EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway
Abstract
:1. Introduction
2. Results
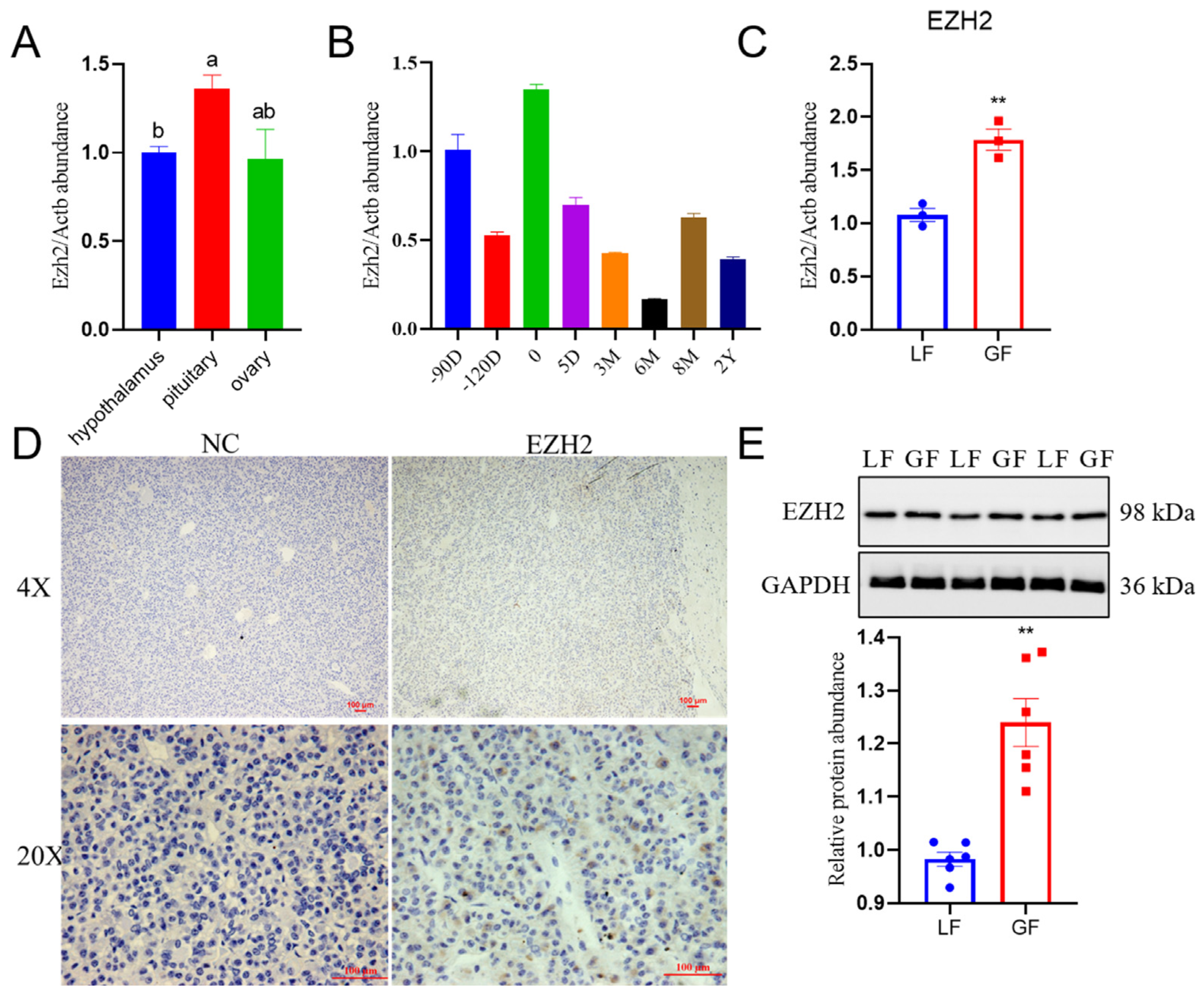
2.1. Profiles for Relative Abundance of EZH2 in the Hu Sheep Pituitary
2.2. Relative EZH2 Abundance in GnRH-Stimulated PCs
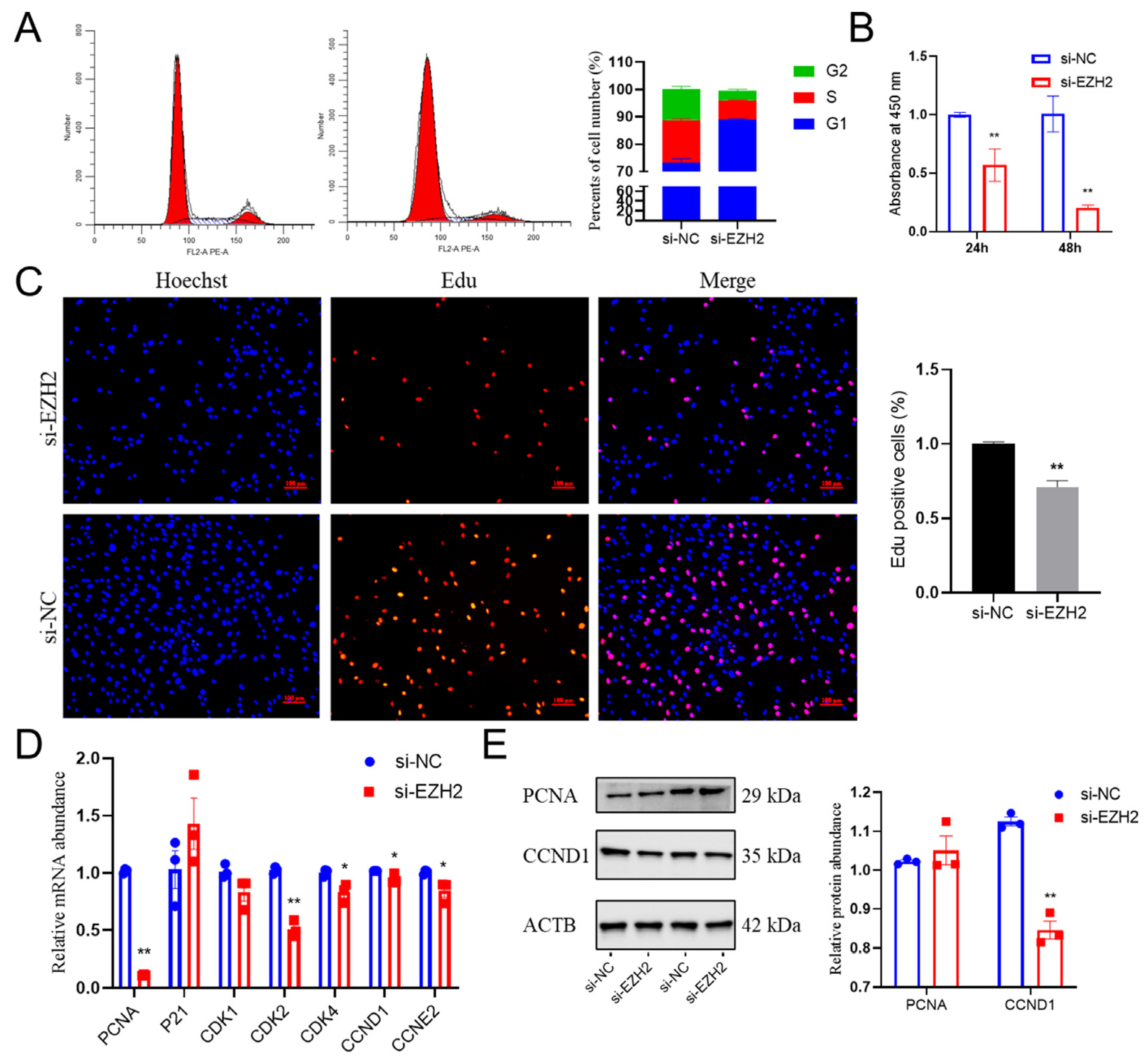
2.3. Effects of EZH2 Abundance Downregulation on Proliferation in PCs
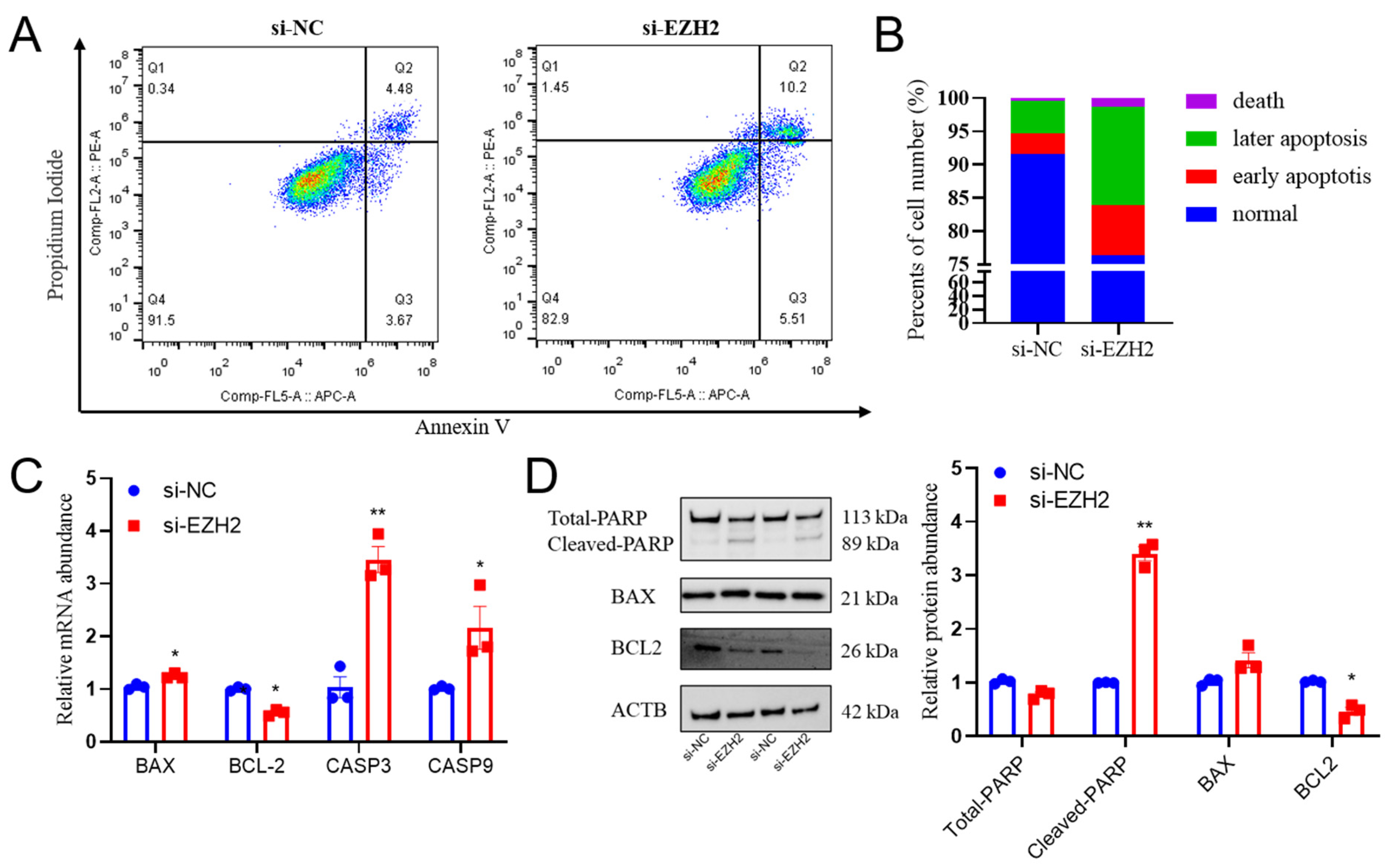
2.4. EZH2 Gene Knockdown Induces Cell Apoptosis in PCs
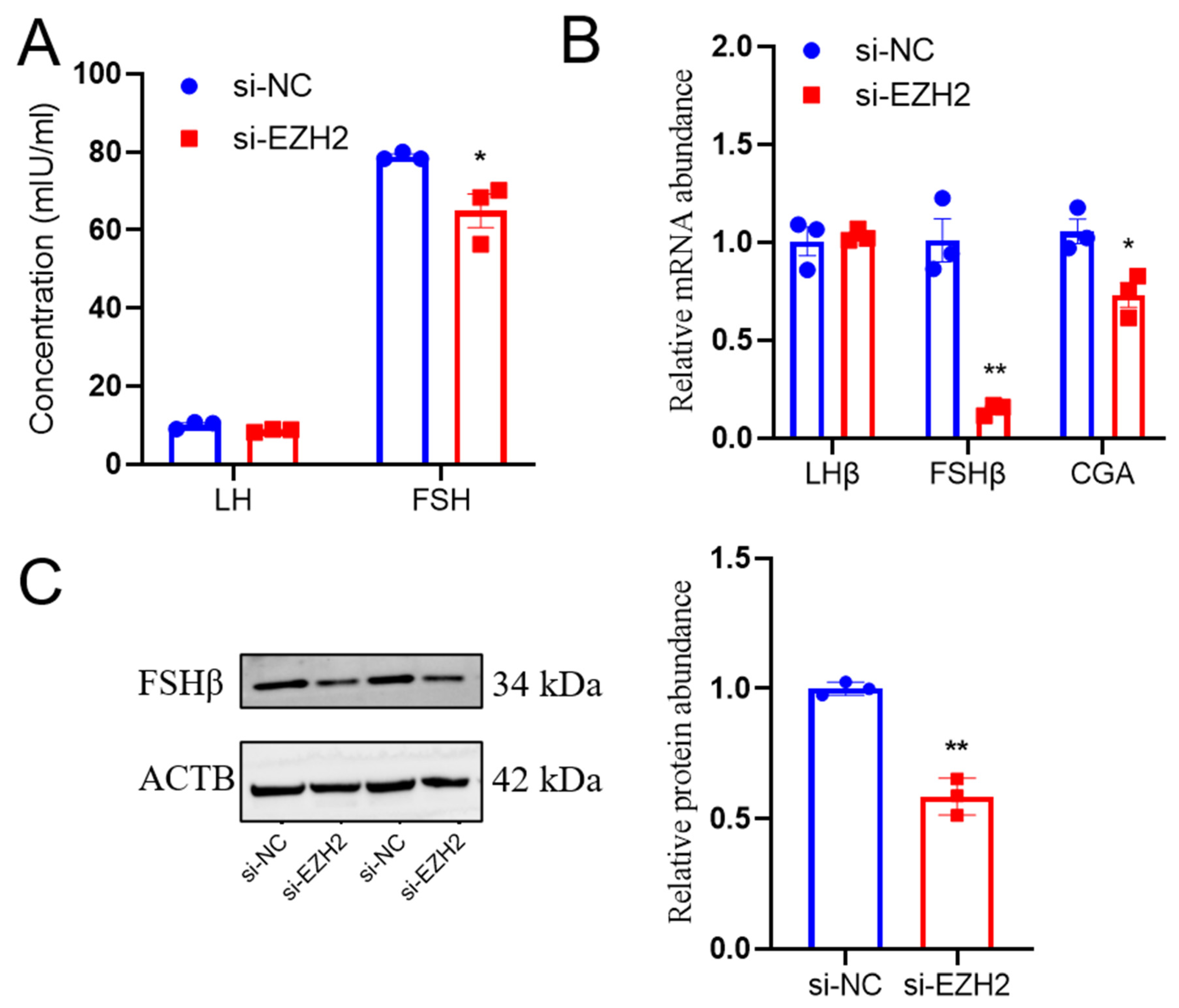
2.5. EZH2 Gene Silencing Led to Reduced FSH Secretion from PCs
2.6. EZH2’s Effects on Regulating the AKT/ERK Pathway in PCs
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Cell Culture and Treatments
4.3. Immunohistochemistry
4.4. Immunofluorescence
4.5. Cell Viability and Proliferation Analysis
4.6. Flow Cytometry Analysis
4.7. ELISA Assay
4.8. Subcellular Localization
4.9. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.10. Western Blot
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Yang, X.; Liu, G.; Deng, M.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. Polymorphisms in BMPR-IB gene and their association with litter size trait in Chinese Hu sheep. Anim. Biotechnol. 2022, 33, 250–259. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Wang, Y.; Chen, X.; Li, F.; Yang, L.; Cui, J.; Li, R.; Cao, B.; An, X.; et al. FecB mutation and litter size are associated with a 90-base pair deletion in BMPR1B in East Friesian and Hu crossbred sheep. Anim. Biotechnol. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Schubert, M.; Perez Lanuza, L.; Gromoll, J. Pharmacogenetics of FSH Action in the Male. Front. Endocrinol. 2019, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Christian, C.A.; Moenter, S.M. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr. Rev. 2010, 31, 544–577. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Li, F.; Wang, F.; Zhang, G.; Pang, J.; Ren, C.; Zhang, T.; Yang, H.; Wang, Z.; Zhang, Y. Genome-wide differential expression profiling of mRNAs and lncRNAs associated with prolificacy in Hu sheep. Biosci. Rep. 2018, 38, 2. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Gao, X.; Bao, Y.; El-Samahy, M.A.; Yang, J.; Wang, Z.; Li, X.; Zhang, G.; Zhang, Y.; Liu, W.; et al. lncRNA FDNCR promotes apoptosis of granulosa cells by targeting the miR-543-3p/DCN/TGF-beta signaling pathway in Hu sheep. Mol. Ther. Nucleic Acids 2021, 24, 223–240. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Gao, X.; Li, X.; Yang, H.; Ei-Samahy, M.A.; Bao, Y.; Xiao, S.; Meng, F.; Wang, F. Unconservative_15_2570409 suppresses progesterone receptor expression in the granulosa cells of Hu sheep. Theriogenology 2020, 157, 303–313. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, H.; Cai, Y.; Ma, J.; Cheng, P.; Wang, Z.; Wang, F.; Zhang, Y. Comparative Transcriptomic Analysis of Hu Sheep Pituitary Gland Prolificacy at the Follicular and Luteal Phases. Genes 2022, 13, 440. [Google Scholar] [CrossRef]
- Han, D.X.; Sun, X.L.; Wang, C.J.; Yu, Z.W.; Zheng, Y.; Huang, Y.J.; Wang, W.H.; Jiang, H.; Gao, Y.; Yuan, B.; et al. Differentially expressed lncRNA-m433s1 regulates FSH secretion by functioning as a miRNA sponge in male rat anterior pituitary cellsdagger. Biol. Reprod. 2019, 101, 416–425. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Diets, I.J.; Waanders, E.; Ligtenberg, M.J.; van Bladel, D.A.; Kamping, E.J.; Hoogerbrugge, P.M.; Jongmans, M.C. High Yield of Pathogenic Germline Mutations Causative or Likely Causative of the Cancer Phenotype in Selected Children with Cancer. Clin. Cancer Res. 2018, 24, 1594–1603. [Google Scholar] [CrossRef] [Green Version]
- Nichol, J.N.; Dupéré-Richer, D.; Ezponda, T.; Licht, J.D.; Miller, W.H., Jr. H3K27 Methylation: A Focal Point of Epigenetic Deregulation in Cancer. Adv. Cancer Res. 2016, 131, 59–95. [Google Scholar]
