Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation
Abstract
:1. Introduction
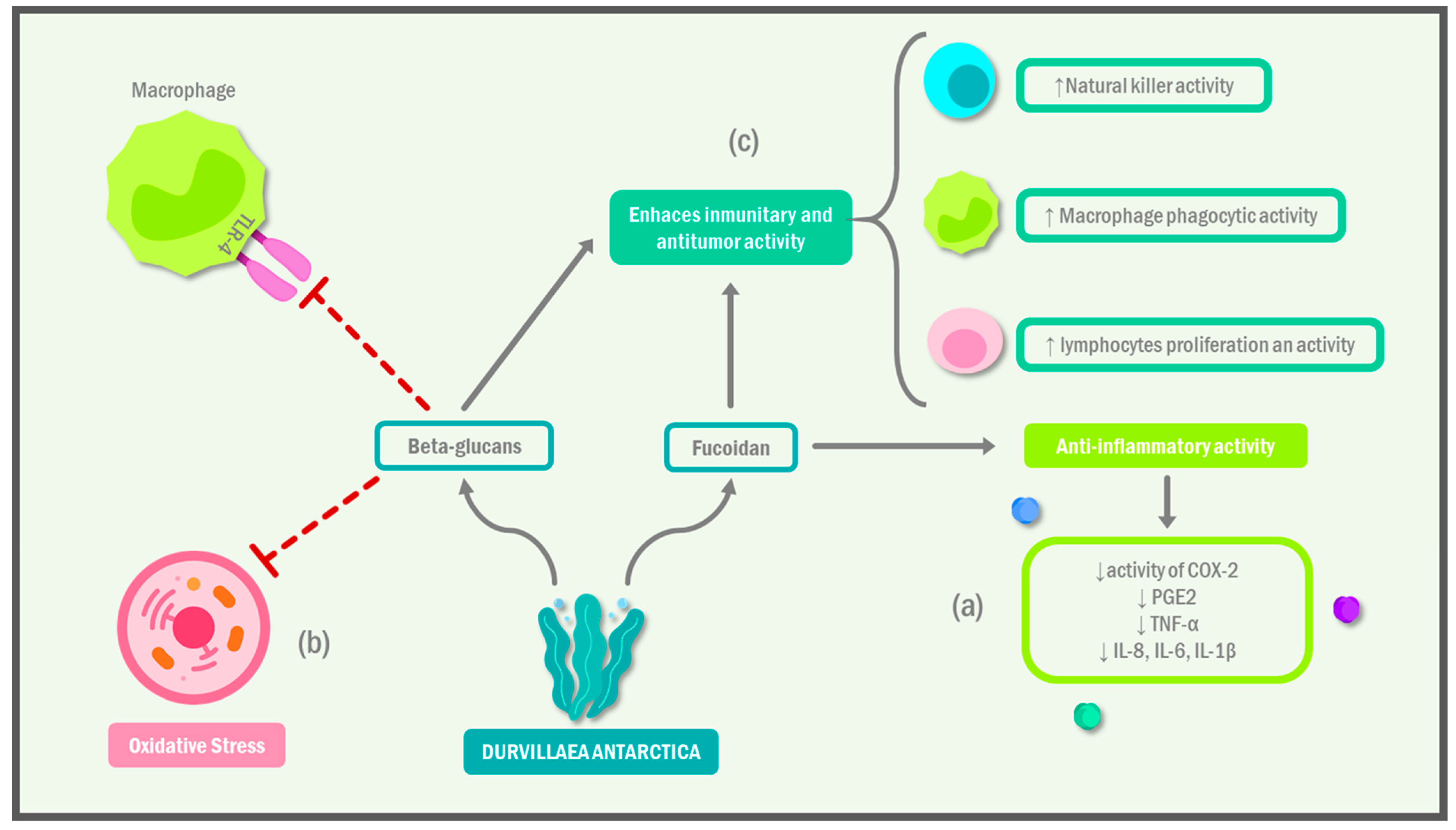
1.1. Immunomodulatory Effect of Durvillaea antarctica
| Author (REF) | Phytochemical Compound Tested | Rationale | Results–Key Findings |
|---|---|---|---|
| Lee et al. [11] | Laminaria japonica extract (LJE) | Study the mitigating LJE on inflammatory reactions of the skin when applied to UVB-induced nc886-PKR pathways, and the regulatory effect of nc886 on the PKR signal transduction channel induced by UVB. | LJE inhibited UVB-induced inflammation in human keratinocytes. LJE treatment reduced the expression of MMP-9, PGE2, IL-8, and TNF-α, and it also inhibited the phosphorylation of p38, SAPK/JNK, c-Jun, ATF-2, and PKR under basal and UVB conditions. |
| Castillo et al. [17] | Macrocystis pyrifera and Durvillaea antarctica aqueous extracts | Evaluate the potential antiviral properties of extracts obtained from two brown macroalgae against both HSV-1 and HSV-2 in humans (HeLa cells) and primary human gingival fibroblasts. | Algae extracts inhibited the growth of both viruses in a dose-dependent manner. The algae extracts also reduced the binding of HSV-1 and HSV-2 to HeLa cells, and decreased the expression of the viral proteins gB and gD. |
| Xu et al. [19] | D. antarctica polysaccharide (DAPP) | Validate how DAPP inhibits EV71 to induce the apoptosis of Vero cell. | DAPP had no toxicity on Vero cells at the concentration of 250 μg/mL. DAPP inhibited the proliferation of EV71 virus in a dose-dependent manner, inhibited the Vero cells’ apoptosis induced by EV71 via P53 signaling pathway, and decreased the expression of proinflammatory cytokines. |
| Qin et al. [20] | Sulfated polysaccharide 4 of D. antarctica (DAP4) | Isolation and purification of DAP4 via methylation analysis and NMR spectrometry analysis. Evaluation of immunomodulatory activity in vitro, including lymphocyte proliferation, phagocytic activity of macrophages, NO production, and NK cell cytotoxicity in vitro. | DAP4 had immunomodulatory activity in vitro. DAP4 was non-toxic to RAW264.7 cells at concentrations of up to 400 μg/mL. DAP4 also enhanced the phagocytic activity of RAW264.7 cells. DAP4 increased the production of NO by RAW264.7 cells. DAP4 also enhanced the proliferation of splenocytes in response to ConA and LPS. DAP4 increased the cytotoxicity of NK cells against YAC-1 cells. |
1.2. Durvillaea antarctica as a Cornerstone for Gut Microbiota Modulation
| Author (REF) | Phytochemical Compound Tested | Rationale | Results–Key Findings |
|---|---|---|---|
| He et al. [23] | Combination of deep-sea water (DSW) and/or fucoidan (CDF) | The combined effect of DSW and fucoidan was investigated on a T2DM rat model induced by a high-fat diet and streptozocin injection. Fecal metabolomics and 16S rDNA analysis were used to explore the relationship between these interventions and identify potential metabolic pathways. | CDF was more effective than DSW or fucoidan alone in improving blood glucose, lipid levels, and histopathological changes in T2DM rats. CDF also enhanced the phosphorylation of Akt and GSK3β, which are important steps in insulin signaling. Fecal metabolomics and 16S rDNA analysis showed that CDF altered the composition of gut microbiota and metabolic pathways. |
