Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue
Abstract
:1. Introduction
2. Results
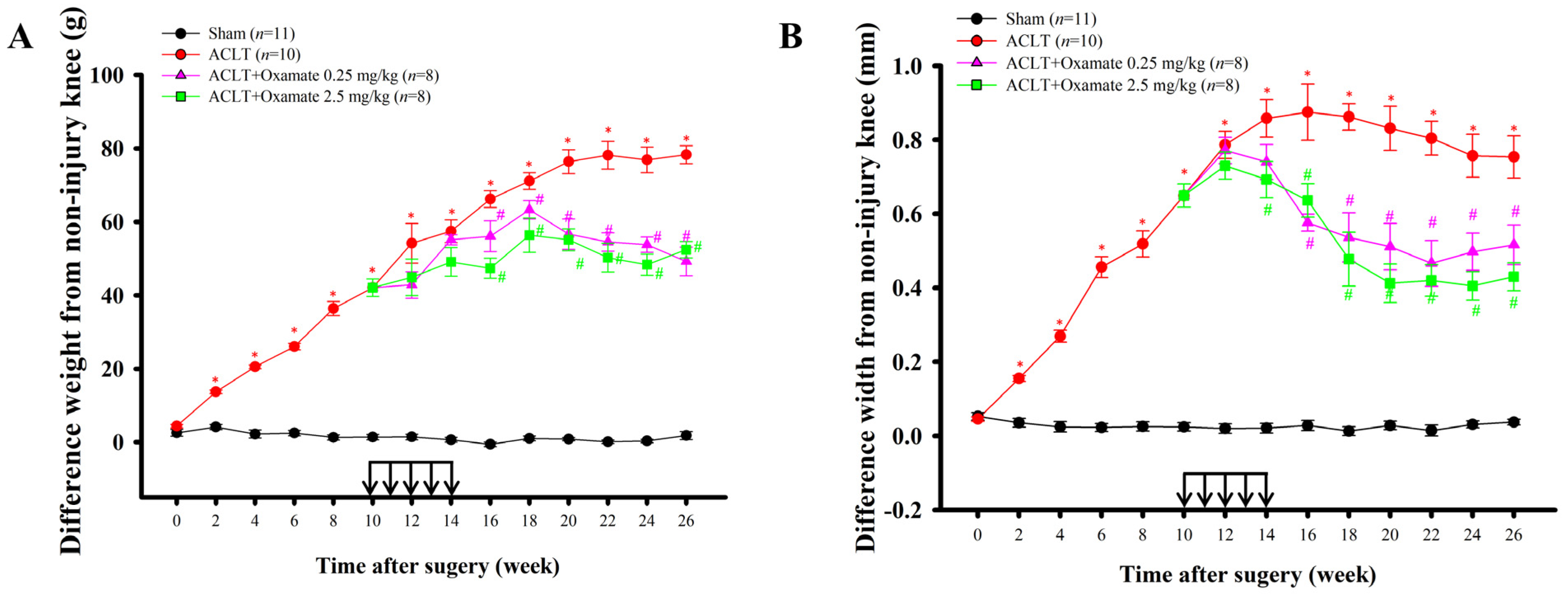
2.1. Effects of Intra-Articular Oxamate Administration on ACLT-Induced Nociception
2.2. Oxamate Attenuates Cartilage Degradation in ACLT-Rats
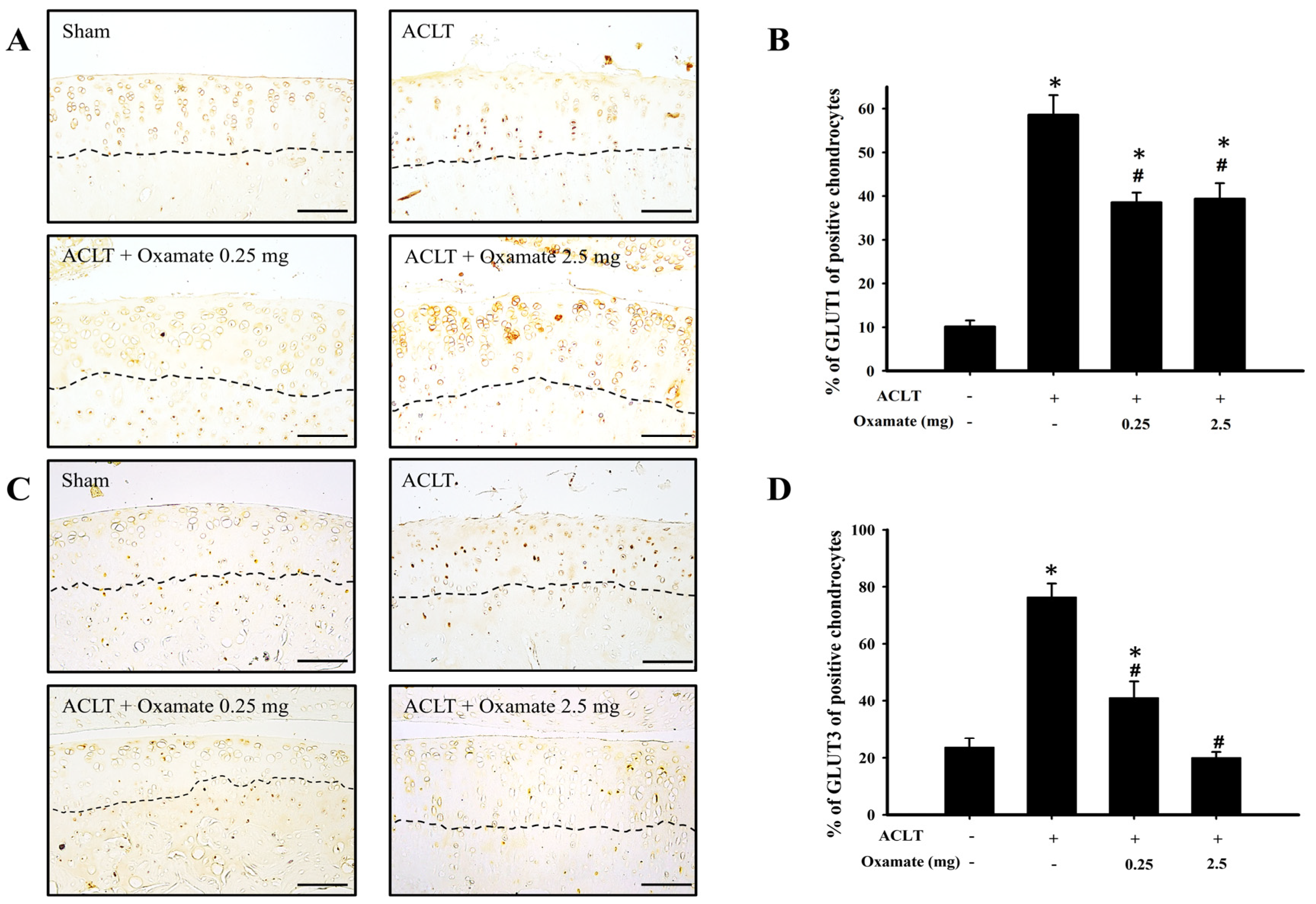
2.3. Oxamate Affects Glucose Transporter 1- and 3 Expression in ACLT-Cartilage
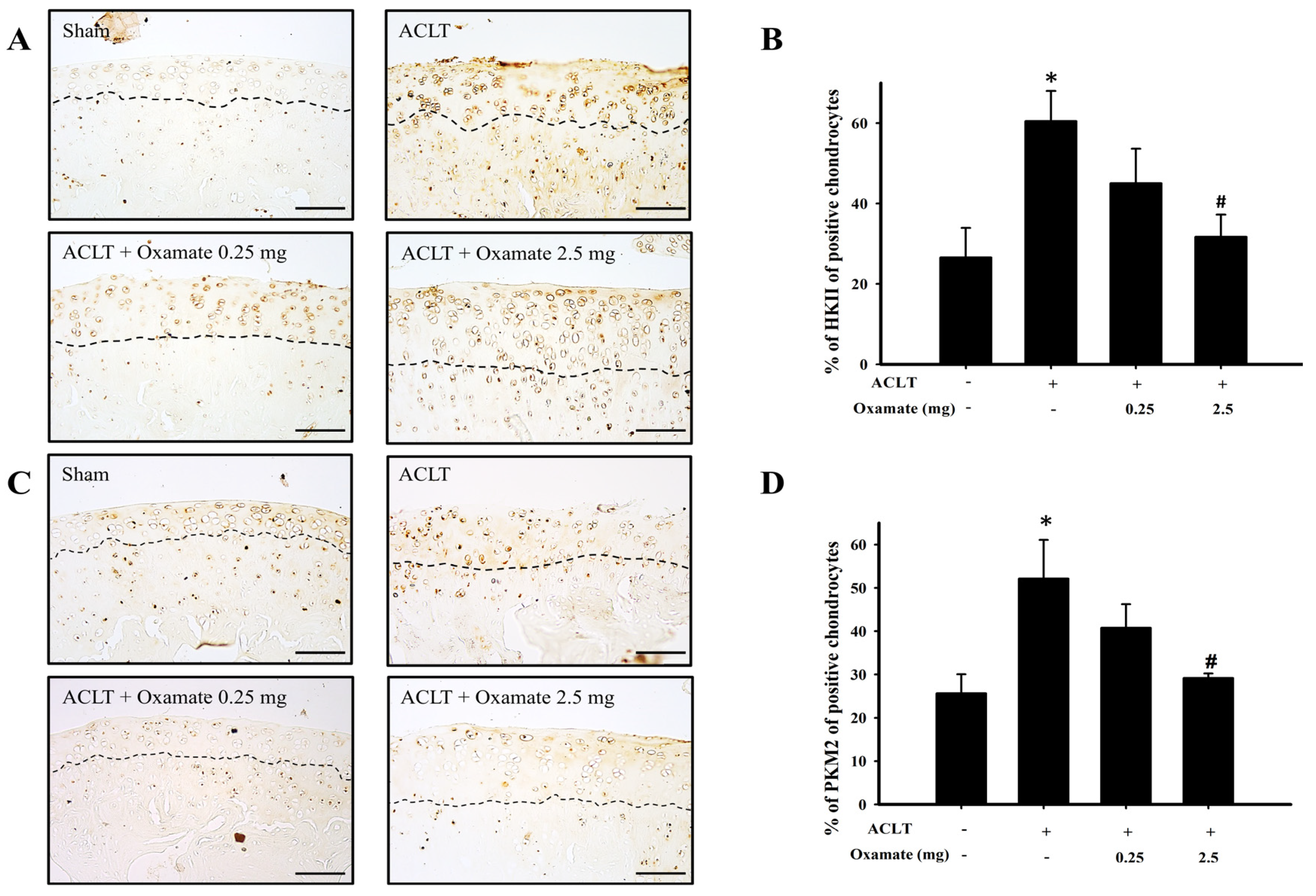
2.4. Oxamate Affects Hexokinase-II Expression in ACLT-Cartilage
2.5. Oxamate Affects Pyruvate Kinase M2 Expression in ACLT Cartilage
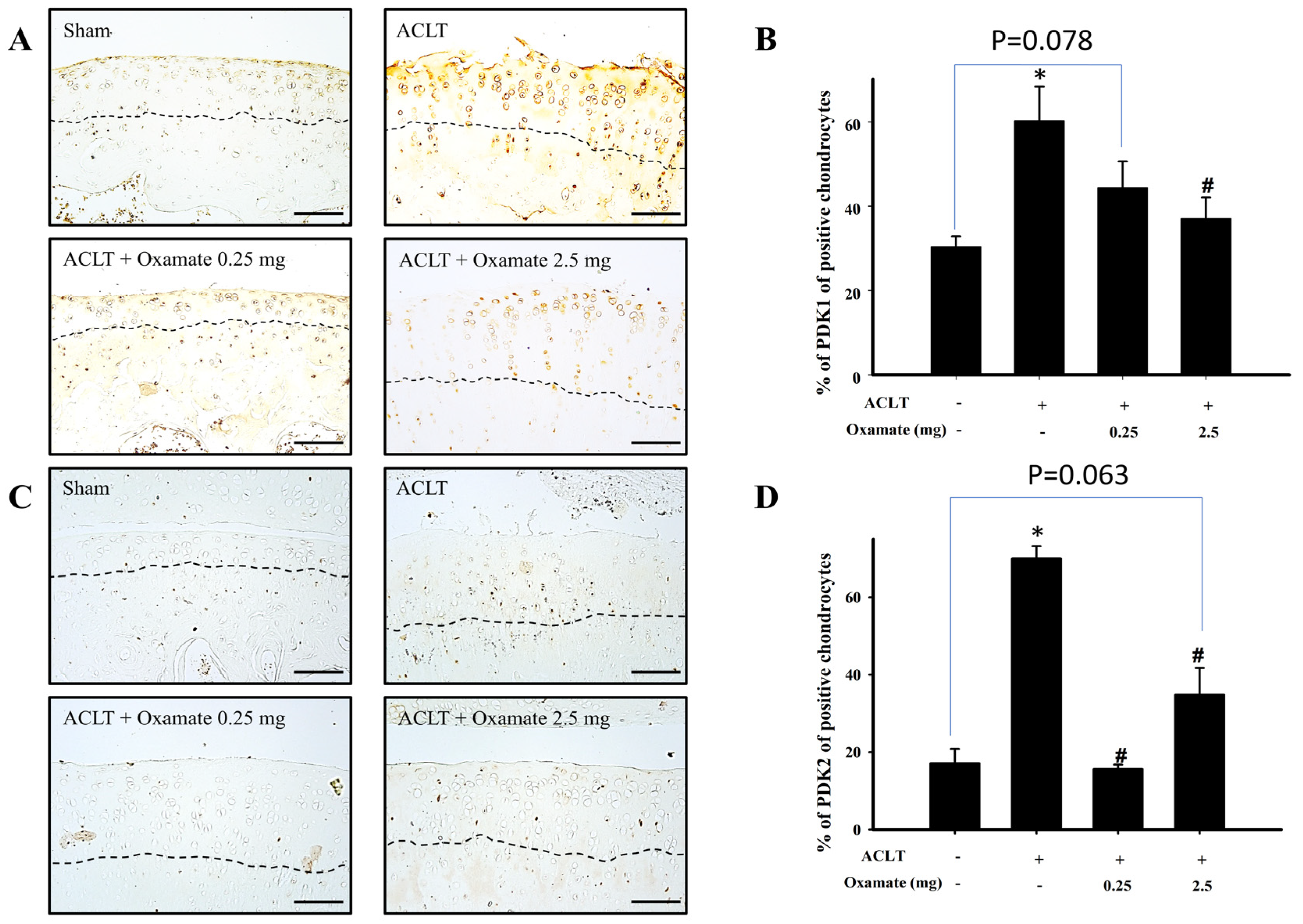
2.6. Oxamate Affects Pyruvate Dehydrogenase Kinase 1- and 2 Expression in ACLT-Cartilage
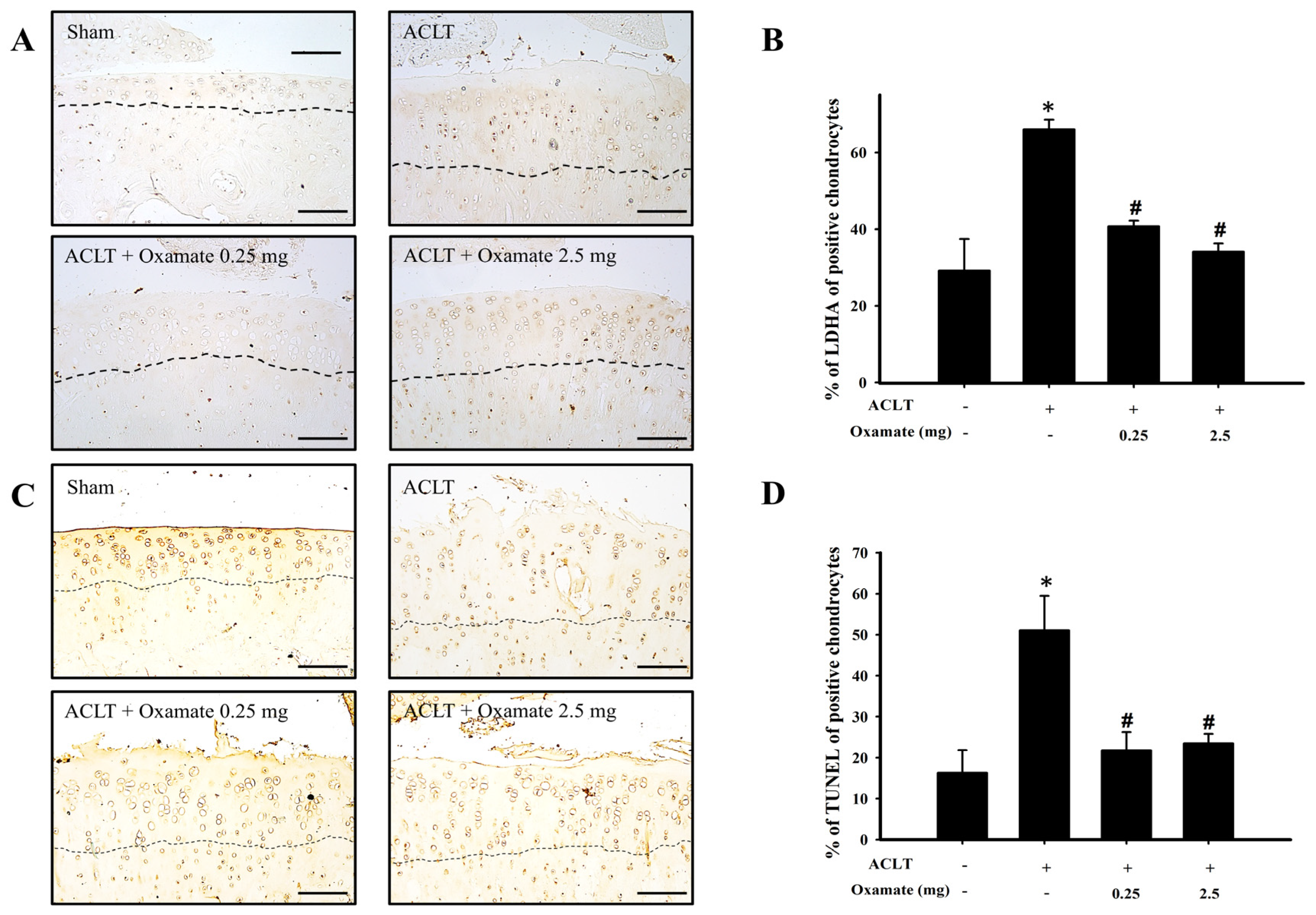
2.7. Oxamate Affects Lactate Dehydrogenase a Expression in ACLT-Cartilage
2.8. Oxamate Affects TUNEL Expression (Apoptosis) in ACLT-Cartilage
3. Discussion
4. Materials and Methods
4.1. Animals and Surgical Technique for OA Induction
4.2. Experimental Design and Oxamate Treatment
4.3. Test for Defects in Hind Limb Weight-Bearing and Knee Swelling (Change of Joint Width) Measurement
4.4. Gross Morphology and Histopathological Examination
4.5. Immunohistochemistry and TUNEL Assay
4.6. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the Immune System and Bone Metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Xuan, Y.; Zhang, X.; Liu, Y.; Yang, S.; Yang, K. Immune cell metabolism and metabolic reprogramming. Mol. Biol. Rep. 2022, 49, 9783–9795. [Google Scholar] [CrossRef] [PubMed]