- Schult, D.; Holsken, A.; Siegel, S.; Buchfelder, M.; Fahlbusch, R.; Kreitschmann-Andermahr, I.; Buslei, R. EZH2 is highly expressed in pituitary adenomas and associated with proliferation. Sci. Rep. 2015, 5, 16965. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Pang, B.; Wang, Q.; Yang, S.; Gao, T.; Ding, Q.; Liu, H.; Yang, Y.; Fan, H.; Zhang, R.; et al. EZH2 upregulation correlates with tumor invasiveness, proliferation, and angiogenesis in human pituitary adenomas. Hum. Pathol. 2017, 66, 101–107. [Google Scholar] [CrossRef]
- Collinson, A.; Collier, A.J.; Morgan, N.P.; Sienerth, A.R.; Chandra, T.; Andrews, S.; Rugg-Gunn, P.J. Deletion of the Polycomb-Group Protein EZH2 Leads to Compromised Self-Renewal and Differentiation Defects in Human Embryonic Stem Cells. Cell Rep. 2016, 17, 2700–2714. [Google Scholar] [CrossRef] [Green Version]
- Nutt, S.L.; Keenan, C.; Chopin, M.; Allan, R.S. EZH2 function in immune cell development. Biol. Chem. 2020, 401, 933–943. [Google Scholar] [CrossRef]
- Wen, Y.; Hou, Y.; Yi, X.; Sun, S.; Guo, J.; He, X.; Li, T.; Cai, J.; Wang, Z. EZH2 activates CHK1 signaling to promote ovarian cancer chemoresistance by maintaining the properties of cancer stem cells. Theranostics 2021, 11, 1795–1813. [Google Scholar] [CrossRef]
- Wei, C.; Ren, H.; Xu, L.; Li, L.; Liu, R.; Zhang, L.; Zhao, F.; Lu, J.; Zhang, X.; Du, L. Signals of Ezh2, Src, and Akt Involve in myostatin-Pax7 pathways regulating the myogenic fate determination during the sheep myoblast proliferation and differentiation. PLoS ONE 2015, 10, e0120956. [Google Scholar] [CrossRef]
- Jiao, W.; Hao, J.; Xie, Y.; Meng, M.; Gao, W. EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovasc. Disord. 2022, 22, 95. [Google Scholar] [CrossRef]
- Yang, H.; Ma, J.; Wan, Z.; Wang, Q.; Wang, Z.; Zhao, J.; Wang, F.; Zhang, Y. Characterization of sheep spermatogenesis through single-cell RNA sequencing. FASEB J. 2021, 35, e21187. [Google Scholar] [CrossRef]
- Xia, Q.; Chu, M.; He, X.; Liu, Q.; Zhang, X.; Zhang, J.; Guo, X.; Di, R. Identification of Photoperiod-Induced LncRNAs and mRNAs in Pituitary Pars Tuberalis of Sheep. Front. Vet. Sci. 2021, 8, 644474. [Google Scholar] [CrossRef]
