Diverse Sphingolipid Profiles in Rectal and Colon Cancer
Abstract
:1. Introduction
2. Results
2.1. Patients
2.2. Content of Sphingolipids in Colorectal Tissue
2.3. Content of Sphingolipids in the Tumor Tissue Depending on the Location: Rectum vs. Colon
2.4. Sphingosine-1-Phosphate to Ceramide Ratio in Colorectal Tissue
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sphingolipid Content in Tissues
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowski, A.R.; Markowska, A.J.; Ustymowicz, W.; Pryczynicz, A.; Guzińska-Ustymowicz, K. Simultaneous analysis of tumor-infiltrating immune cells density, tumor budding status, and presence of lymphoid follicles in CRC tissue. Sci. Rep. 2022, 12, 21732. [Google Scholar] [CrossRef] [PubMed]
- Markowski, A.R.; Błachnio-Zabielska, A.U.; Guzińska-Ustymowicz, K.; Markowska, A.; Pogodzińska, K.; Roszczyc, K.; Zińczuk, J.; Zabielski, P. Ceramides profile identifies patients with more advanced stages of colorectal cancer. Biomolecules 2020, 10, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Solar, V.; Lizardo, D.Y.; Li, N.; Hurst, J.J.; Brais, C.J.; Atilla-Gokcumen, G.E. Di_erential Regulation of Specific Sphingolipids in Colon Cancer Cells during Staurosporine-Induced Apoptosis. Chem. Biol. 2015, 22, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Hady, H.R.; Markowski, A.R.; Kurianiuk, A.; Karwowska, A.; Gorski, J.; Zabielski, P. Inhibition of ceramide de novo synthesis a_ects adipocytokines secretion and improves systemic and adipose tissue insulin sensitivity. Int. J. Mol. Sci. 2018, 19, 3995. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cheng, J.C.; Turner, L.S.; Elojeimy, S.; Beckham, T.H.; Bielawska, A.; Keane, T.E.; Hannun, Y.A.; Norris, J.S. Acid ceramidase upregulation in prostate cancer: Role in tumor development and implications for therapy. Expert Opin. Ther. Targets 2009, 13, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, H.; Li, Y.; Li, L.; Qiu, Y.; Ren, J. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol. Rep. 2015, 34, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Machala, M.; Procházková, J.; Hofmanová, J.; Králiková, L.; Slavík, J.; Tylichová, Z.; Ovesná, P.; Kozubík, A.; Vondráček, J. Colon Cancer and Perturbations of the Sphingolipid Metabolism. Int. J. Mol. Sci. 2019, 20, 6051. [Google Scholar] [CrossRef] [Green Version]
