Efficacy of Carotenoid-Loaded Gelatin Nanoparticles in Reducing Plasma Cytokines and Adipocyte Hypertrophy in Wistar Rats
Abstract
1. Introduction
2. Results
2.1. Characterization of the Crude Extract Rich in Carotenoids from Cantaloupe Melons
2.2. Characterization of Nanoparticles and Incorporation Efficiency (%)
2.3. Preclinical Study
2.3.1. Food Consumption, Caloric Intake, Caloric Efficiency, and Body Weight
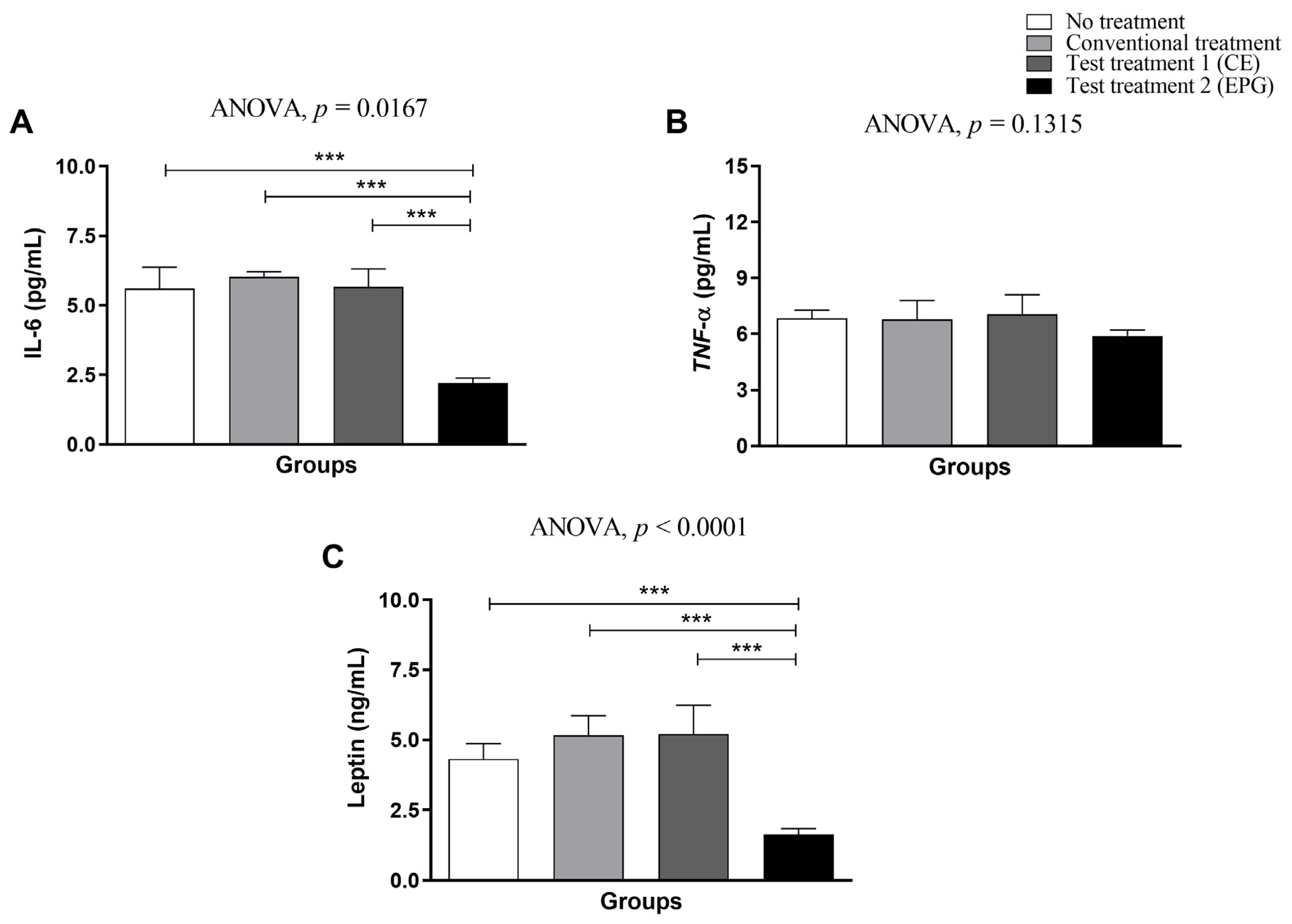
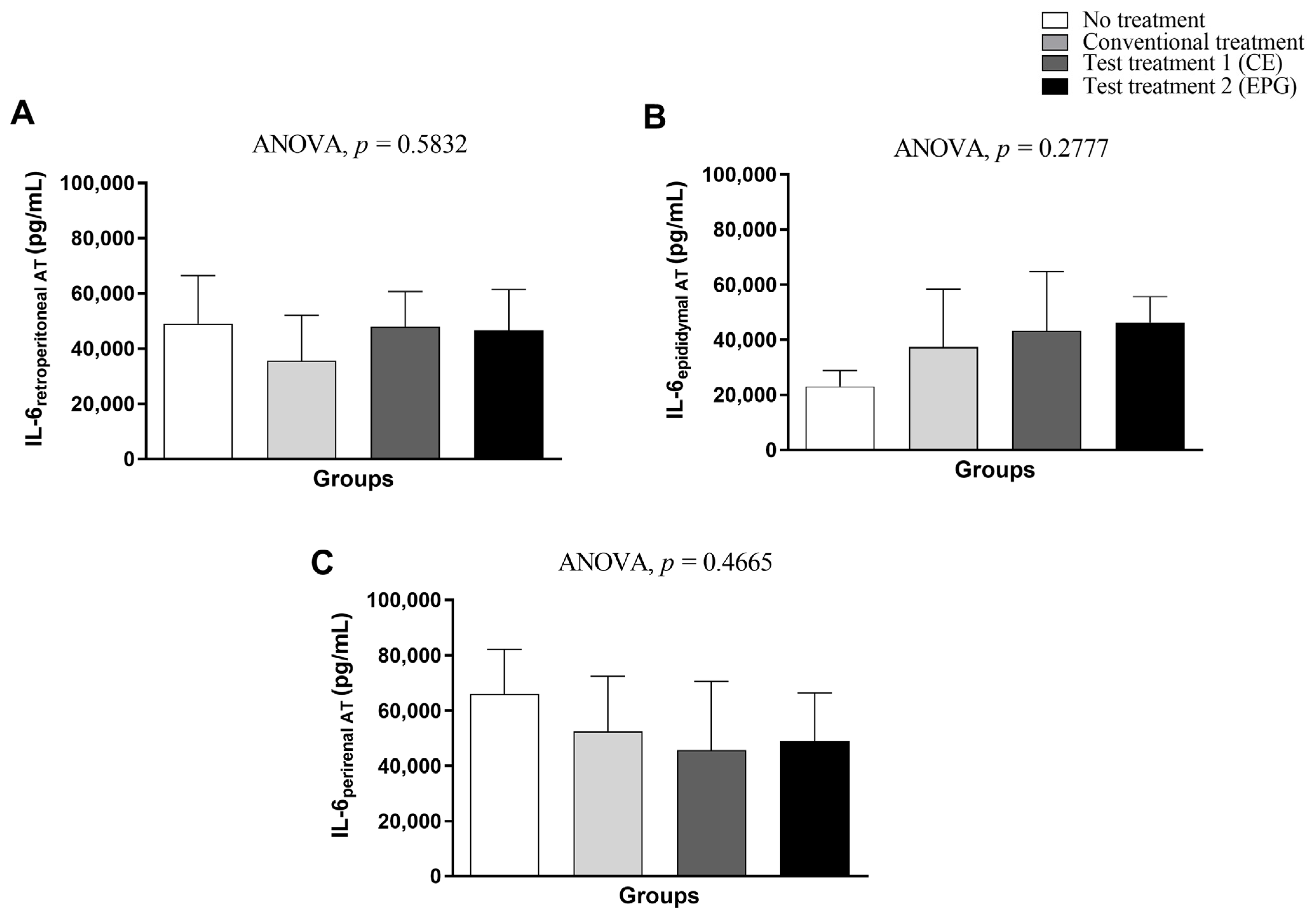
2.3.2. Plasma Concentrations of IL-6, TNF-α and Leptin
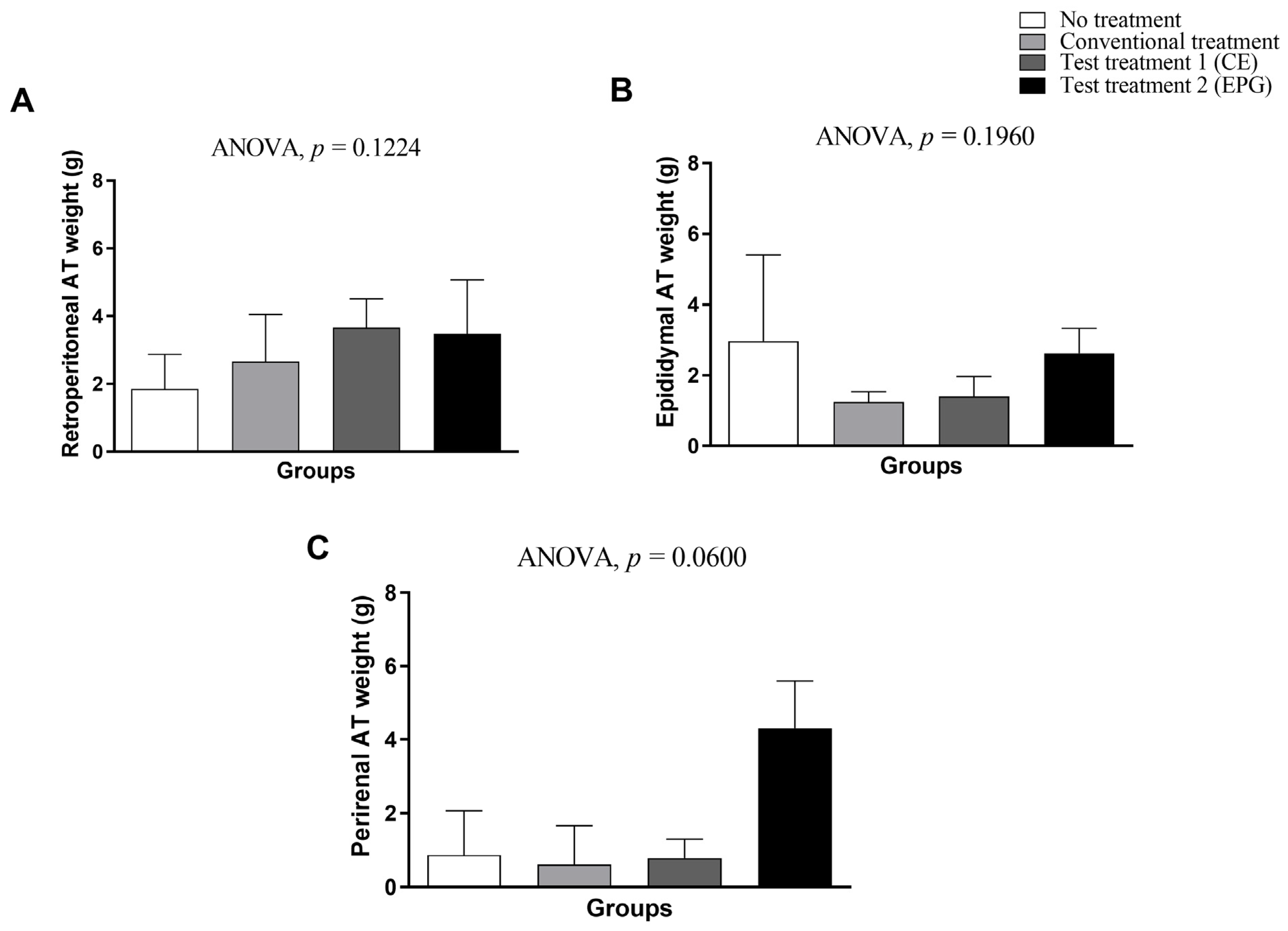
2.3.3. Weight of Visceral Adipose Tissue
2.3.4. Histopathological Analysis of Visceral Adipocytes
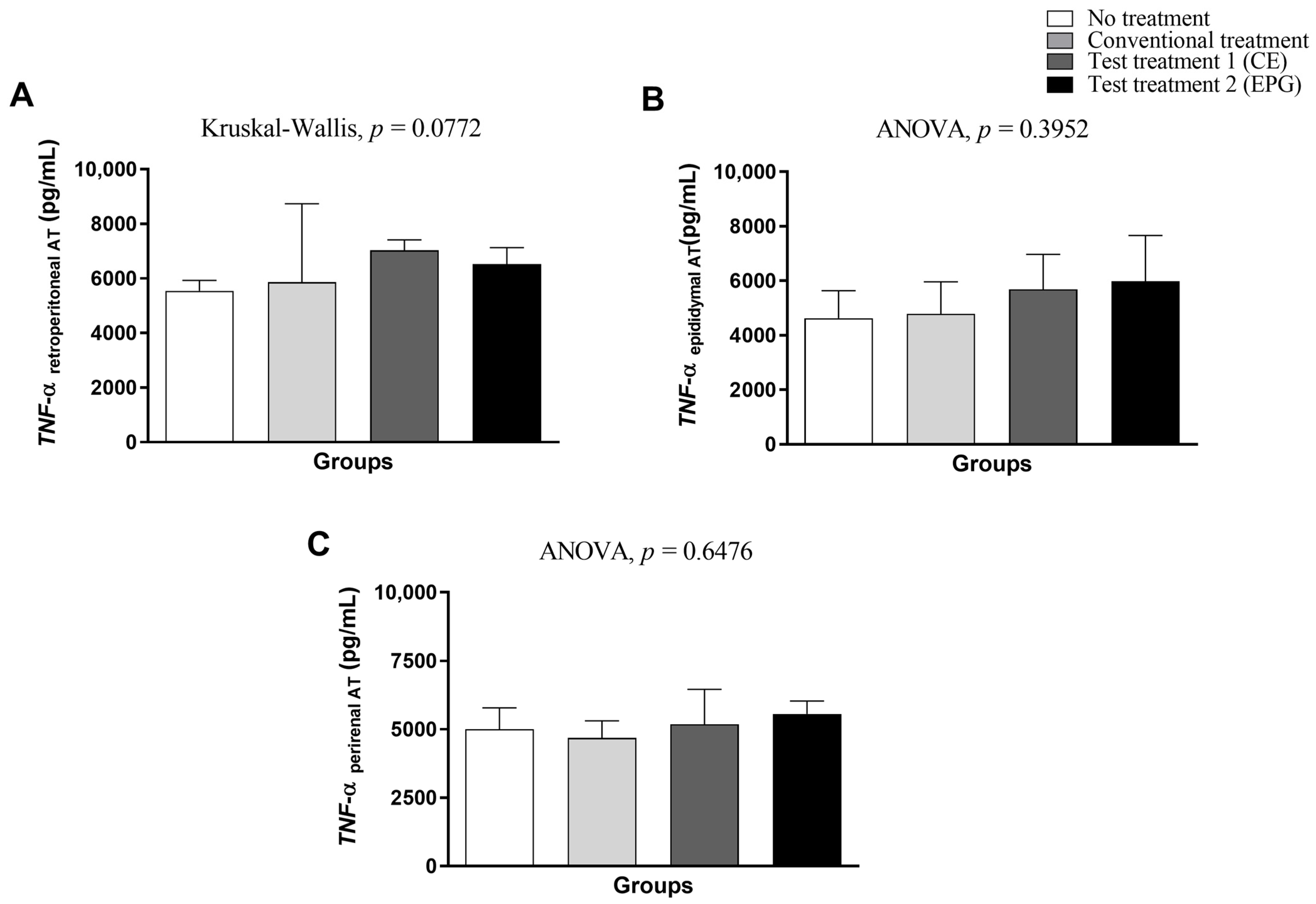
2.3.5. Measurement of Cytokines (TNF-α and IL-6) in Visceral Adipose Tissue
2.3.6. Determination of Thiobarbituric Acid Reactive Substances (TBARS) in Plasma and Visceral Adipose Tissue
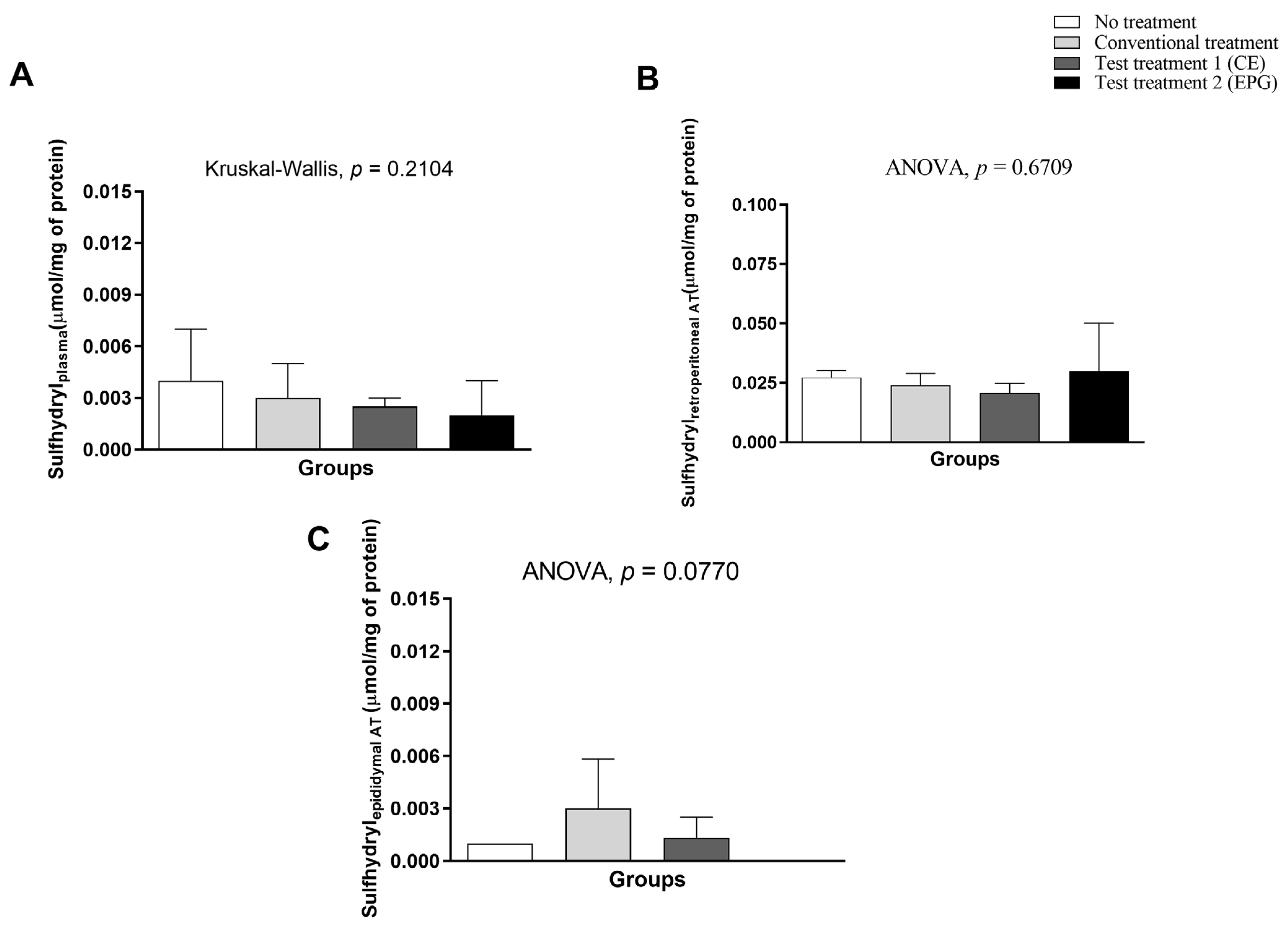
2.3.7. Determination of Sulfhydryls in Plasma and Visceral Adipose Tissue
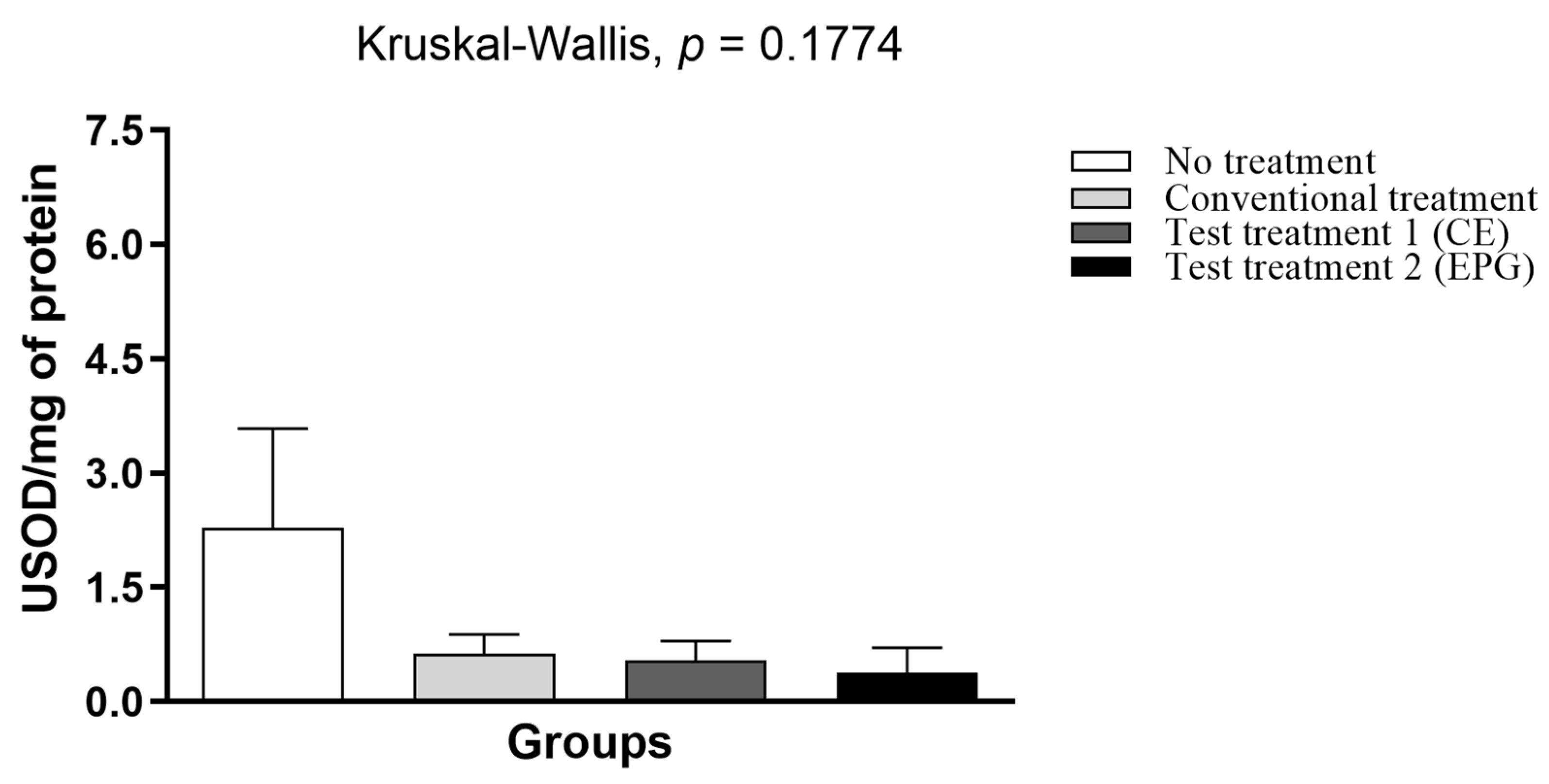
2.3.8. Determination of the Enzymatic Activity of Superoxide Dismutase (SOD) in Plasma
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Obtaining, Characterization, and Nanoencapsulation of the Crude Extract Rich in Carotenoids from Cantaloupe Melons
4.2.1. Characterization of the Nanoparticles (EPG)
4.2.2. Determination of Incorporation Efficiency (IE) (%)
4.3. In Vivo Study
4.3.1. Diets and Experimental Groups
4.3.2. Food Consumption, Caloric Intake, Caloric Efficiency, and Body Weight
4.3.3. Plasma Concentrations of IL-6, TNF-α, and Leptin in an Experimental Model
4.3.4. Visceral Adipose Tissue Weight
4.3.5. Histopathological Analysis of Visceral Adipocytes
4.3.6. Cytokine Concentration (TNF-α and IL-6) in Visceral Adipose Tissue
4.3.7. Quantification of Thiobarbituric Acid Reactive Substances (TBARS) in Visceral Adipose Tissue and Plasma by the Thiobarbituric Acid (TBA) Method