- Kudelko, M.; Chan, C.W.; Sharma, R.; Yao, Q.; Lau, E.; Chu, I.K.; Cheah, K.S.; Tanner, J.A.; Chan, D. Label-Free Quantitative Proteomics Reveals Survival Mechanisms Developed by Hypertrophic Chondrocytes under ER Stress. J. Proteome Res. 2016, 15, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Kubota, S.; Aoyama, E.; Takigawa, M. Impaired glycolytic metabolism causes chondrocyte hypertrophy-like changes via promotion of phospho-Smad1/5/8 translocation into nucleus. Osteoarthr. Cartil. 2013, 21, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, R.S.; Fu, Y.; Matsuzaki, S.; Kinter, M.; Humphries, K.M.; Griffin, T.M. Mitochondrial respiration and redox coupling in articular chondrocytes. Arthritis Res. Ther. 2015, 17, 54. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Tang, B.L. Glucose, glycolysis, and neurodegenerative diseases. J. Cell. Physiol. 2020, 235, 7653–7662. [Google Scholar] [CrossRef]
- Abboud, G.; Choi, S.C.; Kanda, N.; Zeumer-Spataro, L.; Roopenian, D.C.; Morel, L. Inhibition of Glycolysis Reduces Disease Severity in an Autoimmune Model of Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1973. [Google Scholar] [CrossRef] [Green Version]
- McFate, T.; Mohyeldin, A.; Lu, H.; Thakar, J.; Henriques, J.; Halim, N.D.; Wu, H.; Schell, M.J.; Tsang, T.M.; Teahan, O.; et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J. Biol. Chem. 2008, 283, 22700–22708. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Mosquera, M.E.; Rego-Pérez, I.; Soto-Hermida, A.; Fernández-Moreno, M.; Fernández-Tajes, J.; Cortés-Pereira, E.; Relaño-Fernández, S.; Oreiro-Villar, N.; Fernández-López, C.; Blanco, F.J. New insights into the molecular basis of the metabolic alterations in the osteoarthritis (OA) disease. Osteoarthr. Cartil. 