-MB-231 cell line. Folia Histochem. Cytobiol. 2022, 60, 13–23. [Google Scholar] [CrossRef]
- Fan, C.; Lin, Y.; Mao, Y.; Huang, Z.; Liu, A.Y.; Ma, H.; Yu, D.; Maitikabili, A.; Xiao, H.; Zhang, C.; et al. MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA1. Oncotarget 2016, 7, 21825–21839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Lin, B.; Huang, Z.; Cui, D.; Zhu, M.; Ma, Z.; Zhang, Y.; Liu, F.; Liu, Y. MicroRNA-873 inhibits colorectal cancer metastasis by targeting ELK1 and STRN4. Oncotarget 2018, 10, 4192–4204. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Zhao, X.; Deng, M.; Huang, Z.; Wang, J.; Wu, Y.; Cui, D.; Liu, Y.; Liu, R.; Ouyang, G. Bone marrow mesenchymal stromal cell-derived periostin promotes B-ALL progression by modulating CCL2 in leukemia cells. Cell. Rep. 2019, 26, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Ma, Z.; Wu, Y.; Yuan, C.; Zhang, Y.; Liang, Z.; Yang, Y.; Zhang, W.; Jiao, P. MST4 negatively regulated type I interferons production via targeting MAVS-mediated pathway. Cell Commun. Signal. 2022, 20, 103. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, L.; Zhang, J.; Cheng, K.; Zheng, W.; Ma, Z. CC Chemokine 2 Promotes Ovarian Cancer Progression through the MEK/ERK/MAP3K19 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 10652. https://doi.org/10.3390/ijms241310652
Liu W, Wang L, Zhang J, Cheng K, Zheng W, Ma Z. CC Chemokine 2 Promotes Ovarian Cancer Progression through the MEK/ERK/MAP3K19 Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(13):10652. https://doi.org/10.3390/ijms241310652
Chicago/Turabian StyleLiu, Wei, Lei Wang, Jiajia Zhang, Kun Cheng, Wenming Zheng, and Zhenling Ma. 2023. "CC Chemokine 2 Promotes Ovarian Cancer Progression through the MEK/ERK/MAP3K19 Signaling Pathway" International Journal of Molecular Sciences 24, no. 13: 10652. https://doi.org/10.3390/ijms241310652