4.3.8. Protein Quantification in Tissue and Plasma Samples
4.3.9. Determination of Sulfhydryl in Plasma and Visceral Adipose Tissue
4.3.10. Determination of the Enzymatic Activity of Superoxide Dismutase (SOD) in Plasma
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3Rs | Replacement, reduction, and refinement |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline) 6-sulfonic acid |
| BCA | bicinchoninic acid |
| BHT | Butylated hydroxytoluene |
| BSA | Bovine serum albumin |
| CE | Crude extract rich in carotenoids from Cantaloupe melon |
| CEUA | Ethics in Animal Use Committee |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| EPG | Crude extract rich in carotenoids from Cantaloupe melon nanoencapsulated in porcine gelatin |
| FA 1 | Aqueous phase 1 |
| FA 2 | Aqueous phase 2 |
| FTIR | Fourier Transform Infrared Spectrophotometry |
| HGLI diet | High glycemic index diet and high glycemic load |
| HRP | Conjugated peroxidase |
| IC50 | The amount necessary to inhibit by 50% |
| IE | Incorporation efficiency |
| IKK | IkappaB kinase complex (IKKβ and IKKα) |
| IL-10 | interleukin 10 |
| IL-6 | interleukin 6 |
| KCl | potassium chloride |
| MDA | malondialdehyde |
| NaCl | sodium chloride |
| NF-κB | Nuclear factor Kappa B |
| NutriSBioativoS | Nutrition and Bioactive Substances for Health |
| O/W | Oil/Water emulsification |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RXR | Retinoid X receptor |
| SDS | sodium dodecyl sulfate |
| SISGEN | National System of Genetic Heritage Management and Associated Traditional Knowledge |
| SOD | superoxide dismutase |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
| TCA | trichloroacetic acid |
| TNF-α | Tumor Necrosis Factor-alpha |
| UCP1 and UCP2 | Uncoupling proteins 1 and 2 |
| UFRN | Federal University of Rio Grande do Norte |
| UnP | Potiguar University |
| UPLC | Ultra Performance Liquid Chromatography |
| WAT | White adipose tissue |
References
- Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica. Diretrizes Brasileiras de Obesidade, 4th ed.; ABESO: São Paulo, Brazil, 2016. [Google Scholar]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Mol. Cell Biol. Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili Pepper Carotenoids: Nutraceutical Properties and Mechanisms of Action. Molecules 2020, 25, 5573. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Passos, T.; Morais, A. Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques. Nutrients 2021, 13, 1921. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of carotenoids extracted from halophilic Archaea in oil-in-water (O/W) micro- and nano-emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Medeiros, A.K.D.O.C.; Gomes, C.D.C.; Amaral, M.L.Q.D.A.; De Medeiros, L.D.G.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.). Food Chem. 2019, 270, 562–572. [Google Scholar] [CrossRef]
- de Oliveira, G.L.R.; Medeiros, I.; Nascimento, S.S.D.C.; Viana, R.L.S.; Porto, D.L.; Rocha, H.A.O.; Aragão, C.F.S.; Maciel, B.L.L.; de Assis, C.F.; Morais, A.H.D.A.; et al. Antioxidant stability enhancement of carotenoid rich-extract from Cantaloupe melon (Cucumis melo L.) nanoencapsulated in gelatin under different storage conditions. Food Chem. 2021, 348, 129055. [Google Scholar] [CrossRef]
- Medeiros, I.; de Oliveira, G.L.R.; de Queiroz, J.L.C.; de Carvalho Gomes, C.; de Carvalho, F.M.C.; de Souza Lima, M.C.J.; Serquiz, A.C.; de Andrade Santos, P.P.; da Silva Camillo, C.; Maciel, B.L.L.; et al. Safety and bioactive potential of nanoparticles containing Cantaloupe melon (Cucumis melo L.) carotenoids in an experimental model of chronic inflammation. Biotechnol. Rep. 2020, 28, e00567. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.D.C. Estudo Da Vitamina a E Do Beta-Caroteno Nanoencapsulado NA Obesidade. Ph.D. Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2021. [Google Scholar]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef]
