Sp1 Upregulation Bolsters the Radioresistance of Glioblastoma Cells by Promoting Double Strand Breaks Repair
Abstract
:1. Introduction
2. Results
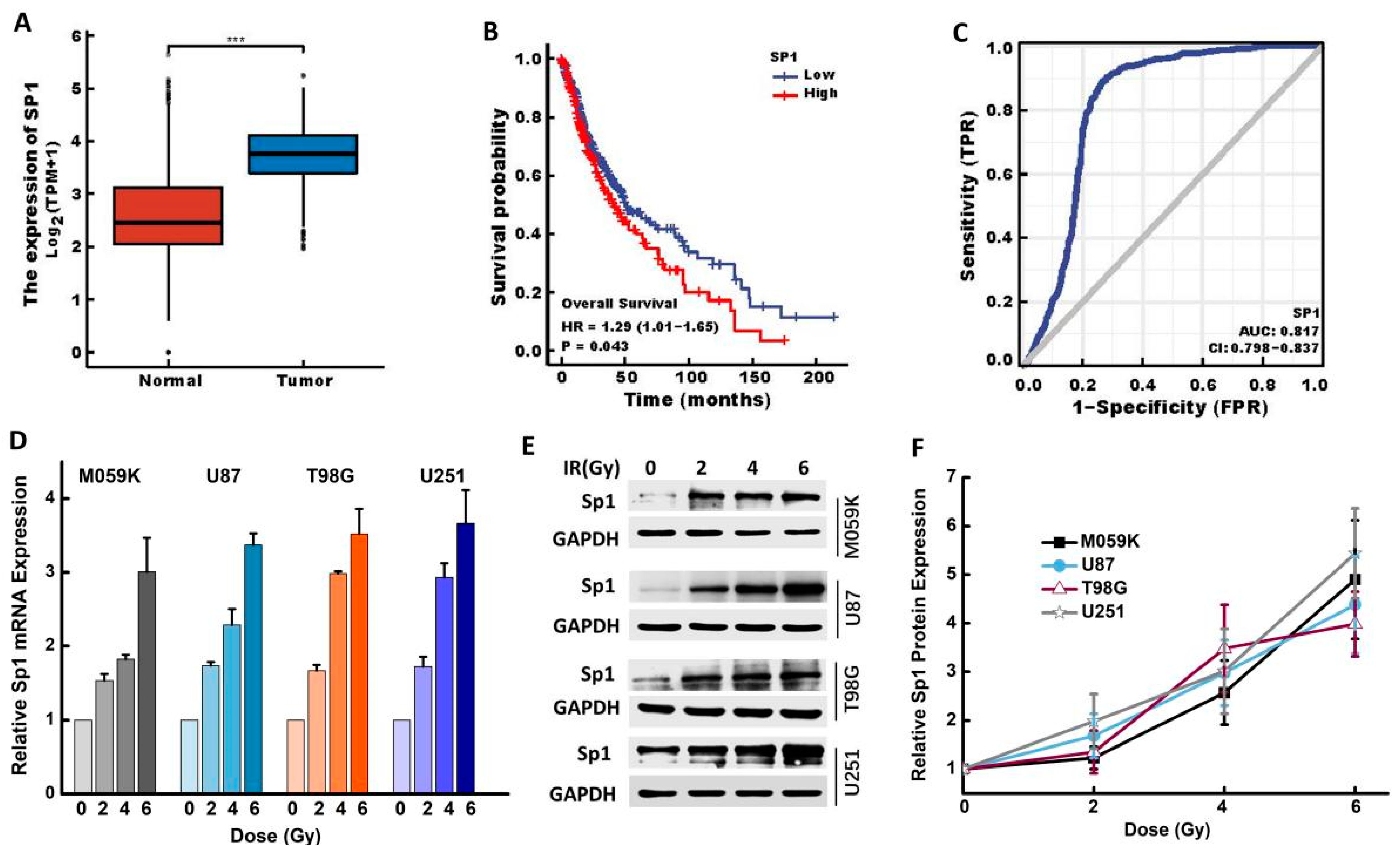
2.1. IR Upregulated Sp1 Expression in GBM Cells
2.2. IR Upregulated Sp1 Expression in GBM Cells In Vivo
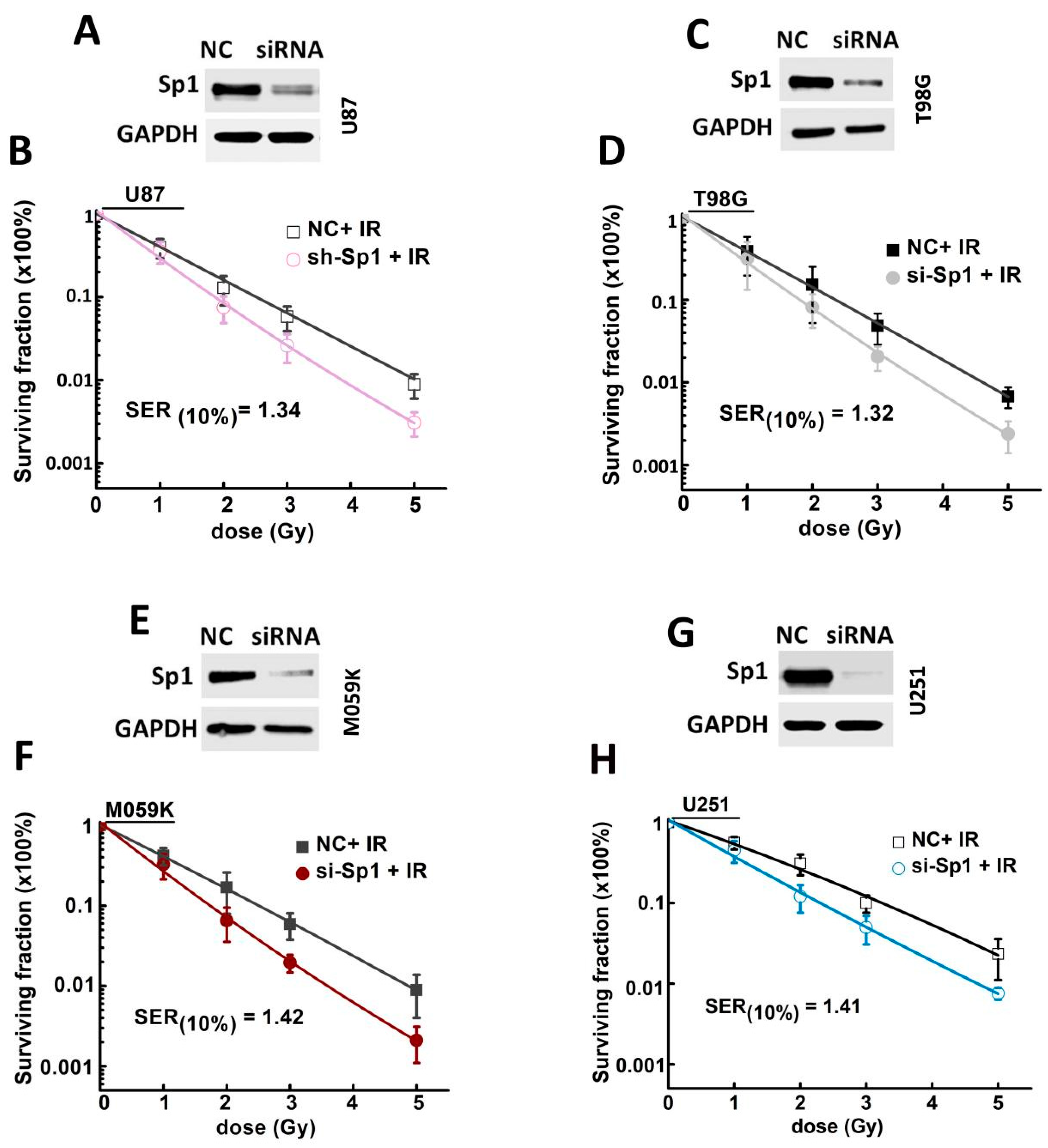
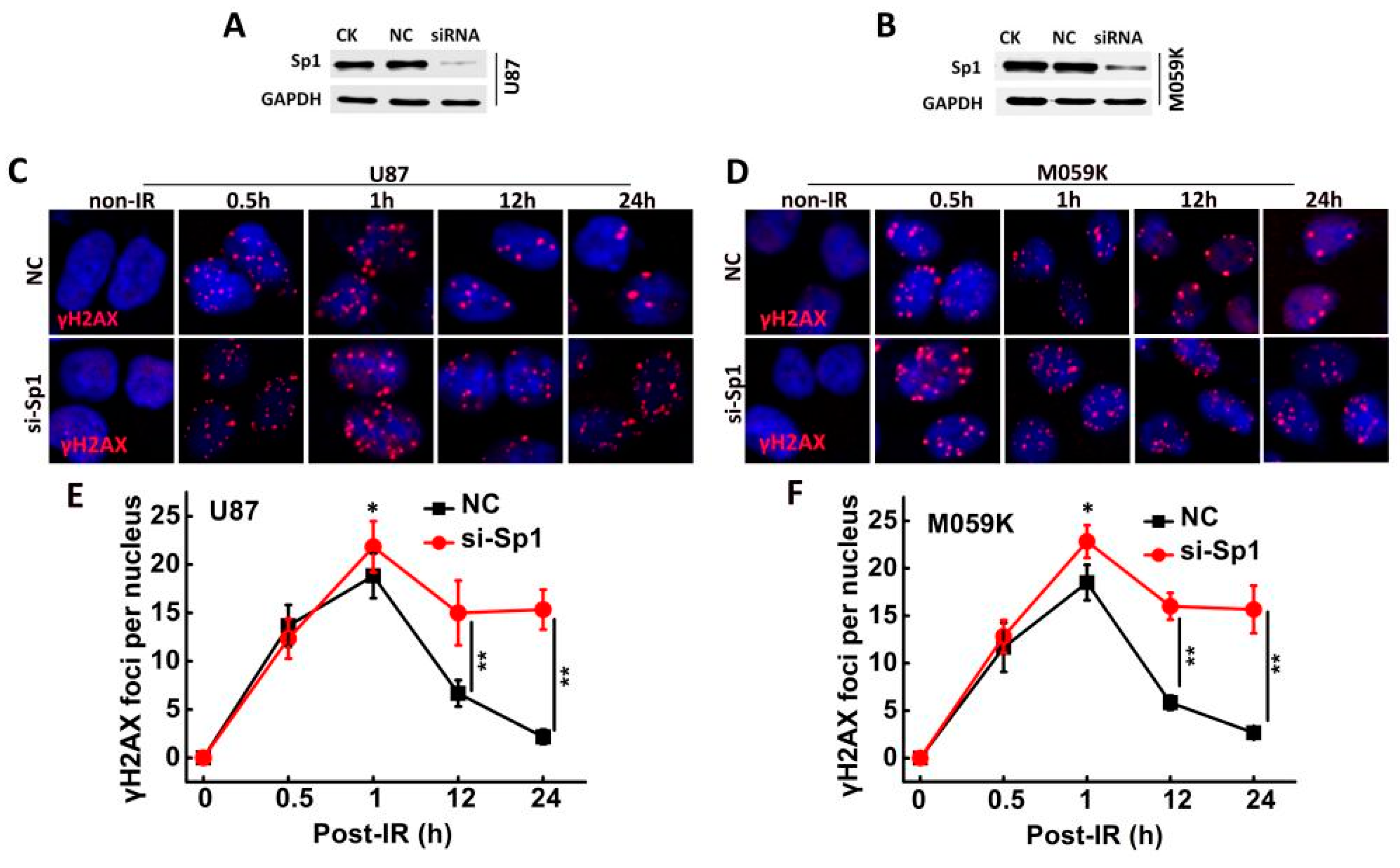
2.3. Sp1 Knockdown Radiosensitized GBM Cells In Vitro
2.4. Sp1 Knockdown Radiosensitized GBM Cells In Vivo
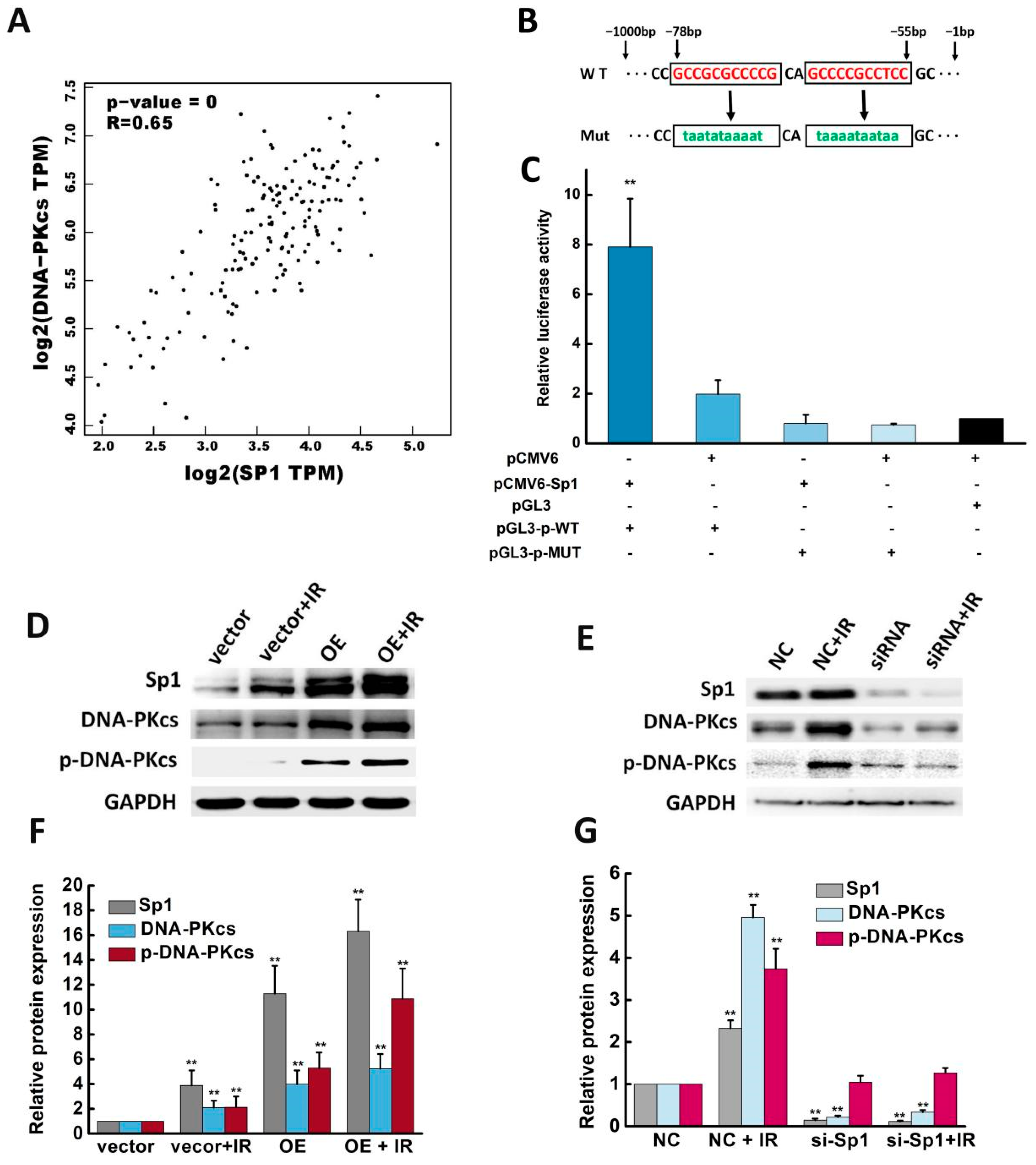
2.5. Sp1 Activated the DNA-PKcs Promoter and Enhanced DNA-PKcs Expression and Activity
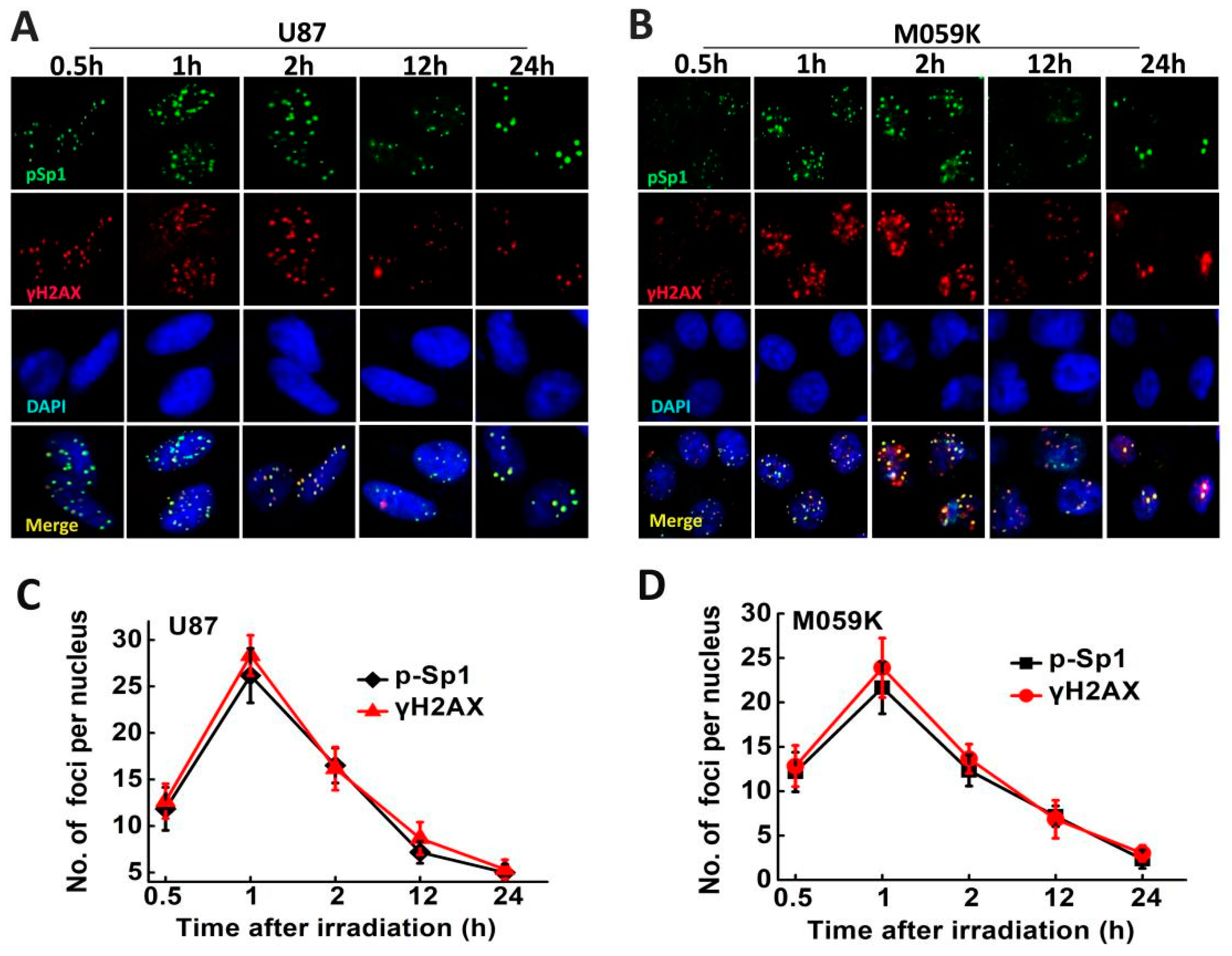
2.6. p-Sp1 Was Located in the DSB Sites
2.7. Sp1 Silencing Delayed IR-Induced DSB Repair
2.8. Sp1 Silencing Aggravated IR-Induced Apoptosis In Vivo and In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Irradiation
4.3. RNA Extraction and RT-PCR
4.4. siRNA Transfection and Establishment of Stable Sp1-Knockdown Cell Lines
4.5. Western Blotting
4.6. Immunofluorescence
4.7. Dual Luciferase Assay
4.8. Clonogenic Assay
4.9. Apoptosis Analysis
4.10. Xenograft Model
4.11. Bioinformatics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| DSB | Double strand breaks |
| Sp1 | Specificity protein 1 |