- Markowski, A.R.; Żbikowski, A.; Zabielski, P.; Chlabicz, U.; Sadowska, P.; Pogodzińska, K.; Błachnio-Zabielska, A.U. The effect of silencing the genes responsible for the level of sphingosine-1-phosphate on the proliferative activity of colon cancer cells. Int. J. Mol. Sci. 2023, 24, 7197. [Google Scholar] [CrossRef]
- Hartmann, D.; Lucks, J.; Fuchs, S.; Schiffmann, S.; Schreiber, Y.; Ferreirós, N.; Merkens, J.; Marschalek, R.; Geisslinger, G.; Grösch, S. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int. J. Biochem. Cell Biol. 2012, 44, 620–628. [Google Scholar] [CrossRef]
- Andrieu, N.; Launoy, G.; Guillois, R.; Ory-Paoletti, C.; Gignoux, M. Estimation of the familial relative risk of cancer by site from a French population based family study on colorectal cancer (CCREF study). Gut 2004, 53, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Bongaerts, B.W.; van den Brandt, P.A.; Goldbohm, R.A.; de Goeij, A.F.; Weijenberg, M.P. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int. J. Cancer. 2008, 123, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Ichimasa, K.; Kudo, S.; Kouyama, Y.; Mochizuki, K.; Takashina, Y.; Misawa, M.; Mori, Y.; Hayashi, T.; Wakamura, K.; Miyachi, H. Tumor Location as a Prognostic Factor in T1 Colorectal Cancer. J. Anus Rectum Colon 2022, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Shun, C.T.; Lin, B.R.; Sanduleanu, S.; Hsu, W.F.; Wu, M.S.; Chiu, H.M. Recurrence Outcomes Less Favorable in T1 Rectal Cancer than in T1 Colon Cancer. Oncologist 2021, 26, e1548–e1554. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Dekkers, N.; le Cessie, S.; van Hooft, J.E.; van Leerdam, M.E.; Oldenburg, P.P.; Flothuis, L.; Schoones, J.W.; Langers, A.M.J.; Hardwick, J.C.H.; et al. Risk and Time Pattern of Recurrences After Local Endoscopic Resection of T1 Colorectal Cancer: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e298–e314. [Google Scholar] [CrossRef]