- Luvizotto, R.D.A.M.; Nascimento, A.F.; Imaizumi, E.; Pierine, D.T.; Conde, S.J.; Correa, C.R.; Yeum, K.-J.; Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013, 110, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The different shades of fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and Fucoxanthin Ameliorate Lipogenesis and Markers of Both Inflammation and Cardiovascular Dysfunction in an Animal Model of Diet-Induced Obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef]
- Amengual, J.; Gouranton, E.; Van Helden, Y.G.J.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-Carotene Reduces Body Adiposity of Mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yoo, J.H.; Lee, B.-C. Therapeutic Effect of Cucumis melo L. Extract on Insulin Resistance and the Gut Microbiome in Lepob/Lepob Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 8159261. [Google Scholar] [CrossRef]
- Gao, M.; Ma, Y.; Liu, D. Rutin Suppresses Palmitic Acids-Triggered Inflammation in Macrophages and Blocks High Fat Diet-Induced Obesity and Fatty Liver in Mice. Pharm. Res. 2013, 30, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Yang, W.-K.; Kim, H.Y.; Min, B.; Caturla, N.; Jones, J.; Park, Y.-C.; Kim, S.-H. Metabolaid® Combination of Lemon Verbena and Hibiscus Flower Extract Prevents High-Fat Diet-Induced Obesity through AMP-Activated Protein Kinase Activation. Nutrients 2018, 10, 1204. [Google Scholar] [CrossRef]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.-F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.S.D.C.; de Queiroz, J.L.C.; de Medeiros, A.F.; Nunes, A.C.D.F.; Piuvezam, G.; Maciel, B.L.L.; Passos, T.S.; Morais, A.H.D.A. Anti-inflammatory agents as modulators of the inflammation in adipose tissue: A systematic review. PLoS ONE 2022, 17, e0273942. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ha, T.-Y.; Kim, S.; Lee, S.-J.; Ahn, J. Red paprika (Capsicum annuum L.) and its main carotenoid capsanthin ameliorate impaired lipid metabolism in the liver and adipose tissue of high-fat diet-induced obese mice. J. Funct. Foods 2017, 31, 131–140. [Google Scholar] [CrossRef]