- Dardente, H.; Lomet, D.; Chesneau, D.; Pellicer-Rubio, M.T.; Hazlerigg, D. Discontinuity in the molecular neuroendocrine response to increasing daylengths in Ile-de-France ewes: Is transient Dio2 induction a key feature of circannual timing. J. Neuroendocrinol. 2019, 31, e12775. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, M.; Liu, Z.; Zhang, G.; Pang, J.; An, S.; Wang, Z.; Zhang, Y.; Wang, F. EZH2 expression and its role in spermatogonial stem cell self-renewal in goats. Theriogenology 2020, 155, 222–231. [Google Scholar] [CrossRef]
- Roof, A.K.; Gutierrez-Hartmann, A. Consider the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol. Cell Endocrinol. 2018, 463, 87–96. [Google Scholar] [CrossRef]
- Chang, M.; Yang, C.; Bao, X.; Wang, R. Genetic and Epigenetic Causes of Pituitary Adenomas. Front. Endocrinol. 2020, 11, 596554. [Google Scholar] [CrossRef]
- Cano, D.A.; Soto-Moreno, A.; Leal-Cerro, A. Genetically engineered mouse models of pituitary tumors. Front. Oncol. 2014, 4, 203. [Google Scholar] [CrossRef] [Green Version]
- Hauser, B.M.; Lau, A.; Gupta, S.; Bi, W.L.; Dunn, I.F. The Epigenomics of Pituitary Adenoma. Front. Endocrinol. 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.; Kalebic, N.; Florio, M.; Lakshmanaperumal, N.; Haffner, C.; Brandl, H.; Henry, I.; Huttner, W.B. Epigenome profiling and editing of neocortical progenitor cells during development. EMBO J. 2017, 36, 2642–2658. [Google Scholar] [CrossRef]
- Grassian, A.R.; Scales, T.M.E.; Knutson, S.K.; Kuntz, K.W.; McCarthy, N.J.; Lowe, C.E.; Moore, J.D.; Copeland, R.A.; Keilhack, H.; Smith, J.J.; et al. A Medium-Throughput Single Cell CRISPR-Cas9 Assay to Assess Gene Essentiality. Biol. Proced. Online 2015, 17, 15. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhang, L.; Gao, T.; Ye, T.; Zhu, Y.; Lei, Q.; Feng, Q.; He, B.; Deng, H.; Yu, L. Selective inhibition of EZH2 by ZLD10A blocks H3K27 methylation and kills mutant lymphoma cells proliferation. Biomed. Pharmacother. 2016, 81, 288–294. [Google Scholar] [CrossRef]