| Bai et al. [25] | Alginate | Alginate overproducing mutant of P. aeruginosa was obtained through transposon mutagenesis libraries. The in vitro functions of human gut microbiota in degrading seaweed and mutant Pseudomonas alginates were comparatively studied. | Both bacterial and seaweed alginates were found to be completely degraded by fecal bacteria isolated from study volunteers. Moreover, their regulatory function on gut microbiota was similar, as they promoted the proliferation of beneficial bifidobacteria while reducing the abundance of pathogenic bacterial strains. |
| Siddiqui et al. [29] | Crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) | The impact of streptozotocin-induced T1DM on gut barrier permeability and gut microbiota dysbiosis. | CDDP treatment increased beneficial bacteria (Firmicutes, Bacteroidetes, Lactobacillus) via 16S rRNA sequencing. Immunohistological analysis confirmed CDDP’s anti-inflammatory effects, restoring colon morphology and maintaining gut structure and barrier permeability. |
| du Preez et al. [39] | Sargassum siliquosum extract | Evaluated the impact of S. siliquosum on metabolic syndrome parameters, including heart/liver function, plasma biochemistry, glucose/insulin responses, body composition, and gut microbiota composition. | S. siliquosum decreased body weight, fat mass, abdominal fat deposition, liver fat vacuole size, and improved glucose tolerance and insulin sensitivity. S. siliquosum also increased the population of beneficial bacteria in the gut and reduced inflammation. |
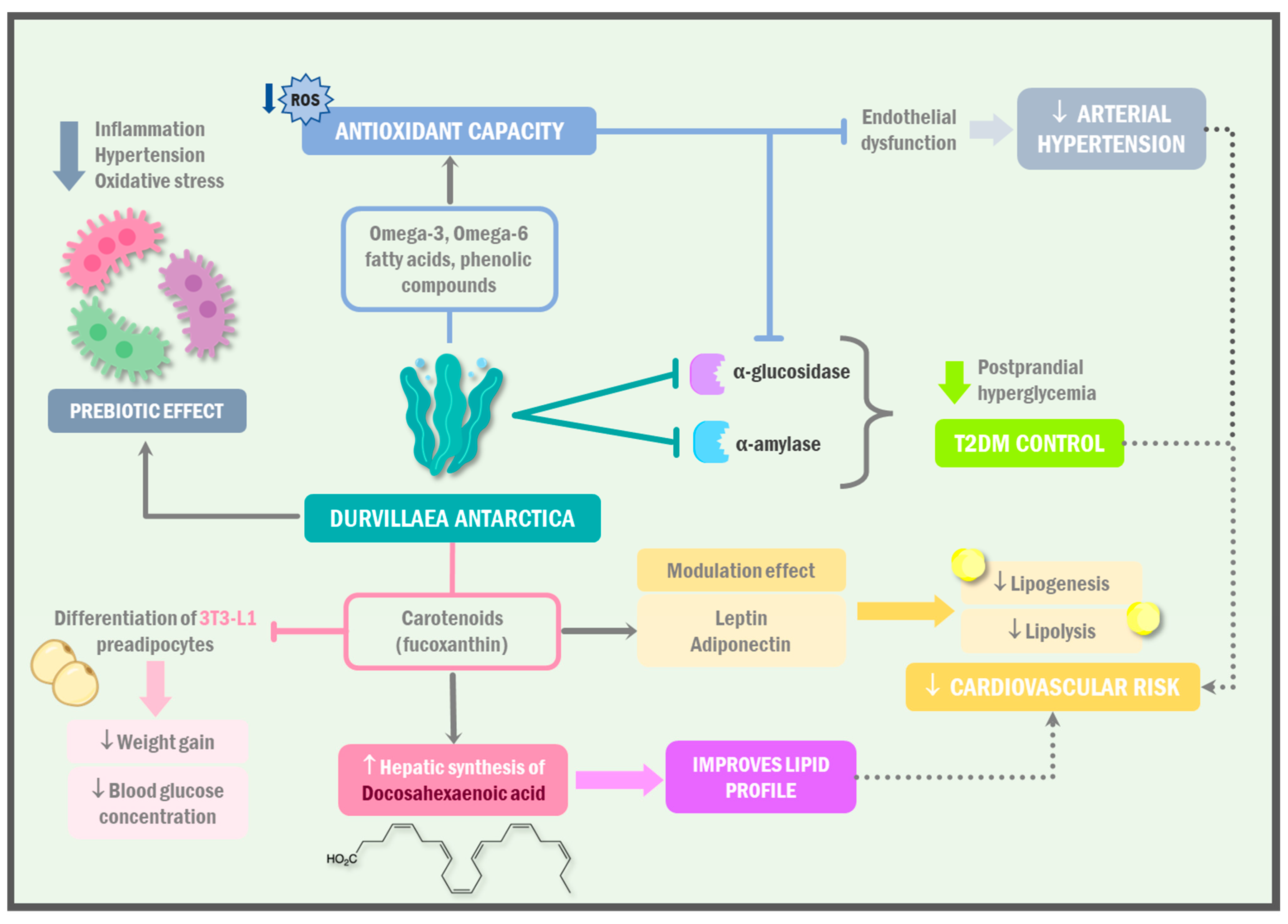
1.3. Cardioprotective Role of Durvillaea antarctica
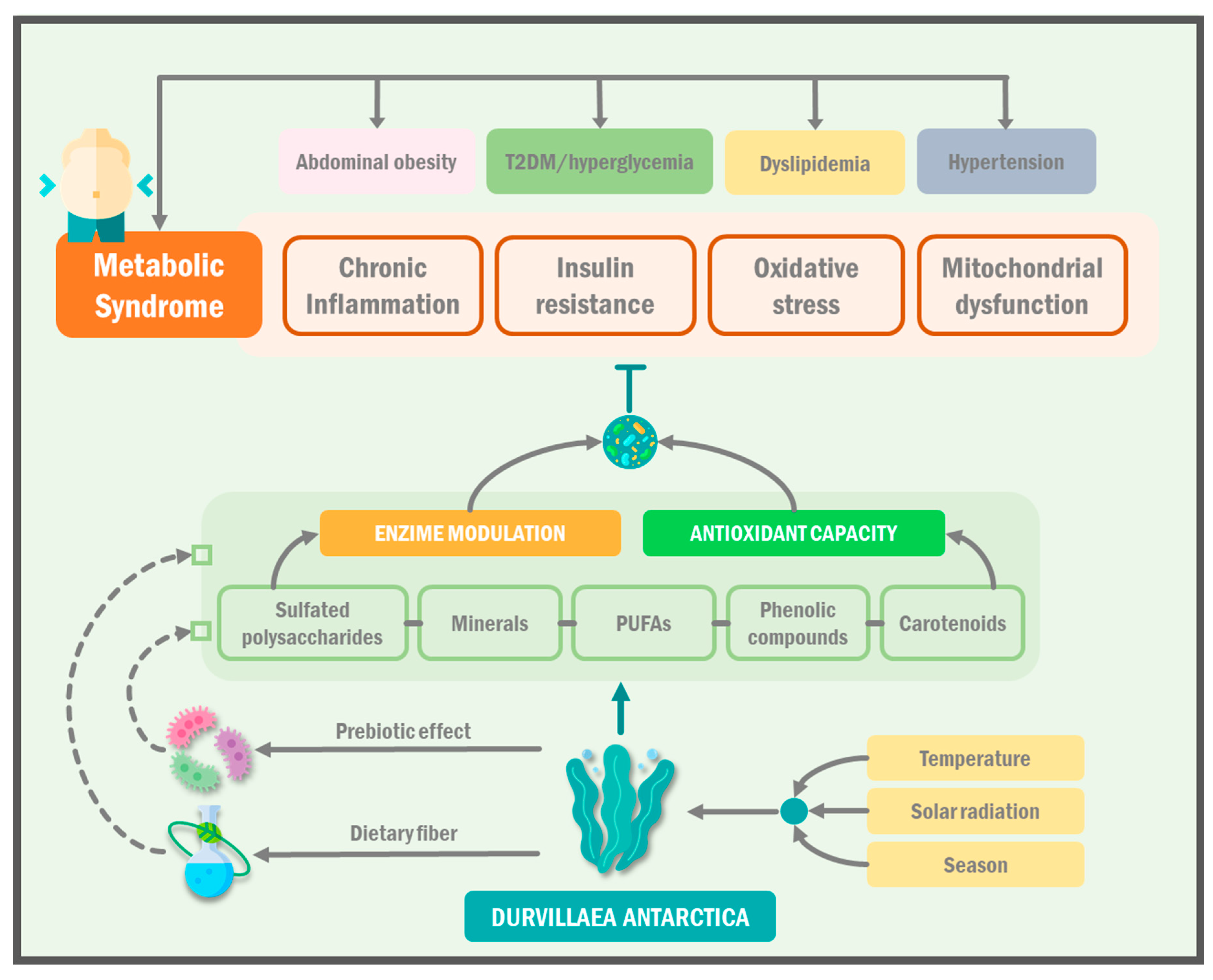
1.4. D. antarctica as a Promising Therapeutic Dietary Agent for the Management of Metabolic Syndrome
| Author (REF) | Phytochemical Compound Tested | Rationale | Results–Key Findings |
|---|---|---|---|
| Stiefvatter et al. [54] | Phaeodactylum tricornutum (PT) | Bioavailability and safety of consuming whole biomass of PT in humans. Intestinal health and microbiota were also assessed. | PT intake increased n-3 PUFA and EPA levels, decreased the n-6:n-3 ratio, and resulted in the uptake of fucoxanthinol (FX) and amarouciaxanthin A (A × A). No adverse effects were observed, supporting PT as a sustainable food source. |
| Chichibu et al. [55] | Seaweed | Seaweed intake was assessed through a 24 h dietary recall survey and categorized into four groups (0, 1–5.5, 5.5–15, and ≥15 g/day). The study examined the incidence of cardiovascular disease within the Circulatory Risk in Communities Study (CIRCS). | Seaweed intake was inversely associated with the risk of total stroke and cerebral infarction among men but not among women. The hazard ratios (95% confidence intervals) for the highest versus the lowest categories of seaweed intake were 0.63 (0.42–0.94; 0.01) for total stroke and 0.59 (0.36–0.97; 0.03) for cerebral infarction. |