| IR | Ionizing radiation |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| FBS | Fetal bovine serum |
| ATM | Ataxia-telangiectasia mutated |
| DDB | Damage-specific DNA-binding protein |
| NFDB1 | Nuclear factor with BRCT domain 1 |
| MDC1 | Mediator of DNA damage checkpoint protein 1 |
| XRCC1 | X-ray repair cross-complementing gene 1 |
| NHEJ | Non-homologous end joining |
| HR | Homologous recombination |
References
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef]
- Aldoghachi, A.F.; Aldoghachi, A.F.; Breyne, K.; Ling, K.-H.; Cheah, P.-S. Recent Advances in the Therapeutic Strategies of Glioblastoma Multiforme. Neuroscience 2022, 491, 240–270. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, E.; Wen, P.Y. Current therapeutic options for glioblastoma and future perspectives. Expert Opin. Pharmacother. 2022, 23, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Amelio, D.; Amichetti, M.; Salvati, M.; Muni, R.; Bozzao, A.; Lanzetta, G.; Scarpino, S.; Arcella, A.; Enrici, R.M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 2010, 97, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Palmer, J.D.; Siedow, M.; Haque, S.J.; Chakravarti, A. Overcoming Radiation Resistance in Gliomas by Targeting Metabolism and DNA Repair Pathways. Int. J. Mol. Sci. 2022, 23, 2246. [Google Scholar] [CrossRef]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015, 282, 224–258. [Google Scholar] [CrossRef]