- Sauvageau, M.; Sauvageau, G. Polycomb group proteins: Multi-faceted regulators of somatic stem cells and cancer. Cell Stem. Cell 2010, 7, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.A.; Dong, J.; Dong, Y.; Dong, Y.; Schor, N.; Tweardy, D.J.; Zhang, L.; Mitch, W.E. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J. Biol. Chem. 2015, 290, 11177–11187. [Google Scholar] [CrossRef] [Green Version]
- Knutson, S.K.; Warholic, N.M.; Wigle, T.J.; Klaus, C.R.; Allain, C.J.; Raimondi, A.; Porter Scott, M.; Chesworth, R.; Moyer, M.P.; Copeland, R.A.; et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. USA 2013, 110, 7922–7927. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Zhang, Y.; Wang, Z.P.; Wang, X.X.; Sun, T.C.; Li, X.Y.; Tang, J.X.; Cheng, J.M.; Li, J.; Chen, S.R.; et al. EZH2 deletion promotes spermatogonial differentiation and apoptosis. Reproduction 2017, 154, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Guo, Y.; Zheng, B.; Shao, B.; Jiang, M.; Wang, G.; Zhou, T.; Wang, L.; Zhou, Z.; Guo, X.; et al. Establishment of a proteome profile and identification of molecular markers for mouse spermatogonial stem cells. J. Cell. Mol. Med. 2015, 19, 521–534. [Google Scholar] [CrossRef]
- Abdalkader, L.; Oka, T.; Takata, K.; Sato, H.; Murakami, I.; Otte, A.P.; Yoshino, T. Aberrant differential expression of EZH1 and EZH2 in Polycomb repressive complex 2 among B- and T/NK-cell neoplasms. Pathology 2016, 48, 467–482. [Google Scholar] [CrossRef]
- Lecona, E.; Rojas, L.A.; Bonasio, R.; Johnston, A.; Fernandez-Capetillo, O.; Reinberg, D. Polycomb protein SCML2 regulates the cell cycle by binding and modulating CDK/CYCLIN/p21 complexes. PLoS Biol. 2013, 11, e1001737. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nature reviews. Cancer 2009, 9, 400–414. [Google Scholar]