- de Queiroz, J.L.C.; Medeiros, I.; Trajano, A.C.; Piuvezam, G.; Nunes, A.C.D.F.; Passos, T.S.; Morais, A.H.D.A. Encapsulation techniques perfect the antioxidant action of carotenoids: A systematic review of how this effect is promoted. Food Chem. 2022, 385, 132593. [Google Scholar] [CrossRef] [PubMed]
- Babin, A.; Saciat, C.; Teixeira, M.; Troussard, J.-P.; Motreuil, S.; Moreau, J.; Moret, Y. Limiting immunopathology: Interaction between carotenoids and enzymatic antioxidant defences. Dev. Comp. Immunol. 2015, 49, 278–281. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Lim, L.-S.; Ma, H.; Li, S.; Zheng, H. Roles of Carotenoids in Invertebrate Immunology. Front. Immunol. 2020, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Sugahara, L.Y.; Leimann, F.V.; de Oliveira, S.M.; Brum, E.D.S.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; Ineu, R.P.; Gonçalves, O.H. Nanodispersions of beta-carotene: Effects on antioxidant enzymes and cytotoxic properties. Food Funct. 2018, 9, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.; Veeraraghavan, V.; Palanisamy, C.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.B.S.; Figueredo, J.B.D.S.; Salviano, B.D.P.D.; Aguiar, A.J.F.C.; Pinheiro, L.G.S.D.; Krause, M.F.D.; Camillo, C.D.S.; Ladd, F.V.L.; Bortolin, R.H.; Silbiger, V.N.; et al. Adipocytes and intestinal epithelium dysfunctions linking obesity to inflammation induced by high glycemic index pellet-diet in Wistar rats. Biosci. Rep. 2018, 38, BSR20180304. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Orellano, L.A.A.; Lazari, M.G.T.; Campos, P.P.; Cortes, M.E.R.D. Sinisterra, Evidence of hypoglycemic, lipid-lowering and hepatoprotective effects of the Bixin and Bixin: β-CD inclusion compound in high-fat-fed obese mice. Biomed. Pharmacother. 2018, 106, 363–372. [Google Scholar] [CrossRef]