2013, 21, S170. [Google Scholar] [CrossRef] [Green Version]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Lu, D.; Guo, H.; Miao, W.; Wu, G.; Zhou, M. PFKFB3 modulates glycolytic metabolism and alleviates endoplasmic reticulum stress in human osteoarthritis cartilage. Clin. Exp. Pharmacol. Physiol. 2016, 43, 312–318. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Mandelin, J.; Li, T.F.; Salo, J.; Lassus, J.; Liljestrom, M.; Hukkanen, M.; Takagi, M.; Virtanen, I.; Santavirta, S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002, 46, 953–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razaq, S.; Wilkins, R.J.; Urban, J.P. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J. 2003, 12, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, R.J.; Hall, A.C. Control of matrix synthesis in isolated bovine chondrocytes by extracellular and intracellular pH. J. Cell. Physiol. 1995, 164, 474–481. [Google Scholar] [CrossRef]
- Hunter, D.J.; McDougall, J.J.; Keefe, F.J. The symptoms of osteoarthritis and the genesis of pain. Rheum. Dis. Clin. North. Am. 2008, 34, 623–643. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.M.; Hoyland, J.A.; Mobasheri, R.; Csaki, C.; Shakibaei, M.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J. Cell. Physiol. 2010, 222, 23–32. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, Y.; Wan, J.; Zhu, R.; Wu, Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol. Rep. 2013, 30, 2983–2991. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, A.M.; Smith, C.O.; Shum, L.C.; Awad, H.; Eliseev, R.A. Lactate Dehydrogenase Inhibition With Oxamate Exerts Bone Anabolic Effect. J. Bone Miner. Res. 2020, 35, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, H.; Debarge, R.; Richou, J.; Selmi, T.A.; Donell, S.T.; Neyret, P.; Dubrana, F. Osteoarthritis in patients