- Yao, X.; Zhang, G.; Guo, Y.; Ei-Samahy, M.; Wang, S.; Wan, Y.; Han, L.; Liu, Z.; Wang, F.; Zhang, Y. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology 2017, 102, 162–173. [Google Scholar] [CrossRef]
- He, Z.; Jiang, J.; Kokkinaki, M.; Dym, M. Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem. Cells 2009, 27, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Mok, C.K.; Chan, M.C.; Zhang, Y.; Nal, B.; Kien, F.; Bruzzone, R.; Sanyal, S. Cell Cycle-independent Role of Cyclin D3 in Host Restriction of Influenza Virus Infection. J. Biol. Chem. 2017, 292, 5070–5088. [Google Scholar] [CrossRef] [Green Version]
- Janjic, M.M.; Previde, R.M.; Fletcher, P.A.; Sherman, A.; Smiljanic, K.; Abebe, D.; Bjelobaba, I.; Stojilkovic, S.S. Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo. Sci. Rep. 2019, 9, 20098. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Yan, H.; Chen, Y.; Zhang, Q.; Xie, X.; Ding, X.; Wang, X.; Qian, Z.; Xiao, F.; Song, Z.; et al. EZH2 upregulation by ERalpha induces proliferation and migration of papillary thyroid carcinoma. BMC Cancer 2019, 19, 1094. [Google Scholar] [CrossRef] [Green Version]
- Wan, D.; Han, X.; Zhang, C.; Zhang, Y.; Ma, Y.; Wang, G. EZH2 promotes the progression of osteosarcoma through the activation of the AKT/GSK3beta pathway. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1179–1186. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, Y. EZH2 enhances proliferation and migration of trophoblast cell lines by blocking GADD45A-mediated p38/MAPK signaling pathway. Bioengineered 2022, 13, 12583–12597. [Google Scholar] [CrossRef]
- Kosalai, S.T.; Morsy, M.H.A.; Papakonstantinou, N.; Mansouri, L.; Stavroyianni, N.; Kanduri, C.; Stamatopoulos, K.; Rosenquist, R.; Kanduri, M. EZH2 upregulates the PI3K/AKT pathway through IGF1R and MYC in clinically aggressive chronic lymphocytic leukaemia. Epigenetics 2019, 14, 1125–1140. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Yang, H.; Chen, P.; Wang, Z.; Cai, Y.; Yao, X.; Wang, F.; Zhang, Y. The Novel Competing Endogenous Long Noncoding RNA SM2 Regulates Gonadotropin Secretion in the Hu Sheep Anterior Pituitary by Targeting the Oar-miR-16b/TGF-β/SMAD2 Signaling Pathway. Cells 2022, 11, 985. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Yang, H.; Yao, X.; Yang, P.; Ren, C.; Wang, F.; Zhang, Y. Pituitary Transcriptomic Study Reveals the Differential Regulation of lncRNAs and mRNAs Related to Prolificacy in Different FecB Genotyping Sheep. Genes 2019, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Liu, Z.; Ren, C.; Zhang, G.; Pang, J.; Zhang, Y.; Wang, F.; Wan, Y. Long noncoding RNAs exchange during zygotic genome activation in goat. Biol. Reprod. 2018, 99, 707–717. [Google Scholar] [CrossRef]


| Genes | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|
| si-negative control (NC) | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| si-EZH2-2165 | GCAGCUUUCUGUUCAACUUTT | AAGUUGAACAGAAAGCUGCTT |
| Gene Name | Primer Sequence (5′–3′) |
|---|---|
| EZH2-F | TGAAGAAGGGTCAGAACCGC |
| EZH2-R | ACATCAGACGGTGCCAACAG |
| FSHβ-F | GCTATTGCTACACCCGGGAC |
| FSHβ-R | AGTGGCTACTGGGTACGTGT |
| LHβ-F | CGGCTACTGCCTCAGCATGAAG |
| LHβ-R | CACGGGGAAGGAGACCATTGGG |
| PCNA-F | CCTTGGTGCAGCTAACCCTT |
| PCNA-R | GCCAAGGTGTCCGCATTATC |
| BAX-F | TTGGCTGAGTCGCTGAAGAGC |
| BAX-R | AACTCCCATGGCCCCCAAAT |
| BCL2-F | CCTTTCGTTTGCTCGTGCTC |
| BCL2-R | ACCTCCTCCGTGATGTGGTAT |
| PI3K-F | TGAAGCAATGGGTGGAGCTCA |
| PI3K-R | TGAGTCCTGATTCACACATAGCATCT |
| AKT1-F | CAGGAGGAGGAGACGATGGACTTC |
| AKT1-R | CCCAGCAGCTTCAGGTACTCAAAC |
| AKT2-F | CGACGGCTCCTTCATTGGCTATAAG |
| AKT2-R | AGACAGCGAATGACGAAGGTATTGG |
| AKT3-F | GCAGCAGCAGAGAATCCAAAC |
| AKT3-R | CTGCTACAGCCTGGATAGCTT |
| mTOR-F | CCCCAGCTGATTCCACACAT |
| mTOR-R | GTCTCTAGCGCGGCCTTTC |
| CCND1-F | ACATGGAGCTGGTCCTGGTGA |
| CCND1-R | GGAGGGTGGGTTGGAAATGAA |
| CDK1-F | CAACCTTAGAGGGGCAAGGG |
| CDK1-R | GGTAGCGTTGGTGAGGATCT |