- Lei, S.; Ge, Y.; Tian, S.; Cai, B.; Gao, X.; Wang, N.; Wang, G.; Wang, L.; Wang, Z. Colorectal cancer metastases to brain or bone and the relationship to primary tumor location: A population-based study. J. Gastrointest. Surg. 2020, 24, 1833–1842. [Google Scholar] [CrossRef]
- Brouwer, N.P.M.; van der Kruijssen, D.E.W.; Hugen, N.; de Hingh, I.; Nagtegaal, I.D.; Verhoeven, R.H.A.; Koopman, M.; de Wilt, J.H.W. The impact of primary tumor location in synchronous metastatic colorectal cancer: Differences in metastatic sites and survival. Ann. Surg. Oncol. 2020, 27, 1580–1588. [Google Scholar] [CrossRef] [Green Version]
- Shida, D.; Inoue, M.; Tanabe, T.; Moritani, K.; Tsukamoto, S.; Yamauchi, S.; Sugihara, K.; Kanemitsu, Y. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: A nationwide multicenter retrospective study. J. Gastroenterol. 2020, 55, 958–968. [Google Scholar] [CrossRef]
- Franssen, R.F.W.; Strous, M.T.A.; Bongers, B.C.; Vogelaar, F.J.; Janssen-Heijnen, M.L.G. The Association Between Treatment Interval and Survival in Patients With Colon or Rectal Cancer: A Systematic Review. World J. Surg. 2021, 45, 2924–2937. [Google Scholar] [CrossRef]
- Ahn, E.H.; Schroeder, J.J. Induction of apoptosis by sphingosine, sphinganine, and C(2)-ceramide in human colon cancer cells, but not by C(2)-dihydroceramide. Anticancer Res. 2010, 30, 2881–2884. [Google Scholar] [PubMed]
- Tylichová, Z.; Slavík, J.; Ciganek, M.; Ovesná, P.; Krčmář, P.; Straková, N.; Machala, M.; Kozubík, A.; Hofmanová, J.; Vondráček, J. Butyrate and docosahexaenoic acid interact in alterations of specific lipid classes in differentiating colon cancer cells. J. Cell Biochem. 2018, 119, 4664–4679. [Google Scholar] [CrossRef]