| Kishida et al. [56] | Seaweed | Association between seaweed intake frequency and CVD mortality, including stroke subtypes and coronary heart disease, among Japanese participants in the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. | Regular seaweed consumption was associated with lower hazard ratios for cardiovascular disease, stroke, and cerebral infarction in both men and women. The multivariable-adjusted hazard ratios were 0.72 (0.55–0.95; 0.001) for total cardiovascular disease, 0.70 (0.46–1.06; 0.01) for total stroke, and 0.49 (0.27–0.90; 0.22) for cerebral infarction. |
| Pacheco et al. [64] | Durvillaea antarctica, Gelidium sp., Lessonia spicata, Nothogenia sp., Mazzaella laminarioides, Pyropia sp. | Assess the anti-glycemic potential of seaweeds from southern Chile. HPLE was compared to acetone extraction for obtaining polyphenol-rich extracts for functional food development. | The acetone extract of D. antarctica had the highest TP content, while the HPLE ethanol/water extract exhibited the highest antioxidant activity. Cochayuyo extracts showed significant anti-enzymatic capacity against α-glucosidase and α-amylase. No extract affected cell viability. |
| Shih et al. [73] | Durvillaea antarctica | The potential of enzymatic hydrolysates from D. antarctica as natural antioxidants. Three hydrolysates, Dur-A, Dur-B, and Dur-C, were produced using viscozyme, cellulase, and α-amylase enzymes, respectively. | All of the following extracts demonstrated inhibitory effects on key enzymes related to metabolic syndrome: angiotensin I-converting enzyme (ACE), α-amylase, α-glucosidase, and pancreatic lipase. Dur-B showed superior antioxidant and anti-metabolic syndrome effects compared to the other extracts. |
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva Lactuca and Durvillaea Antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary Fiber Intake in Relation to Coronary Heart Disease and All-Cause Mortality over 40 y: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Margozzini Maira, P.; Passi Solar, A. Encuesta Nacional de Salud, ENS 2016-2017: Un aporte a la planificación sanitaria y políticas públicas en Chile. ARS Med. 2018, 43, 30–34. [Google Scholar] [CrossRef]
- Obesidad y Sobrepeso. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 March 2023).