- Safe, S.; Imanirad, P.; Sreevalsan, S.; Nair, V.; Jutooru, I. Transcription factor Sp1, also known as specificity protein 1 as a therapeutic target. Expert Opin. Ther. Targets 2014, 18, 759–769. [Google Scholar] [CrossRef]
- Guan, H.; Cai, J.; Zhang, N.; Wu, J.; Yuan, J.; Li, J.; Li, M. Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int. J. Cancer 2011, 130, 593–601. [Google Scholar] [CrossRef]
- Wang, J.; Kang, M.; Qin, Y.-T.; Wei, Z.-X.; Xiao, J.-J.; Wang, R.-S. Sp1 is over-expressed in nasopharyngeal cancer and is a poor prognostic indicator for patients receiving radiotherapy. Int. J. Clin. Exp. Pathol. 2015, 8, 6936–6943. [Google Scholar]
- Iwahori, S.; Yasui, Y.; Kudoh, A.; Sato, Y.; Nakayama, S.; Murata, T.; Isomura, H.; Tsurumi, T. Identification of phosphorylation sites on transcription factor Sp1 in response to DNA damage and its accumulation at damaged sites. Cell. Signal. 2008, 20, 1795–1803. [Google Scholar] [CrossRef]
- Fletcher, S.; Grou, C.; Legrand, A.J.; Chen, X.; Söderström, K.; Poletto, M.; Dianov, G.L. Sp1 phosphorylation by ATM downregulates BER and promotes cell elimination in response to persistent DNA damage. Nucleic Acids Res. 2017, 46, 1834–1846. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, B.A.; Kelly, C.M.; Kim, J.; Hornsby, S.M.; Azizkhan-Clifford, J. Phosphorylation of Sp1 in Response to DNA Damage by Ataxia Telangiectasia-Mutated Kinase. Mol. Cancer Res. 2007, 5, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Mizutani, N.; Inoue, M.; Omori, Y.; Tamiya-Koizumi, K.; Takagi, A.; Kojima, T.; Suzuki, M.; Nozawa, Y.; Minami, Y.; et al. Phosphorylated Sp1 is the regulator of DNA-PKcs and DNA ligase IV transcription of daunorubicin-resistant leukemia cell lines. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2014, 1839, 265–274. [Google Scholar] [CrossRef]
- Liu, X.; Li, P.; Hirayama, R.; Niu, Y.; Liu, X.; Chen, W.; Jin, X.; Zhang, P.; Ye, F.; Zhao, T.; et al. Genistein sensitizes glioblastoma cells to carbon ions via inhibiting DNA-PKcs phosphorylation and subsequently repressing NHEJ and delaying HR repair pathways. Radiother. Oncol. 2018, 129, 84–94. [Google Scholar] [CrossRef]
- Hosoi, Y.; Watanabe, T.; Nakagawa, K.; Matsumoto, Y.; Enomoto, A.; Morita, A.; Nagawa, H.; Suzuki, N. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int. J. Oncol. 2004, 25, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xue, X.; Zhou, H.; Zhang, G. A molecular view of the radioresistance of gliomas. Oncotarget 2017, 8, 100931–100941. [Google Scholar] [CrossRef] [Green Version]