- Campbell, W.J.; Spence, R.A.; Parks, T.G. Familial adenomatous polyposis. Br. J. Surg. 1994, 81, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nicholson, R.J.; Summers, S.A. Ceramide signaling in the gut. Mol. Cell. Endocrinol. 2022, 544, 111554. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwiler, A.; Pfeilschifter, J. Altering the sphingosine-1-phosphate/ceramide balance: A promising approach for tumor therapy. Curr. Pharm. Des. 2006, 12, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Speirs, M.M.P.; Swensen, A.C.; Chan, T.Y.; Jones, P.M.; Holman, J.; Harris, M.B.; Maschek, J.A.; Cox, J.E.; Carson, R.H.; Hill, J.T.; et al. Imbalanced sphingolipid signaling is maintained as a core proponent of a cancerous phenotype in spite of metabolic pressure and epigenetic drift. Oncotarget 2019, 10, 449–479. [Google Scholar] [CrossRef] [Green Version]
- Baran, Y.; Salas, A.; Senkal, C.E.; Gunduz, U.; Bielawski, J.; Obeid, L.M.; Ogretmen, B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J. Biol. Chem. 2007, 282, 10922–10934. [Google Scholar] [CrossRef] [Green Version]
- Rosa, R.; Marciano, R.; Malapelle, U.; Formisano, L.; Nappi, L.; D’Amato, C.; D’Amato, V.; Damiano, V.; Marfè, G.; Del Vecchio, S.; et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin. Cancer Res. 2013, 19, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Kurek, K.; Łukaszuk, B.; Świdnicka-Siergiejko, A.; Rogalski, P.; Wróblewski, E.; Chabowski, A.; Dąbrowski, A.; Żendzian-Piotrowska, M. Sphingolipid metabolism in colorectal adenomas varies depending on histological architecture of polyps and grade of nuclear dysplasia. Lipids 2015, 50, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahatay, S.; Thomas, K.; Koybasi, S.; Senkal, C.E.; ElOjeimy, S.; Liu, X.; Bielawski, J.; Day, T.A.; Gillespie, M.B.; Sinha, D.; et al. Clinical Relevance of Ceramide Metabolism in the Pathogenesis of Human Head and Neck Squamous Cell Carcinoma (HNSCC): Attenuation of C18-ceramide in HNSCC Tumors Correlates with Lymphovascular Invasion and Nodal Metastasis. Cancer Lett. 2007, 256, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blachnio-Zabielska, A.U.; Persson, X.M.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. 2012, 26, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| 1A. Sphingolipids in Colorectal Cancer (CRC). | Adjacent Tissue (A). n = 30. | Tumor Tissue (T). n = 30. | T vs. A |