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Menshova, R.; Shevchenko, N.; Imbs, T.; Zvyagintseva, T.; Malyarenko, O.; Zaporozhets, T.A.; Besednova, N.; Ermakova, S. Fucoidans from Brown Alga Fucus Evanescens: Structure and Biological Activity. Front. Mar. Sci. 2016, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Isnansetyo, A.; Fikriyah, A.; Kasanah, N.; Murwantoko. Non-Specific Immune Potentiating Activity of Fucoidan from a Tropical Brown Algae (Phaeophyceae), Sargassum Cristaefolium in Tilapia (Oreochromis niloticus). Aquacult. Int. 2016, 24, 465–477. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and Enzymatic Fractionation of Cell Walls from Fucales: Insights into the Structure of the Extracellular Matrix of Brown Algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; Cho, E.; Weon, J.B.; Park, D.; Fréchet, M.; Chajra, H.; Jung, E. Inhibition of UVB-Induced Inflammation by Laminaria Japonica Extract via Regulation of Nc886-PKR Pathway. Nutrients 2020, 12, 1958. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. Anti-Inflammatory Activity of Fucoidan with Blocking NF-ΚB and STAT1 in Human Keratinocytes Cells. Nat. Prod. Sci. 2015, 21, 205–209. [Google Scholar]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1,6-Glucan in Seaweed from the Southern Hemisphere and Its Immunomodulatory Effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Song, Q.; Zhang, C.; Xu, X.; Li, M.; Yao, D.; Wu, L.; Qu, X.; Guan, H.; Yu, G.; et al. A β-1,3/1,6-Glucan from Durvillaea Antarctica Inhibits Tumor Progression in Vivo as an Immune Stimulator. Carbohydr. Polym. 2019, 222, 114993. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Li, J.; Jiang, H.; Shan, X.; Wang, Y.; Ma, W.; Hao, J.; Yu, G. A β-Glucan from Durvillaea Antarctica Has Immunomodulatory Effects on RAW264.7 Macrophages via Toll-like Receptor 4. Carbohydr. Polym. 2018, 191, 255–265. [Google Scholar] [CrossRef]
- Castillo, E.; Duarte, L.F.; Corrales, N.; Álvarez, D.M.; Farías, M.A.; Henríquez, A.; Smith, P.C.; Agurto-Muñoz, C.; González, P.A. Anti-Herpetic Activity of Macrocystis Pyrifera and Durvillaea Antarctica Algae Extracts Against HSV-1 and HSV-2. Front. Microbiol. 2020, 11, 2006. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. IJMS 2016, 17, 1988. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Wu, H.-L.; Chen, H.; Wu, H.; Guo, M.; Zhao, M.; Wang, C.; Lin, T.; Lin, Z.; et al. The Inhibition of Enterovirus 71 Induced Apoptosis by Durvillaea Antarctica through P53 and STAT1 Signaling Pathway. J. Med. Virol. 2021, 93, 3532–3538. [Google Scholar] [CrossRef]
- Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, Chemical Characterization and Immunomodulatory Activity of a Sulfated Polysaccharide from Marine Brown Algae Durvillaea Antarctica. Mar. Drugs 2022, 20, 223. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A Critical Review on the Impacts of β-Glucans on Gut Microbiota and Human Health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef]