- de Sousa Rodrigues, M.E.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; do Nascimento, C.M.O.; Barnum, C.J.; Tansey, M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav. Immun. 2017, 59, 158–172. [Google Scholar] [CrossRef]
- De Souza, D.C.; Matos, V.; Dos Santos, V.O.A.; Medeiros, I.F.; Marinho, C.S.R.; Nascimento, P.R.P.; Dorneles, G.P.; Peres, A.; Müller, C.H.; Krause, M.; et al. Effects of High-Intensity Interval and Moderate-Intensity Continuous Exercise on Inflammatory, Leptin, IgA, and Lipid Peroxidation Responses in Obese Males. Front. Physiol. 2018, 9, 567. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Himmelfarb, J.; McMonagle, E.; McMenamin, E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000, 58, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. Sampling Techniques; John Wiley: Hoboken, NJ, USA, 1963; Volume 7, p. 203. [Google Scholar]

 ) primarily intact, showing focal areas of membrane destruction (
) primarily intact, showing focal areas of membrane destruction ( ), extensive regions of lipolysis channels (
), extensive regions of lipolysis channels ( ), vast areas of fibrosis consisting of dense non-patterned connective tissue in areas of adipocyte destruction (
), vast areas of fibrosis consisting of dense non-patterned connective tissue in areas of adipocyte destruction ( ), and the presence of the focal regions of multilocular adipocytes (
), and the presence of the focal regions of multilocular adipocytes ( ). No treatment: HGLI diet + 1 mL of water by gavage; conventional treatment: nutritionally adequate diet (Labina® feed) + 1 mL of water per gavage; test treatment 1: HGLI diet + 1 mL of CE at a concentration of 12.5 mg/kg by gavage; test treatment 2: HGLI diet + 1 mL of EPG at a concentration of 50 mg/kg by gavage; HGLI diet: mixture composed of Labina®, condensed milk and sugar (1:1:0.21 w/w/w); HGLI: high glycemic index and high glycemic load diet; CE: crude extract rich in carotenoids from Cantaloupe melons; EPG: crude extract rich in carotenoids from Cantaloupe melons nanoencapsulated in porcine gelatin.