- Olive, P.L. Retention of γH2AX foci as an indication of lethal DNA damage. Radiother. Oncol. 2011, 101, 18–23. [Google Scholar] [CrossRef]
- Ali, Y.; Oliva, C.R.; Noman, A.S.M.; Allen, B.G.; Goswami, P.C.; Zakharia, Y.; Monga, V.; Spitz, D.R.; Buatti, J.M.; Griguer, C.E. Radioresistance in Glioblastoma and the Development of Radiosensitizers. Cancers 2020, 12, 2511. [Google Scholar] [CrossRef]
- Ahmed, S.U.; Carruthers, R.; Gilmour, L.; Yildirim, S.; Watts, C.; Chalmers, A.J. Selective Inhibition of Parallel DNA Damage Response Pathways Optimizes Radiosensitization of Glioblastoma Stem-like Cells. Cancer Res. 2015, 75, 4416–4428. [Google Scholar] [CrossRef] [Green Version]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Aiyappa-Maudsley, R.; Chalmers, A.J.; Parsons, J.L. Factors affecting the radiation response in glioblastoma. Neuro Oncol. Adv. 2022, 4, vdac156. [Google Scholar] [CrossRef]
- Grinstein, E.; Jundt, F.; Weinert, I.; Wernet, P.; Royer, H.-D. Sp1 as G1 cell cycle phase specific transcription factor in epithelial cells. Oncogene 2002, 21, 1485–1492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-P.; Zhang, H.; Wang, H.-B.; Li, Y.-X.; Liu, G.-H.; Xing, S.; Li, M.-Z.; Zeng, M.