- He, S.; Peng, W.-B.; Zhou, H.-L.; Fu, X.-J.; Sun, Y.-H.; Wang, Z.-G. A Combination of Deep-Sea Water and Fucoidan Alleviates T2DM through Modulation of Gut Microbiota and Metabolic Pathways. Pharmaceuticals 2023, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Ejima, R.; Akiyama, M.; Sato, H.; Tomioka, S.; Yakabe, K.; Kimizuka, T.; Seki, N.; Fujimura, Y.; Hirayama, A.; Fukuda, S.; et al. Seaweed Dietary Fiber Sodium Alginate Suppresses the Migration of Colonic Inflammatory Monocytes and Diet-Induced Metabolic Syndrome via the Gut Microbiota. Nutrients 2021, 13, 2812. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chen, H.; Zhu, L.; Liu, W.; Yu, H.D.; Wang, X.; Yin, Y. Comparative Study on the in Vitro Effects of Pseudomonas Aeruginosa and Seaweed Alginates on Human Gut Microbiota. PLoS ONE 2017, 12, e0171576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Han, Y.; Ding, Y.; Zhu, B.; Song, S.; Xiao, H. Health Effects of Dietary Sulfated Polysaccharides from Seafoods and Their Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2882–2913. [Google Scholar] [CrossRef]
- Bermano, G.; Stoyanova, T.; Hennequart, F.; Wainwright, C.L. Seaweed-Derived Bioactives as Potential Energy Regulators in Obesity and Type 2 Diabetes. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 87, pp. 205–256. ISBN 978-0-12-820185-5. [Google Scholar]
- Yang, C.; Lai, S.; Chen, Y.; Liu, D.; Liu, B.; Ai, C.; Wan, X.; Gao, L.; Chen, X.; Zhao, C. Anti-Diabetic Effect of Oligosaccharides from Seaweed Sargassum Confusum via JNK-IRS1/PI3K Signalling Pathways and Regulation of Gut Microbiota. Food Chem. Toxicol. 2019, 131, 110562. [Google Scholar] [CrossRef]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; khan, A.I.; Farooqui, N.A.; Zang, S.; Xin, Y.; Wang, L. Effect of Crude Polysaccharide from Seaweed, Dictyopteris Divaricata (CDDP) on Gut Microbiota Restoration and Anti-Diabetic Activity in Streptozotocin (STZ)-Induced T1DM Mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Li, Y.; Wang, Y.; Wang, F.; Qiu, X.; Liu, X.; Li, H. Sodium Alginate Modulates Immunity, Intestinal Mucosal Barrier Function, and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. J. Agric. Food Chem. 2021, 69, 7064–7073. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in Gut Microbiota in Rats Fed a High Fat Diet Correlate with Obesity-Associated Metabolic Parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ai, C.; Wen, C.; Qin, Y.; Liu, Z.; Wang, L.; Gong, Y.; Su, C.; Wang, Z.; Song, S. Fucoidan Isolated from Ascophyllum nodosum Alleviates Gut Microbiota Dysbiosis and Colonic Inflammation in Antibiotic-Treated Mice. Food Funct. 2020, 11, 5595–5606. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, N.; Wang, J.; Geng, L.; Yue, Y.; Wang, F.; Zhang, Q. Low Molecular Weight Fucoidan Fraction LF2 Improves Metabolic Syndrome via Up-Regulating PI3K-AKT-MTOR Axis and Increasing the Abundance of Akkermansia Muciniphila in the Gut Microbiota. Int. J. Biol. Macromol. 2021, 193, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kandasamy, S.; Zhang, J.; Kirby, C.W.; Karakach, T.; Hafting, J.; Critchley, A.T.; Evans, F.; Prithiviraj, B. Prebiotic Effects of Diet Supplemented with the Cultivated Red Seaweed Chondrus Crispus or with Fructo-Oligo-Saccharide on Host Immunity, Colonic Microbiota and Gut Microbial Metabolites. BMC Complement. Altern. Med. 2015, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.; Drago, S.; de Medina, F.