). No treatment: HGLI diet + 1 mL of water by gavage; conventional treatment: nutritionally adequate diet (Labina® feed) + 1 mL of water per gavage; test treatment 1: HGLI diet + 1 mL of CE at a concentration of 12.5 mg/kg by gavage; test treatment 2: HGLI diet + 1 mL of EPG at a concentration of 50 mg/kg by gavage; HGLI diet: mixture composed of Labina®, condensed milk and sugar (1:1:0.21 w/w/w); HGLI: high glycemic index and high glycemic load diet; CE: crude extract rich in carotenoids from Cantaloupe melons; EPG: crude extract rich in carotenoids from Cantaloupe melons nanoencapsulated in porcine gelatin.
 ) primarily intact, showing focal areas of membrane destruction (
) primarily intact, showing focal areas of membrane destruction ( ), extensive regions of lipolysis channels (
), extensive regions of lipolysis channels ( ), vast areas of fibrosis consisting of dense non-patterned connective tissue in areas of adipocyte destruction (
), vast areas of fibrosis consisting of dense non-patterned connective tissue in areas of adipocyte destruction ( ), and the presence of the focal regions of multilocular adipocytes (
), and the presence of the focal regions of multilocular adipocytes ( ). No treatment: HGLI diet + 1 mL of water by gavage; conventional treatment: nutritionally adequate diet (Labina® feed) + 1 mL of water per gavage; test treatment 1: HGLI diet + 1 mL of CE at a concentration of 12.5 mg/kg by gavage; test treatment 2: HGLI diet + 1 mL of EPG at a concentration of 50 mg/kg by gavage; HGLI diet: mixture composed of Labina®, condensed milk and sugar (1:1:0.21 w/w/w); HGLI: high glycemic index and high glycemic load diet; CE: crude extract rich in carotenoids from Cantaloupe melons; EPG: crude extract rich in carotenoids from Cantaloupe melons nanoencapsulated in porcine gelatin.
). No treatment: HGLI diet + 1 mL of water by gavage; conventional treatment: nutritionally adequate diet (Labina® feed) + 1 mL of water per gavage; test treatment 1: HGLI diet + 1 mL of CE at a concentration of 12.5 mg/kg by gavage; test treatment 2: HGLI diet + 1 mL of EPG at a concentration of 50 mg/kg by gavage; HGLI diet: mixture composed of Labina®, condensed milk and sugar (1:1:0.21 w/w/w); HGLI: high glycemic index and high glycemic load diet; CE: crude extract rich in carotenoids from Cantaloupe melons; EPG: crude extract rich in carotenoids from Cantaloupe melons nanoencapsulated in porcine gelatin.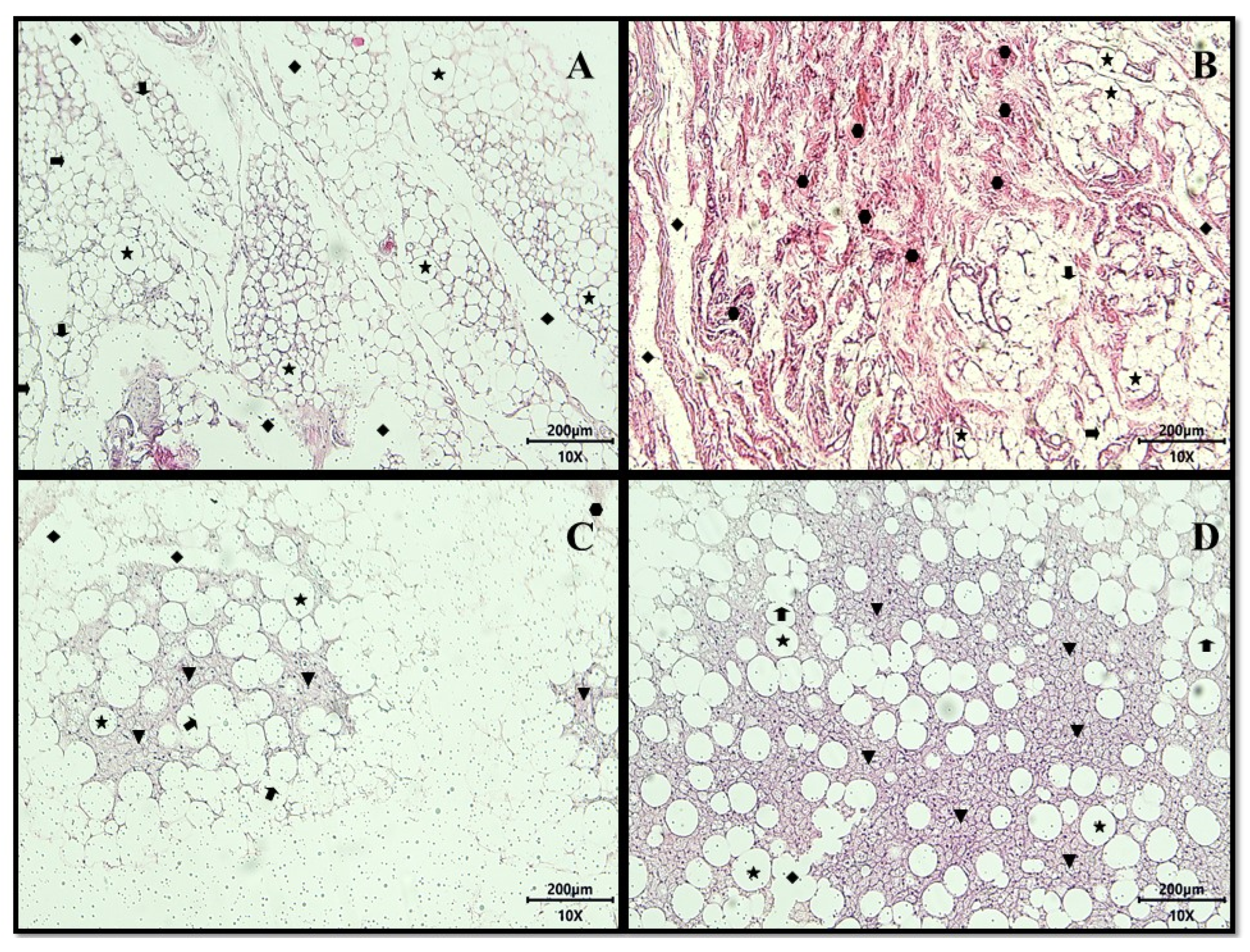