| M ± SE | M ± SE | p | |
| Sph | 2.38 ± 0.22 | ↑ 6.09 ± 0.73 | 0.0001 * |
| S1P | 0.02 ± 0.001 | ↑ 0.05 ± 0.01 | 0.0006 * |
| SPA | 0.70 ± 0.1 | ↑ 1.37 ± 0.14 | 0.0003 * |
| C14:0-Cer | 1.17 ± 0.1 | ↑ 1.66 ± 0.13 | 0.0031 * |
| C16:0-Cer | 106.33 ± 8.09 | 126.75 ± 10.19 | 0.1220 |
| C18:1-Cer | 0.30 ± 0.02 | 0.31 ± 0.03 | 0.8100 |
| C18:0-Cer | 3.91 ± 0.26 | 3.21 ± 0.21 ↓ | 0.0366 * |
| C20:0-Cer | 1.29 ± 0.09 | 0.92 ± 0.08 ↓ | 0.0020 * |
| C22:0-Cer | 3.11 ± 0.16 | 3.12 ± 0.22 | 0.9764 |
| C24:1-Cer | 13.53 ± 0.85 | 14.00 ± 0.96 | 0.7150 |
| C24:0-Cer | 4.69 ± 0.27 | ↑ 6.41 ± 0.48 | 0.0027 * |
| Total Cer | 134.34 ± 9.18 | 156.38 ± 11.23 | 0.1341 |
| 1B. Sphingolipids in rectal cancer (RC). | Adjacent tissue (A). n = 16. | Tumor tissue (T). n = 16. | T vs. A |
| M ± SE | M ± SE | p | |
| Sph | 2.38 ± 0.34 | ↑ 5.16 ± 0.85 | 0.0050 * |
| S1P | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.1244 |
| SPA | 0.69 ± 0.18 | 1.05 ± 0.15 | 0.1343 |
| C14:0-Cer | 1.11 ± 0.14 | 1.43 ± 0.17 | 0.1656 |
| C16:0-Cer | 100.16 ± 11.42 | 107.73 ± 13.0 | 0.6646 |
| C18:1-Cer | 0.30 ± 0.04 | 0.29 ± 0.04 | 0.7525 |
| C18:0-Cer | 3.96 ± 0.39 | 2.92 ± 0.21 ↓ | 0.0270 * |
| C20:0-Cer | 1.37 ± 0.12 | 0.91 ± 0.11 ↓ | 0.0091 * |
| C22:0-Cer | 3.18 ± 0.22 | 2.56 ± 0.24 | 0.0712 |
| C24:1-Cer | 13.90 ± 1.26 | 14.08 ± 1.62 | 0.9306 |
| C24:0-Cer | 4.67 ± 0.38 | 5.17 ± 0.6 | 0.4839 |
| Total Cer | 128.66 ± 13.09 | 135.10 ± 14.78 | 0.7463 |
| 1C. Sphingolipids in colon cancer (CC). | Adjacent tissue (A). n = 14. | Tumor tissue (T). n = 14. | T vs. A |
| M ± SE | M ± SE | p | |
| Sph | 2.39 ± 0.26 | ↑ 7.17 ± 1.19 | 0.0006 * |
| S1P | 0.02 ± 0.001 | ↑ 0.06 ± 0.01 | 0.0011 * |
| SPA | 0.72 ± 0.07 | ↑ 1.73 ± 0.21 | 0.0001 * |
| C14:0-Cer | 1.23 ± 0.13 | ↑ 1.92 ± 0.16 | 0.0030 * |
| C16:0-Cer | 113.38 ± 11.58 | 148.48 ± 14.32 | 0.0678 |
| C18:1-Cer | 0.30 ± 0.03 | 0.34 ± 0.04 | 0.3921 |