with anterior cruciate ligament rupture: A review of risk factors. Knee 2009, 16, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Lin, Y.Y.; Hsieh, S.P.; Lee, H.P.; Lin, S.C.; Chen, W.F.; Jean, Y.H. Calcitonin attenuates cartilage degeneration and nociception in an experimental rat model of osteoarthritis: Role of TGF-beta in chondrocytes. Sci. Rep. 2016, 6, 28862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Chen, C.H.; Wu, S.C.; Hsieh, S.P.; Hsieh, C.S.; Wang, K.Y.; Lin, S.Y.; et al. Intra-articular injection of the selective cyclooxygenase-2 inhibitor meloxicam (Mobic) reduces experimental osteoarthritis and nociception in rats. Osteoarthr. Cartil. 2013, 21, 1976–1986. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26. [Google Scholar] [CrossRef]
- Jean, Y.H.; Wen, Z.H.; Chang, Y.C.; Lee, H.S.; Hsieh, S.P.; Wu, C.T.; Yeh, C.C.; Wong, C.S. Hyaluronic acid attenuates osteoarthritis development in the anterior cruciate ligament-transected knee: Association with excitatory amino acid release in the joint dialysate. J. Orthop. Res. 2006, 24, 1052–1061. [Google Scholar] [CrossRef]
- Jean, Y.H.; Wen, Z.H.; Chang, Y.C.; Hsieh, S.P.; Tang, C.C.; Wang, Y.H.; Wong, C.S. Intra-articular injection of the cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transected knee in rats: Role of excitatory amino acids. Osteoarthr. Cartil. 2007, 15, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.L.; Gale, D.G.; Chaisson, C.E.; Skinner, K.; Kazis, L.; Gale, M.E.; Felson, D.T. Knee effusions, popliteal cysts, and synovial thickening: Association with knee pain in osteoarthritis. J. Rheumatol. 2001, 28, 1330–1337. [Google Scholar]