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, P.; O’Doherty, J.V.; Pierce, K.M.; Callan, J.J.; O’Sullivan, J.T.; Sweeney, T. The Effects of Seaweed Extract Inclusion on Gut Morphology, Selected Intestinal Microbiota, Nutrient Digestibility, Volatile Fatty Acid Concentrations and the Immune Status of the Weaned Pig. Animal 2008, 2, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum Fusiforme Fucoidan Modifies the Gut Microbiota during Alleviation of Streptozotocin-Induced Hyperglycemia in Mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The Role of the Intestinal Microbiota in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef]
- Du Preez, R.; Magnusson, M.; Majzoub, M.E.; Thomas, T.; Praeger, C.; Glasson, C.R.K.; Panchal, S.K.; Brown, L. Brown Seaweed Sargassum Siliquosum as an Intervention for Diet-Induced Obesity in Male Wistar Rats. Nutrients 2021, 13, 1754. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, L.; Liu, B.; He, N. Unsaturated Alginate Oligosaccharides Attenuated Obesity-Related Metabolic Abnormalities by Modulating Gut Microbiota in High-Fat-Diet Mice. Food Funct. 2020, 11, 4773–4784. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural Characterization and in Vitro Fermentation of a Novel Polysaccharide from Sargassum Thunbergii and Its Impact on Gut Microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Fernández-García, V.; González-Ramos, S.; Martín-Sanz, P.; Portillo, F.G.; Laparra, J.M.; Boscá, L. NOD1 in the Interplay between Microbiota and Gastrointestinal Immune Adaptations. Pharmacol. Res. 2021, 171, 105775. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.J.; Zhou, J.Y.; Geddes, K.; Rubino, S.J.; Cho, J.H.; Girardin, S.E.; Philpott, D.J. Nod1 and Nod2 Signaling Does Not Alter the Composition of Intestinal Bacterial Communities at Homeostasis. Gut Microbes 2013, 4, 222–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Zoete, M.R.; Flavell, R.A. Interactions between Nod-Like Receptors and Intestinal Bacteria. Front. Immunol. 2013, 4, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The Differential Effects of EPA and DHA on Cardiovascular Risk Factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, J.; Díaz, R.; Dantagnan, P.; Hernández, A.; Valdes, M.; Lorenzo, J.M.; Cancino, D.; Sepúlveda, N.; Farías, J.G. Dietary Inclusion of Durvillaea Antarctica Meal and Rapeseed (Brassica napus) Oil on Growth, Feed Utilization and Fillet Quality of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2021, 530, 735882. [Google Scholar] [CrossRef]
- Athukorala, Y.; Lee, K.-W.; Kim, S.-K.; Jeon, Y.-J. Anticoagulant Activity of Marine Green and Brown Algae Collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef]