| C18:0-Cer | 3.86 ± 0.33 | 3.53 ± 0.36 | 0.5112 |
| C20:0-Cer | 1.20 ± 0.13 | 0.93 ± 0.10 | 0.1066 |
| C22:0-Cer | 3.03 ± 0.25 | 3.76 ± 0.31 | 0.0783 |
| C24:1-Cer | 13.10 ± 1.15 | 13.91 ± 0.96 | 0.5980 |
| C24:0-Cer | 4.71 ± 0.39 | ↑ 7.82 ± 0.57 | 0.0001 * |
| Total Cer | 140.83 ± 13.07 | 180.69 ± 15.15 | 0.0570 |
| 2A. Sphingolipids in Tumor Tissue (T) | Rectal Cancer (RC). n = 16. | Colon Cancer (CC). n = 14. | RC vs. CC |
| M ± SE | M ± SE | p | |
| Sph | 5.16 ± 0.85 | 7.17 ± 1.19 | 0.172 |
| S1P | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.162 |
| SPA | 1.05 ± 0.15 ↓ | 1.73 ± 0.21 | 0.013 * |
| C14:0-Cer | 1.43 ± 0.17 | 1.92 ± 0.16 | 0.053 |
| C16:0-Cer | 107.73 ± 13.00 ↓ | 148.48 ± 14.32 | 0.044 * |
| C18:1-Cer | 0.29 ± 0.04 | 0.34 ± 0.04 | 0.312 |
| C18:0-Cer | 2.92 ± 0.21 | 3.53 ± 0.36 | 0.151 |
| C20:0-Cer | 0.91 ± 0.11 | 0.93 ± 0.10 | 0.879 |
| C22:0-Cer | 2.56 ± 0.24 ↓ | 3.76 ± 0.31 | 0.005 * |
| C24:1-Cer | 14.08 ± 1.62 | 13.91 ± 0.96 | 0.928 |
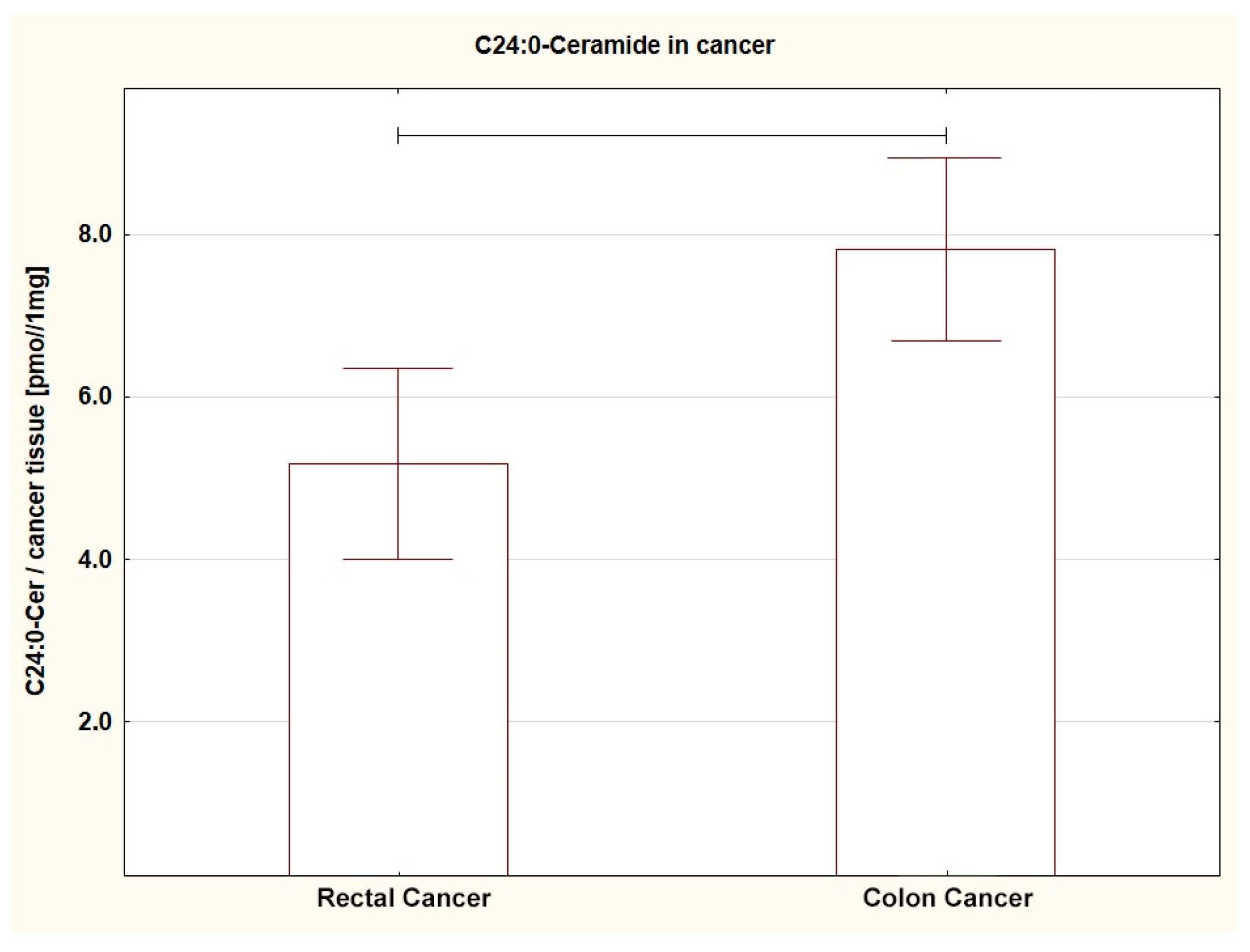
| C24:0-Cer | 5.17 ± 0.60 ↓ | 7.82 ± 0.57 | 0.004 * |
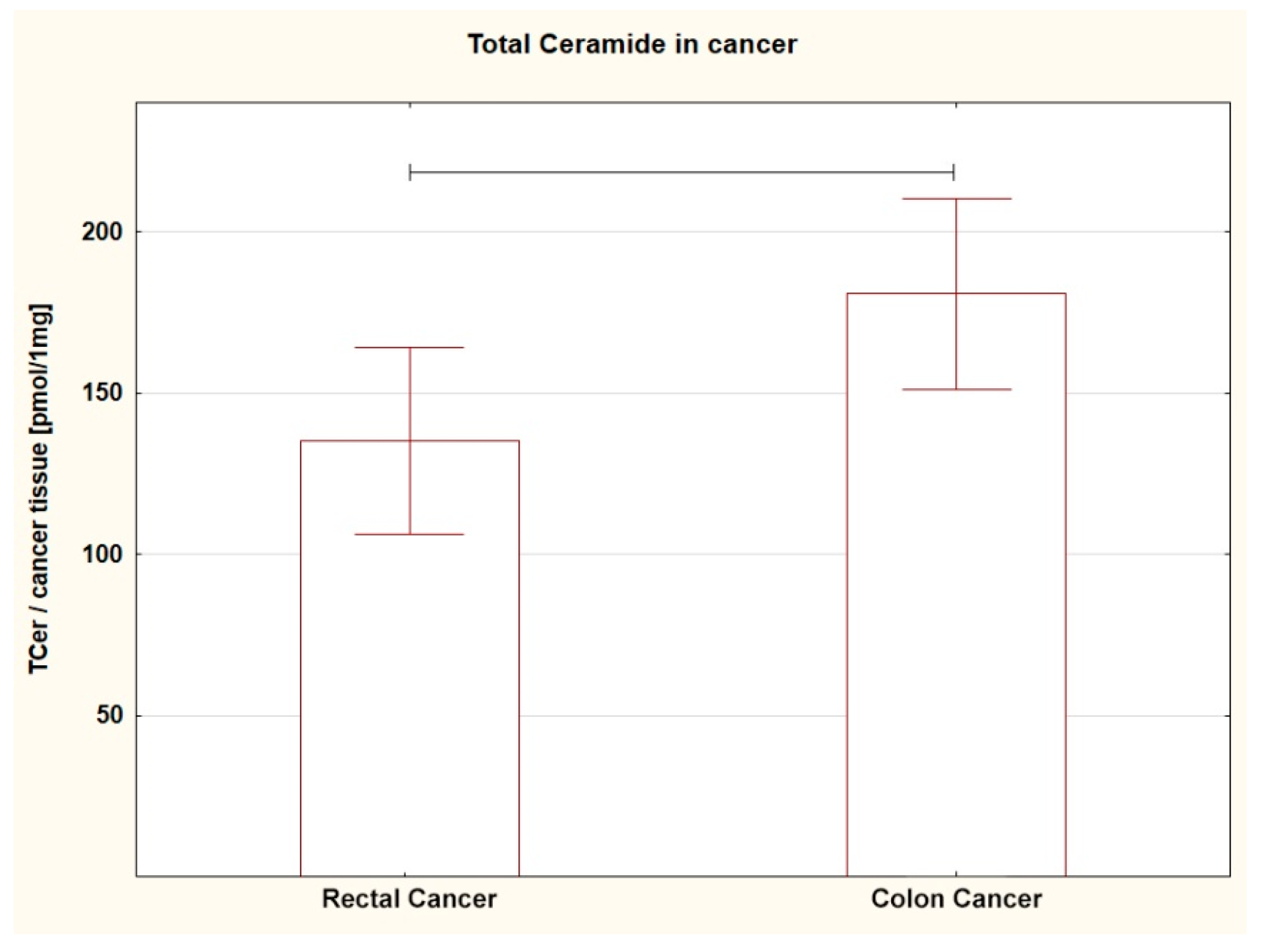
| Total Cer | 135.10 ± 14.78 ↓ | 180.69 ± 15.15 | 0.041 * |
| 2B. Sphingolipids in adjacent tissue (A) | Rectal cancer (RC). n = 16. | Colon cancer (CC). n = 14. | RC vs. CC |
| M ± SE | M ± SE | p | |
| Sph | 2.38 ± 0.34 | 2.39 ± 0.26 | 0.971 |
| S1P | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.400 |
| SPA | 0.69 ± 0.18 | 0.72 ± 0.07 | 0.861 |
| C14:0-Cer | 1.11 ± 0.14 | 1.23 ± 0.13 | 0.547 |
| C16:0-Cer | 100.16 ± 11.42 | 113.38 ± 11.58 | 0.425 |
| C18:1-Cer | 0.30 ± 0.04 | 0.30 ± 0.03 | 1.000 |
| C18:0-Cer | 3.96 ± 0.39 | 3.86 ± 0.33 | 0.847 |
| C20:0-Cer | 1.37 ± 0.12 | 1.20 ± 0.13 | 0.344 |
| C22:0-Cer | 3.18 ± 0.22 | 3.03 ± 0.25 | 0.661 |
| C24:1-Cer | 13.90 ± 1.26 | 13.10 ± 1.15 | 0.647 |
| C24:0-Cer | 4.67 ± 0.38 | 4.71 ± 0.39 | 0.939 |
| Total Cer | 128.66 ± 13.09 | 140.83 ± 13.07 | 0.518 |
| 3A. S1P/Cer in Colorectal Cancer (CRC). | Adjacent Tissue (A). n = 30. | Tumor Tissue (T). n = 30. | T vs. A |
|---|---|---|---|
| M ± SE | M ± SE | p | |