| Groups | ∆Dietary Intake (g) Mean (SD) | Caloric Intake (kJ/Kcal of Intake) Mean (SD) | Caloric Efficiency (kJ/g of Weight) Mean (SD) |
|---|---|---|---|
| No treatment | 3.90 (2.61) a | 255.48 (18.22) a | 21.97 (62.91) a |
| Conventional treatment | 4.13 (6.73) a | 290.70 (64.77) a | 1.33 (58.57) a |
| Test treatment 1 (CE) | 0.40 (4.52) a | 270.22 (25.32) a | −11.10 (72.80) a |
| Test treatment 2 (EPG) | −3.00 (3.03) a | 239.51 (33.64) a | −38.20 (31.98) a |
| Groups | ∆Weight (g) Mean (SD) | Average Weight Loss (g) | Average Weight Gain (g) |
|---|---|---|---|
| No treatment | 1.40 (9.61) a | 8.00 (1.41) | 7.70 (6.03) |
| Conventional treatment | −3.00 (10.46) a | 11.00 (8.49) | 5.00 (0.00) |
| Test treatment 1 (CE) | −5.20 (11.82) a | 12.30 (9.29) | 5.50 (2.12) |
| Test treatment 2 (EPG) | −11.00 (8.40) a | 11.00 (8.40) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, J.L.C.d.; Medeiros, I.; Lima, M.S.R.; Carvalho, F.M.C.d.; Camillo, C.S.; Santos, P.P.d.A.; Guerra, G.C.B.; da Silva, V.C.; Schroeder, H.T.; Krause, M.; et al. Efficacy of Carotenoid-Loaded Gelatin Nanoparticles in Reducing Plasma Cytokines and Adipocyte Hypertrophy in Wistar Rats. Int. J. Mol. Sci. 2023, 24, 10657. https://doi.org/10.3390/ijms241310657
Queiroz JLCd, Medeiros I, Lima MSR, Carvalho FMCd, Camillo CS, Santos PPdA, Guerra GCB, da Silva VC, Schroeder HT, Krause M, et al. Efficacy of Carotenoid-Loaded Gelatin Nanoparticles in Reducing Plasma Cytokines and Adipocyte Hypertrophy in Wistar Rats. International Journal of Molecular Sciences. 2023; 24(13):10657. https://doi.org/10.3390/ijms241310657
Chicago/Turabian StyleQueiroz, Jaluza Luana C. de, Isaiane Medeiros, Mayara S. R. Lima, Fabiana Maria C. de Carvalho, Christina S. Camillo, Pedro Paulo de A. Santos, Gerlane C. B. Guerra, Valéria C. da Silva, Helena T. Schroeder, Mauricio Krause, and et al. 2023. "Efficacy of Carotenoid-Loaded Gelatin Nanoparticles in Reducing Plasma Cytokines and Adipocyte Hypertrophy in Wistar Rats" International Journal of Molecular Sciences 24, no. 13: 10657. https://doi.org/10.3390/ijms241310657
APA StyleQueiroz, J. L. C. d., Medeiros, I., Lima, M. S. R., Carvalho, F. M. C. d., Camillo, C. S., Santos, P. P. d. A., Guerra, G. C. B., da Silva, V. C., Schroeder, H. T., Krause, M., Morais, A. H. d. A., & Passos, T. S. (2023). Efficacy of Carotenoid-Loaded Gelatin Nanoparticles in Reducing Plasma Cytokines and Adipocyte Hypertrophy in Wistar Rats. International Journal of Molecular Sciences, 24(13), 10657. https://doi.org/10.3390/ijms241310657