- Onofrejová, L.; Vasícková, J.; Klejdus, B.; Stratil, P.; Misurcová, L.; Krácmar, S.; Kopecký, J.; Vacek, J. Bioactive Phenols in Algae: The Application of Pressurized-Liquid and Solid-Phase Extraction Techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Škrovánková, S. Chapter 28—Seaweed Vitamins as Nutraceuticals. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 357–369. [Google Scholar]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Quitral, R.V.; Morales, G.C.; Sepúlveda, L.M.; Schwartz, M.M. Propiedades Nutritivas y Saludables de Algas Marinas y Su Potencialidad Como Ingrediente Funcional. Rev. Chil. De Nutr. ÓN 2012, 39, 196–202. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum Tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Chichibu, H.; Yamagishi, K.; Kishida, R.; Maruyama, K.; Hayama-Terada, M.; Shimizu, Y.; Muraki, I.; Umesawa, M.; Cui, R.; Imano, H.; et al. Seaweed Intake and Risk of Cardiovascular Disease: The Circulatory Risk in Communities Study (CIRCS). JAT 2021, 28, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Kishida, R.; Yamagishi, K.; Muraki, I.; Sata, M.; Tamakoshi, A.; Iso, H.; JACC Study Group. Frequency of Seaweed Intake and Its Association with Cardiovascular Disease Mortality: The JACC Study. JAT 2020, 27, 1340–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome Update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Raut, S.K.; Khullar, M. Oxidative Stress in Metabolic Diseases: Current Scenario and Therapeutic Relevance. Mol. Cell. Biochem. 2023, 478, 185–196. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.; Abdalbasit, A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axmann, M.; Strobl, W.M.; Plochberger, B.; Stangl, H. Cholesterol Transfer at the Plasma Membrane. Atherosclerosis 2019, 290, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Shi, J.; Yu, Y.; Lu, H.; Yu, L.; Liu, Y.; Zhang, F. Diosgenin Regulates Cholesterol Metabolism in Hypercholesterolemic Rats by Inhibiting NPC1L1 and Enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2019, 1864, 1124–1133. [Google Scholar] [CrossRef]
- Valério Filho, A.; Santana, L.R.; Motta, N.G.; Passos, L.F.; Ines Wolke, S.; Mansilla, A.; Astorga-España, M.S.; Becker, E.M.; de Pereira, C.M.P.; Carreno, N.L.V. Extraction of Fatty Acids and Cellulose from the Biomass of Algae Durvillaea Antarctica and Ulva Lactuca: An Alternative for Biorefineries. Algal Res. 2023, 71, 103084. [Google Scholar] [CrossRef]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of Drying Methods on Bioactive Compounds, Nutritional, Antioxidant, and Antidiabetic Potential of Brown Alga Durvillaea Antarctica. Dry. Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Cuesta, R.G.; García, K.L.G.; del, R.; Valdés Iglesias, O.; Rivera, Y.H.; Suárez, Y.A. Algas marinas como fuente de compuestos bioactivos en beneficio de la salud humana: Un artículo de revisión/Seaweeds as sources of bioactive compounds in the benefit of human health: A review. Biotecnia 2016, 18, 20–27. [Google Scholar] [CrossRef]