| S1P/C14:0-Cer | 0.0275 ± 0.007 | 0.0378 ± 0.007 | 0.2899 |
| S1P/C16:0-Cer | 0.0004 ± 0.001 | 0.0005 ± 0.001 | 0.3157 |
| S1P/C18:1-Cer | 0.0070 ± 0.001 | ↑ 0.2202 ± 0.048 | 0.0000 * |
| S1P/C18:0-Cer | 0.0081 ± 0.002 | ↑ 0.0200 ± 0.005 | 0.0241 * |
| S1P/C20:0-Cer | 0.0219 ± 0.004 | ↑ 0.0715 ± 0.015 | 0.0018 * |
| S1P/C22:0-Cer | 0.0085 ± 0.002 | ↑ 0.0184 ± 0.003 | 0.0081 * |
| S1P/C24:1-Cer | 0.0020 ± 0.001 | ↑ 0.0044 ± 0.001 | 0.0081 * |
| S1P/C24:0-Cer | 0.0055 ± 0.001 | ↑ 0.0089 ± 0.001 | 0.0499 * |
| S1P/TCer | 0.0002 ± 0.001 | 0.0004 ± 0.001 | 0.0769 |
| 3B. S1P/Cer in rectal cancer (RC). | Adjacent tissue (A). n = 16. | Tumor tissue (T). n = 16. | T vs. A |
| M ± SE | M ± SE | p | |
| S1P/C14:0-Cer | 0.0338 ± 0.011 | 0.0450 ± 0.008 | 0.5075 |
| S1P/C16:0-Cer | 0.0005 ± 0.001 | 0.0007 ± 0.001 | 0.3813 |
| S1P/C18:1-Cer | 0.0077 ± 0.002 | ↑ 0.2885 ± 0.066 | 0.0023 * |
| S1P/C18:0-Cer | 0.0105 ± 0.004 | 0.0251 ± 0.007 | 0.1199 |
| S1P/C20:0-Cer | 0.0233 ± 0.006 | ↑ 0.0920 ± 0.024 | 0.0122 * |
| S1P/C22:0-Cer | 0.0093 ± 0.002 | ↑ 0.0224 ± 0.006 | 0.0337 * |
| S1P/C24:1-Cer | 0.0022 ± 0.001 | ↑ 0.0054 ± 0.001 | 0.0340 * |
| S1P/C24:0-Cer | 0.0061 ± 0.001 | 0.0107 ± 0.002 | 0.0967 |
| S1P/TCer | 0.0003 ± 0.001 | 0.0005 ± 0.001 | 0.1545 |
| 3C. S1P/Cer in colon cancer (CC). | Adjacent tissue (A). n = 14. | Tumor tissue (T). n = 14. | T vs. A |
| M ± SE | M ± SE | p | |
| S1P/C14:0-Cer | 0.0204 ± 0.006 | 0.0296 ± 0.012 | 0.2339 |
| S1P/C16:0-Cer | 0.0003 ± 0.001 | 0.0003 ± 0.001 | 0.5820 |
| S1P/C18:1-Cer | 0.0062 ± 0.001 | ↑ 0.1421 ± 0.072 | 0.0000 * |
| S1P/C18:0-Cer | 0.0054 ± 0.001 | ↑ 0.0142 ± 0.006 | 0.0089 * |
| S1P/C20:0-Cer | 0.0204 ± 0.005 | ↑ 0.0482 ± 0.016 | 0.0299 * |
| S1P/C22:0-Cer | 0.0075 ± 0.002 | 0.0138 ± 0.003 | 0.1028 |
| S1P/C24:1-Cer | 0.0018 ± 0.001 | 0.0033 ± 0.001 | 0.0984 |
| S1P/C24:0-Cer | 0.0048 ± 0.001 | 0.0069 ± 0.002 | 0.2983 |
| S1P/TCer | 0.0002 ± 0.001 | 0.0003 ± 0.001 | 0.1485 |
| 3D. S1P/Cer in tumor tissue (T) | Rectal cancer (RC). n = 16. | Colon cancer (CC). n = 14. | RC vs. CC |