-S. Down-regulation of Sp1 suppresses cell proliferation, clonogenicity and the expressions of stem cell markers in nasopharyngeal carcinoma. J. Transl. Med. 2014, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wang, X.; Xia, Z.; Yang, L.; Ding, Z.; Chen, S.; Lai, B.; Zhang, N. Transcriptional factor specificity protein 1 (SP1) promotes the proliferation of glioma cells by up-regulating midkine (MDK). Mol. Biol. Cell 2015, 26, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Cao, F.; Xiong, Y.; Zhou, H. SP1 transcriptionally activates NLRP6 inflammasome and induces immune evasion and radioresistance in glioma cells. Int. Immunopharmacol. 2021, 98, 107858. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing Radiation-Induced DNA Damage, Response, and Repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Deycmar, S.; Faccin, E.; Kazimova, T.; Knobel, P.A.; Telarovic, I.; Tschanz, F.; Waller, V.; Winkler, R.; Yong, C.; Zingariello, D.; et al. The relative biological effectiveness of proton irradiation in dependence of DNA damage repair. Br. J. Radiol. 2020, 93, 20190494. [Google Scholar] [CrossRef]
- Bu, Y.; Suenaga, Y.; Ono, S.; Koda, T.; Song, F.; Nakagawara, A.; Ozaki, T. Sp1-mediated transcriptional regulation of NFBD1/MDC1 plays a critical role in DNA damage response pathway. Genes Cells 2008, 13, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.F.; Itoh, T.; Zolezzi, F.; Hutsell, S.; Linn, S. Basal transcriptional regulation of human damage-specific DNA-binding protein genes DDB1 and DDB2 by Sp1, E2F, N-myc and NF1 elements. Nucleic Acids Res. 2003, 31, 562–569. [Google Scholar] [CrossRef]
- Fang, X.; Huang, Z.; Zhai, K.; Huang, Q.; Tao, W.; Kim, L.; Wu, Q.; Almasan, A.; Yu, J.S.; Li, X.; et al. Inhibiting DNA-PK induces glioma stem cell differentiation and sensitizes glioblastoma to radiation in mice. Sci. Transl. Med. 2021, 13, eabc7275. [Google Scholar] [CrossRef]
- Sibanda, B.L.; Chirgadze, D.Y.; Ascher, D.B.; Blundell, T.L. DNA-PKcs structure suggests an allosteric mechanism modulating DNA double-strand break repair. Science 2017, 355, 520–524. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Zheng, X.; Zhao, T.; Li, Q. Genistein sensitizes sarcoma cells in vitro and in vivo by enhancing apoptosis and by inhibiting DSB repair pathways. J. Radiat. Res. 2016, 57, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Iwahori, S.; Shirata, N.; Kawaguchi, Y.; Weller, S.K.; Sato, Y.; Kudoh, A.; Nakayama, S.; Isomura, H.; Tsurumi, T. Enhanced Phosphorylation of Transcription Factor Sp1 in Response to Herpes Simplex Virus Type 1 Infection Is Dependent on the Ataxia Telangiectasia-Mutated Protein. J. Virol. 2007, 81, 9653–9664. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef] [Green Version]