- Seca, A.; Pinto, D. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.; Pereira, O.; Seca, A.; Pinto, D.; Silva, A. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Shih, M.-K.; Hou, C.-Y.; Dong, C.-D.; Patel, A.K.; Tsai, Y.-H.; Lin, M.-C.; Xu, Z.-Y.; Perumal, P.K.; Kuo, C.-H.; Huang, C.-Y. Production and Characterization of Durvillaea Antarctica Enzyme Extract for Antioxidant and Anti-Metabolic Syndrome Effects. Catalysts 2022, 12, 1284. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Bastida, S.; Ruiz-Capillas, C.; Bravo, L.; Larrea, M.T.; Sánchez-Muniz, F.; Cofrades, S.; Jiménez-Colmenero, F. Composition and Antioxidant Capacity of Low-Salt Meat Emulsion Model Systems Containing Edible Seaweeds. Meat Sci. 2009, 83, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Ksouda, G.; Benslima, A.; Nasri, R.; Rinaudo, M.; Nasri, M.; Hajji, M. Enhancing Colour and Oxidative Stabilities of Reduced-Nitrite Turkey Meat Sausages during Refrigerated Storage Using Fucoxanthin Purified from the Tunisian Seaweed Cystoseira Barbata. Food Chem. Toxicol. 2017, 107, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Tala, F.; López, B.A.; Velásquez, M.; Jeldres, R.; Macaya, E.C.; Mansilla, A.; Ojeda, J.; Thiel, M. Long-Term Persistence of the Floating Bull Kelp Durvillaea Antarctica from the South-East Pacific: Potential Contribution to Local and Transoceanic Connectivity. Mar. Environ. Res. 2019, 149, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Astorga-España, M.S.; Mansilla, A.; Ojeda, J.; Marambio, J.; Rosenfeld, S.; Mendez, F.; Rodriguez, J.P.; Ocaranza, P. Nutritional Properties of Dishes Prepared with Sub-Antarctic Macroalgae—An Opportunity for Healthy Eating. J. Appl. Phycol. 2017, 29, 2399–2406. [Google Scholar] [CrossRef]
- Khandaker, M.U.; Chijioke, N.O.; Heffny, N.A.B.; Bradley, D.A.; Alsubaie, A.; Sulieman, A.; Faruque, M.R.I.; Sayyed, M.I.; Al-mugren, K.S. Elevated Concentrations of Metal (Loids) in Seaweed and the Concomitant Exposure to Humans. Foods 2021, 10, 381. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Wyss, M.; Yans, C.; Boscán-González, A.; Duran, P.; Parra-Soto, S.; Angarita, L. Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation. Int. J. Mol. Sci. 2023, 24, 10779. https://doi.org/10.3390/ijms241310779
Guerrero-Wyss M, Yans C, Boscán-González A, Duran P, Parra-Soto S, Angarita L. Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation. International Journal of Molecular Sciences. 2023; 24(13):10779. https://doi.org/10.3390/ijms241310779
Chicago/Turabian StyleGuerrero-Wyss, Marion, Caroline Yans, Arturo Boscán-González, Pablo Duran, Solange Parra-Soto, and Lissé Angarita. 2023. "Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation" International Journal of Molecular Sciences 24, no. 13: 10779. https://doi.org/10.3390/ijms241310779
APA StyleGuerrero-Wyss, M., Yans, C., Boscán-González, A., Duran, P., Parra-Soto, S., & Angarita, L. (2023). Durvillaea antarctica: A Seaweed for Enhancing Immune and Cardiometabolic Health and Gut Microbiota Composition Modulation. International Journal of Molecular Sciences, 24(13), 10779. https://doi.org/10.3390/ijms241310779