| CDK4-F | GCTGCTGCTGGAGATGCTGAC |
| CDK4-R | CTCTGCGTCACCTTCTGCCTTG |
| ACTB-F | CGCAAGTACTCCGTGTGGAT |
| ACTB-R | TAACGCAGCTAACAGTCCGC |
| CGA-F | AAGGCCACAGTGATGGGAAA |
| CGA-R | CAACCATCATCAACATGGCCC |
| Antibodies | Cat NO. | Source | Dilution | Observed Band (kDa) |
|---|---|---|---|---|
| Anti-KMT6/EZH2 antibody | ab191250 | Abcam (Boston, MA, USA) | 1:5000 | 98 |
| Anti-PCNA antibody | ab18197 | Abcam (MA, USA) | 1:500 | 29 |
| BAX rabbit polyclonal antibody | 50599-2-Ig | Protein Tech (Rosemont, IL, USA) | 1:2000 | 24 |
| BCL2 | 12789-1-AP | Protein Tech (IL, USA) | 1:1000 | 26 |
| AKT | 60203-2-Ig | Protein Tech (IL, USA) | 1:5000 | 56 |
| Phospho-pan-AKT1/2/3 (Ser473) | AF0016 | Affinity biosciences (Melbourne, VIC, Australia) | 1:1000 | 56 |
| mTOR | 2983 | CST | 1:1000 | 289 |
| P-mTOR (Ser2248) | 5586 | CST | 1:1000 | 289 |
| ERK1/2 | 11257-1-AP | Protein Tech (IL, USA) | 1:1000 | 42 |
| P-ERK1/2 (Thr202/Tyr204) | 28733-1-AP | Protein Tech (IL, USA) | 1:1000 | 42 |
| GAPDH | 60004-1-Ig | Protein Tech (IL, USA) | 1:8000 | 36 |
| TUBLIN | 66031-1-Ig | Protein Tech (IL, USA) | 1:10,000 | 55 |
| β-Actin antibody | ab8227 | Abcam (MA, USA) | 1:4000 | 42 |
| Goat anti-rabbit IgG | 31460 | Pierce | 1:10,000 | - |
| Goat anti-mouse IgG | 20536-1-AP | Protein Tech (IL, USA) | 1:2000 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Chen, P.; Xu, H.; Li, S.; Zhao, B.; Fan, Y.; Wang, F.; Zhang, Y. EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 10656. https://doi.org/10.3390/ijms241310656
Cai Y, Chen P, Xu H, Li S, Zhao B, Fan Y, Wang F, Zhang Y. EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(13):10656. https://doi.org/10.3390/ijms241310656
Chicago/Turabian StyleCai, Yu, Peiyong Chen, Hui Xu, Shanglai Li, Bingru Zhao, Yixuan Fan, Feng Wang, and Yanli Zhang. 2023. "EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway" International Journal of Molecular Sciences 24, no. 13: 10656. https://doi.org/10.3390/ijms241310656