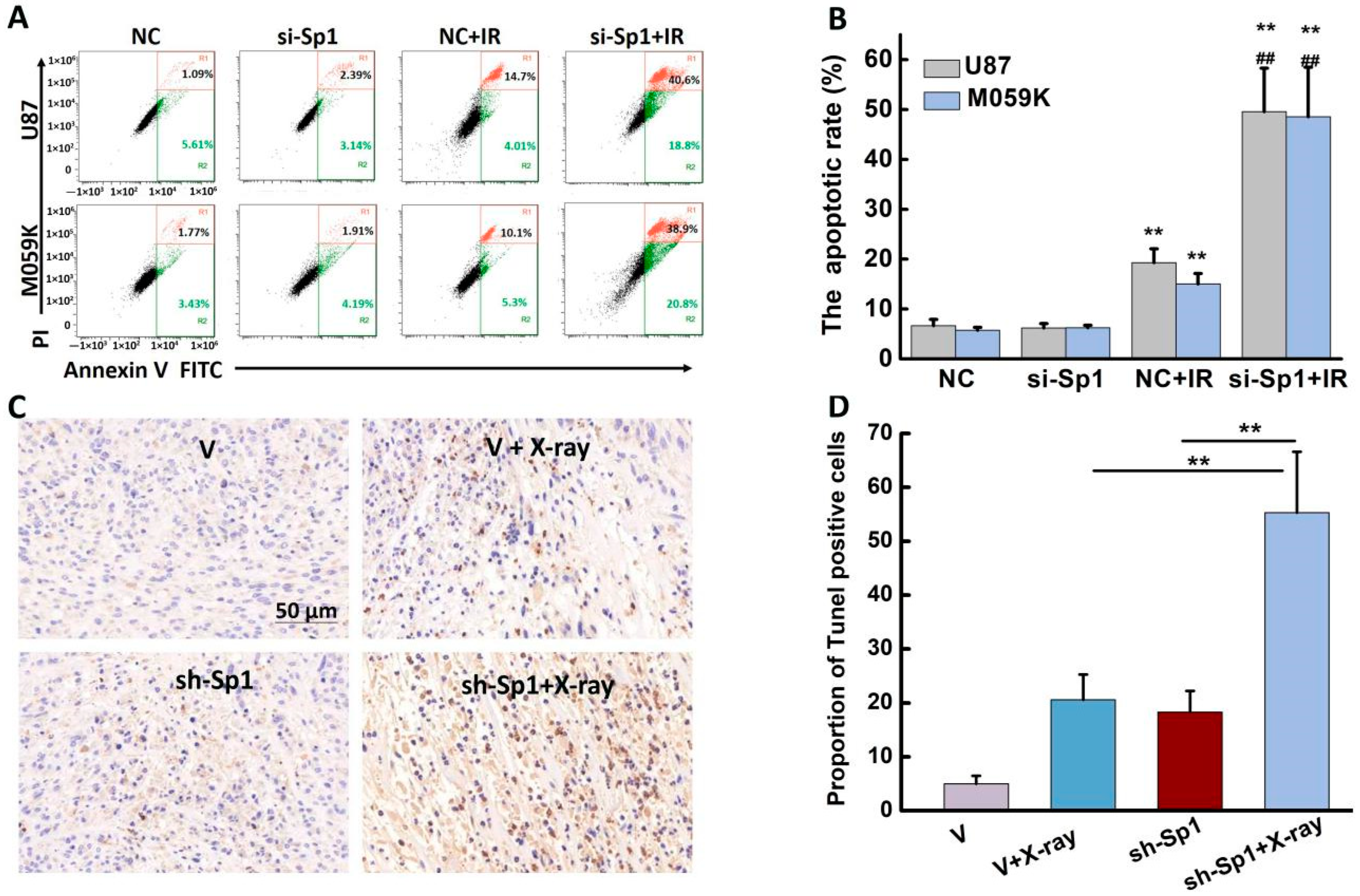
| Total Apoptotic Cells (%) | ||
|---|---|---|
| U87 | M059K | |
| NC | 6.67 ± 1.26 | 5.76 ± 0.56 |
| si-SP1 | 6.20 ± 0.87 | 6.26 ± 0.48 |
| NC + IR | 19.72 ± 2.79 | 15.03 ± 2.07 |
| si-Sp1 + IR | 49.57 ± 8.71 ***### | 48.53 ± 9.93 ***### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Sun, C.; Wang, Q.; Li, P.; Zhao, T.; Li, Q. Sp1 Upregulation Bolsters the Radioresistance of Glioblastoma Cells by Promoting Double Strand Breaks Repair. Int. J. Mol. Sci. 2023, 24, 10658. https://doi.org/10.3390/ijms241310658
Liu X, Sun C, Wang Q, Li P, Zhao T, Li Q. Sp1 Upregulation Bolsters the Radioresistance of Glioblastoma Cells by Promoting Double Strand Breaks Repair. International Journal of Molecular Sciences. 2023; 24(13):10658. https://doi.org/10.3390/ijms241310658
Chicago/Turabian StyleLiu, Xiongxiong, Chao Sun, Qiqi Wang, Ping Li, Ting Zhao, and Qiang Li. 2023. "Sp1 Upregulation Bolsters the Radioresistance of Glioblastoma Cells by Promoting Double Strand Breaks Repair" International Journal of Molecular Sciences 24, no. 13: 10658. https://doi.org/10.3390/ijms241310658