- Wu, X.; Fan, X.; Crawford, R.; Xiao, Y.; Prasadam, I. The Metabolic Landscape in Osteoarthritis. Aging Dis. 2022, 13, 1166–1182. [Google Scholar] [CrossRef]
- Kan, S.; Duan, M.; Liu, Y.; Wang, C.; Xie, J. Role of Mitochondria in Physiology of Chondrocytes and Diseases of Osteoarthritis and Rheumatoid Arthritis. Cartilage 2021, 13, 1102S–1121S. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasheri, A.; Neama, G.; Bell, S.; Richardson, S.; Carter, S.D. Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell. Biol. Int. 2002, 26, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell. Death Differ. 2015, 22, 364. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Oliveira, P.G.; Garcia-Carbonell, R.; Croft, A.P.; Smith, J.M.; Serrano, R.L.; Sanchez-Lopez, E.; Liu, X.; Kisseleva, T.; Hay, N.; et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1636–1643. [Google Scholar] [CrossRef]
- Zhou, K.L.; Zhu, Z.H.; Zhou, J.P.; Zhao, J.J.; Zhang, Y.; Jiang, B. Increased hexokinase-2 as a novel biomarker for the diagnosis and correlating with disease severity in rheumatoid arthritis. Medicine 2021, 100, e26504. [Google Scholar] [CrossRef]
- Wang, C.; Silverman, R.M.; Shen, J.; O’Keefe, R.J. Distinct metabolic programs induced by TGF-beta1 and BMP2 in human articular chondrocytes with osteoarthritis. J. Orthop. Translat. 2018, 12, 66–73. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Zhao, X.; Chen, L.; Li, W.; Ran, J.; Wu, L. Pyruvate Kinase M2 Modulates the Glycolysis of Chondrocyte and Extracellular Matrix in Osteoarthritis. DNA Cell. Biol. 2018, 37, 271–277. [Google Scholar] [CrossRef]