| M ± SE | M ± SE | p | |
| S1P/C14:0-Cer | 0.0450 ± 0.008 | 0.0296 ± 0.012 | 0.2798 |
| S1P/C16:0-Cer | 0.0007 ± 0.001 | 0.0003 ± 0.001 | 0.1122 |
| S1P/C18:1-Cer | 0.2885 ± 0.066 | 0.1421 ± 0.072 | 0.1300 |
| S1P/C18:0-Cer | 0.0251 ± 0.007 | 0.0142 ± 0.006 | 0.2340 |
| S1P/C20:0-Cer | 0.0920 ± 0.024 | 0.0482 ± 0.016 | 0.1383 |
| S1P/C22:0-Cer | 0.0224 ± 0.006 | 0.0138 ± 0.003 | 0.1989 |
| S1P/C24:1-Cer | 0.0054 ± 0.001 | 0.0033 ± 0.001 | 0.1989 |
| S1P/C24:0-Cer | 0.0107 ± 0.002 | 0.0069 ± 0.002 | 0.2057 |
| S1P/TCer | 0.0005 ± 0.001 | 0.0003 ± 0.001 | 0.1211 |
| 3E. S1P/Cer in adjacent tissue (A) | Rectal cancer (RC). n = 16. | Colon cancer (CC). n = 14. | RC vs. CC |
| M ± SE | M ± SE | p | |
| S1P/C14:0-Cer | 0.0338 ± 0.011 | 0.0204 ± 0.006 | 0.3131 |
| S1P/C16:0-Cer | 0.0005 ± 0.001 | 0.0003 ± 0.001 | 0.4654 |
| S1P/C18:1-Cer | 0.0077 ± 0.002 | 0.0062 ± 0.001 | 0.4908 |
| S1P/C18:0-Cer | 0.0105 ± 0.004 | 0.0054 ± 0.001 | 0.3067 |
| S1P/C20:0-Cer | 0.0233 ± 0.006 | 0.0204 ± 0.005 | 0.7263 |
| S1P/C22:0-Cer | 0.0093 ± 0.002 | 0.0075 ± 0.002 | 0.5505 |
| S1P/C24:1-Cer | 0.0022 ± 0.001 | 0.0018 ± 0.001 | 0.5464 |
| S1P/C24:0-Cer | 0.0061 ± 0.001 | 0.0048 ± 0.001 | 0.4952 |
| S1P/TCer | 0.0003 ± 0.001 | 0.0002 ± 0.001 | 0.3662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowski, A.R.; Błachnio-Zabielska, A.U.; Pogodzińska, K.; Markowska, A.J.; Zabielski, P. Diverse Sphingolipid Profiles in Rectal and Colon Cancer. Int. J. Mol. Sci. 2023, 24, 10867. https://doi.org/10.3390/ijms241310867
Markowski AR, Błachnio-Zabielska AU, Pogodzińska K, Markowska AJ, Zabielski P. Diverse Sphingolipid Profiles in Rectal and Colon Cancer. International Journal of Molecular Sciences. 2023; 24(13):10867. https://doi.org/10.3390/ijms241310867
Chicago/Turabian StyleMarkowski, Adam R., Agnieszka U. Błachnio-Zabielska, Karolina Pogodzińska, Anna J. Markowska, and Piotr Zabielski. 2023. "Diverse Sphingolipid Profiles in Rectal and Colon Cancer" International Journal of Molecular Sciences 24, no. 13: 10867. https://doi.org/10.3390/ijms241310867