- Smolle, M.; Prior, A.E.; Brown, A.E.; Cooper, A.; Byron, O.; Lindsay, J.G. A new level of architectural complexity in the human pyruvate dehydrogenase complex. J. Biol. Chem. 2006, 281, 19772–19780. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Biosci. Rep. 2021, 41, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell. Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudoh, K.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: Involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2005, 7, R904–R914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeoung, N.H. Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers. Diabetes Metab. J. 2015, 39, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Martinez, G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 19, 307–313. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Yang, H.; Wen, Y.; Zhang, M.; Liu, Q.; Zhang, H.; Zhang, J.; Lu, L.; Ye, T.; Bai, X.; Xiao, G.; et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy 2020, 16, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Teeple, E.; Jay, G.D.; Elsaid, K.A.; Fleming, B.C. Animal models of osteoarthritis: Challenges of model selection and analysis. AAPS J. 2013, 15, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [Green Version]
- Radin, E.L.; Rose, R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 1986, 213, 34–40. [Google Scholar] [CrossRef]
- Wen, Z.H.; Huang, J.S.; Lin, Y.Y.; Yao, Z.K.; Lai, Y.C.; Chen, W.F.; Liu, H.T.; Lin, S.C.; Tsai, Y.C.; Tsai, T.C.; et al. Chondroprotective Effects of a Histone Deacetylase Inhibitor, Panobinostat, on Pain Behavior and Cartilage Degradation in Anterior Cruciate Ligament Transection-Induced Experimental Osteoarthritic Rats. Int. J. Mol. Sci. 2021, 22, 7290. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.M.; Sophocleous, A. Quantitative analysis of bone and soft tissue by micro-computed tomography: Applications to ex vivo and in vivo studies. Bonekey Rep. 2014, 3, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoop, R.; Buma, P.; van der Kraan, P.M.; Hollander, A.P.; Billinghurst, R.C.; Meijers, T.H.; Poole, A.R.; van den Berg, W.B. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthr. Cartil. 2001, 9, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Bove, S.E.; Laemont, K.D.; Brooker, R.M.; Osborn, M.N.; Sanchez, B.M.; Guzman, R.E.; Hook, K.E.; Juneau, P.L.; Connor, J.R.; Kilgore, K.S. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthr. Cartil. 2006, 14, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.H.; Lin, Y.Y.; Chang, Y.C.; Tang, C.C.; Hsieh, S.P.; Lee, H.P.; Sung, C.S.; Chen, W.F.; Lee, C.H.; Hsuan Jean, Y. The COX-2 inhibitor etoricoxib reduces experimental osteoarthritis and nociception in rats: The roles of TGF-beta1 and NGF expressions in chondrocytes. Eur. J. Pain. 2020, 24, 209–222. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.C.; Martel-Pelletier, J.; Otterness, I.G.; Lopez-Anaya, A.; Mineau, F.; Tardif, G.; Pelletier, J.P. Effects of tenidap on canine experimental osteoarthritis. I. Morphologic and metalloprotease analysis. Arthritis Rheum. 1995, 38, 1290–1303. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Boileau, C.; Martel-Pelletier, J.; Brunet, J.; Schrier, D.; Flory, C.; Boily, M.; Pelletier, J.P. PD-0200347, an alpha2delta ligand of the voltage gated calcium channel, inhibits in vivo activation of the Erk1/2 pathway in osteoarthritic chondrocytes: A PKCalpha dependent effect. Ann. Rheum. Dis. 2006, 65, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primary Antibody | Host | Supplier | Catalog # | Dilution Ratio |
|---|---|---|---|---|
| GLUT1 | Rabbit | Abcam, Cambridge, UK | ab652 | 1:1000 |
| GLUT3 | Rabbit | Biorbyt, Cambridge, UK | orb10727 | 1:1000 |
| HK II | Rabbit | GeneTex, Irvine, CA, USA | gtx111525 | 1:3000 |
| PKM2 | Rabbit | Cell Signaling Technology, Danvers, MA, USA | 4053s | 1:800 |
| PDK1 | Mouse | Abcam | ab110025 | 1:1000 |
| PDK2 | Rabbit | Abcam | ab68164 | 1:800 |
| LDHA | Rabbit | Novus, Littleton, CO, USA | NBP2-67483 | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Z.-H.; Sung, C.-S.; Lin, S.-C.; Yao, Z.-K.; Lai, Y.-C.; Liu, Y.-W.; Wu, Y.-Y.; Sun, H.-W.; Liu, H.-T.; Chen, W.-F.; et al. Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue. Int. J. Mol. Sci. 2023, 24, 10770. https://doi.org/10.3390/ijms241310770
Wen Z-H, Sung C-S, Lin S-C, Yao Z-K, Lai Y-C, Liu Y-W, Wu Y-Y, Sun H-W, Liu H-T, Chen W-F, et al. Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue. International Journal of Molecular Sciences. 2023; 24(13):10770. https://doi.org/10.3390/ijms241310770
Chicago/Turabian StyleWen, Zhi-Hong, Chun-Sung Sung, Sung-Chun Lin, Zhi-Kang Yao, Yu-Cheng Lai, Yu-Wei Liu, Yu-Yan Wu, Hsi-Wen Sun, Hsin-Tzu Liu, Wu-Fu Chen, and et al. 2023. "Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue" International Journal of Molecular Sciences 24, no. 13: 10770. https://doi.org/10.3390/ijms241310770
APA StyleWen, Z. -H., Sung, C. -S., Lin, S. -C., Yao, Z. -K., Lai, Y. -C., Liu, Y. -W., Wu, Y. -Y., Sun, H. -W., Liu, H. -T., Chen, W. -F., & Jean, Y. -H. (2023). Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue. International Journal of Molecular Sciences, 24(13), 10770. https://doi.org/10.3390/ijms241310770






