The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses
Abstract
:1. Flies Are a Powerful Model to Study Addiction
1.1. What Is Addiction?
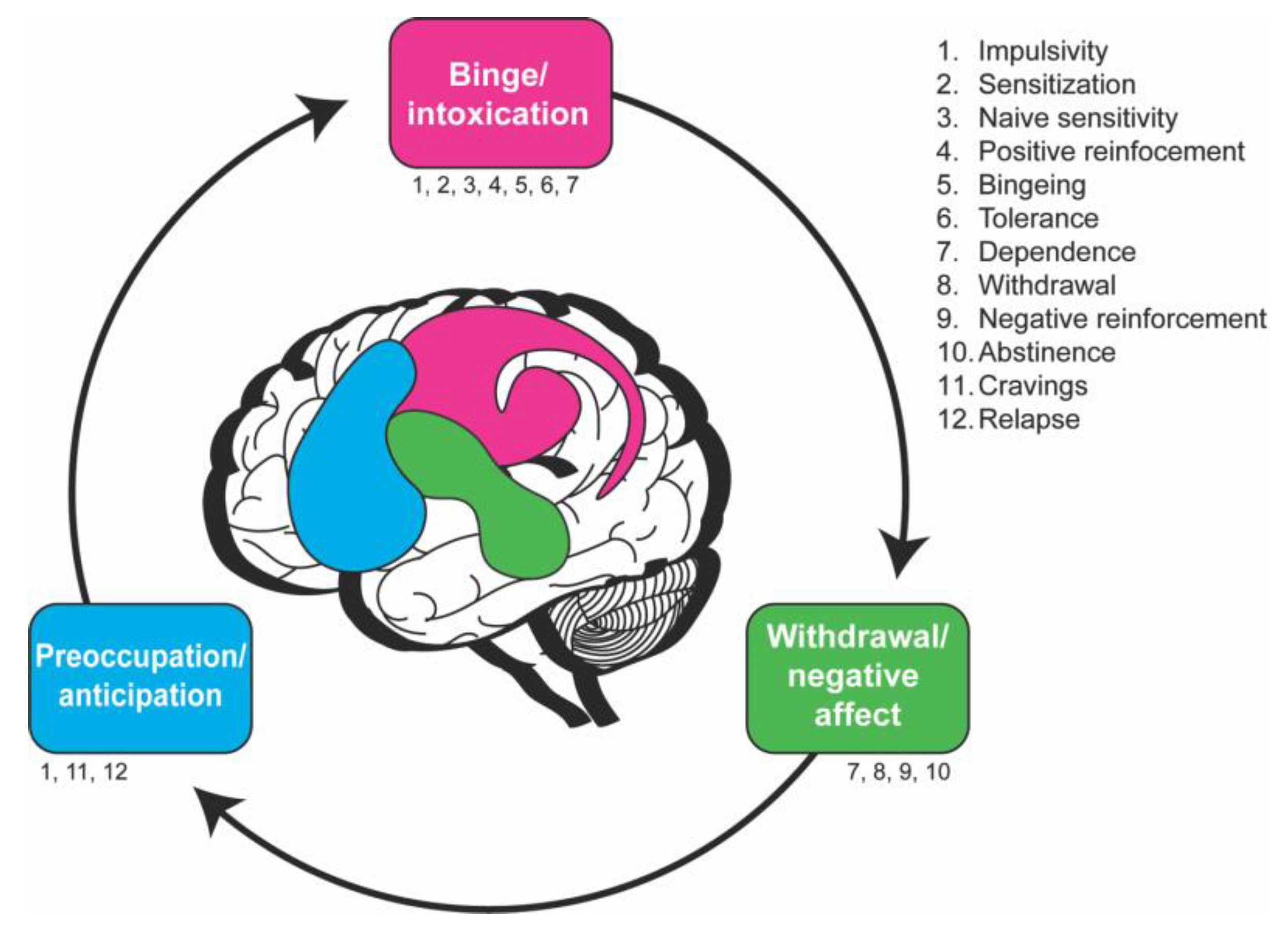
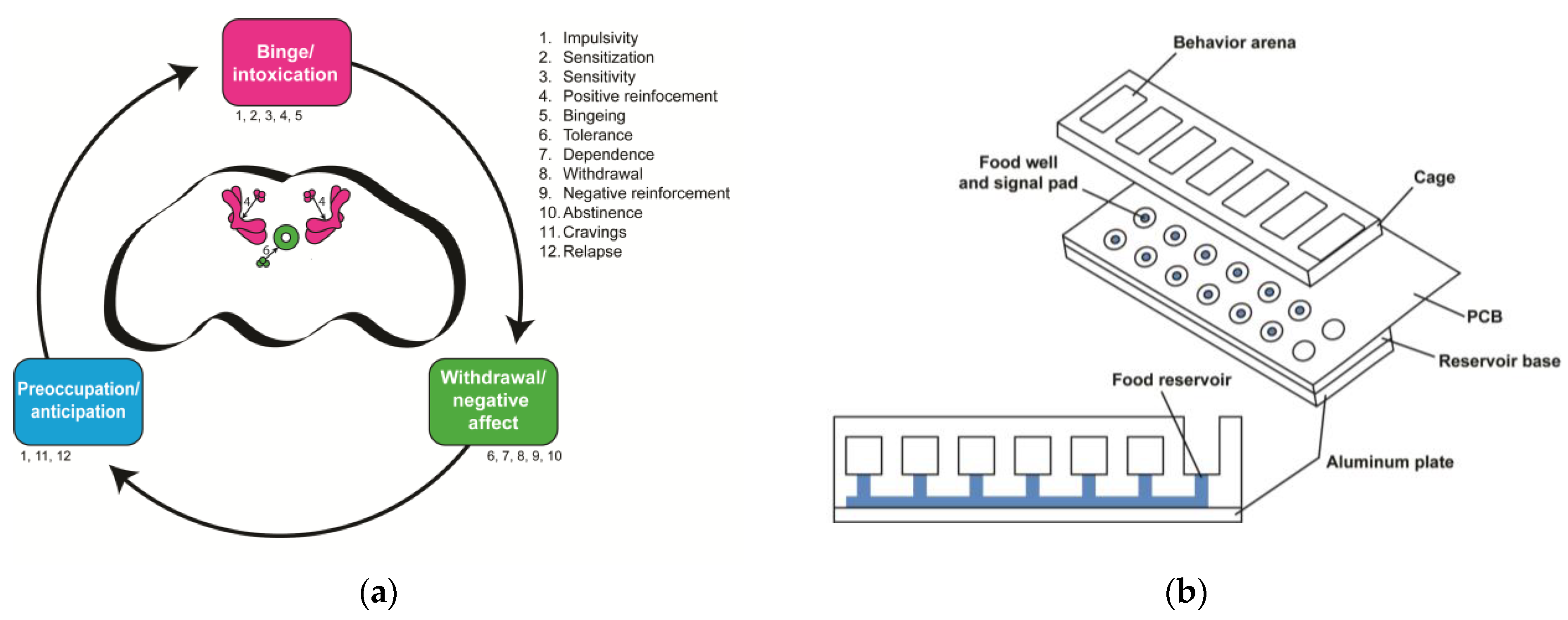
1.2. The Neurobiological Stage-Based Model Breaks Down Addiction into Behavioral Components
1.3. Benefits of Drosophila to Study Addiction
1.4. Genetic Approaches to Studying Addiction Using Flies
2. Using the Fly to Understand the Literature Gaps in Addiction
| Endophenotypes | Neurobiological Stage(s) of Addiction | Drosophila Assays | References |
|---|---|---|---|
| Sensitization/tolerance | Binge/Intoxication, Withdrawal/Negative affect | Booze-o-mat (E) | [40,41] |
| (Drug exposure through forced vaporization; determine locomotion). | |||
| Maples assay (E) | [42] | ||
| (Drug exposure through forced vaporization; determine loss of righting). | |||
| Inebriometer (E) | [36,43] | ||
| (Drug exposure through forced vaporization; determine loss of postural control). | |||
| FlyBong (C, M) | [44,45] | ||
| (Drug exposure through vaporization and forced administration). | |||
| CApillary FEeder assay (CAFÉ; E) | [46] | ||
| (Drug exposure through voluntary 2-choice food consumption; determine volume consumed and preference). | |||
| FlyCafe (M) | [43] | ||
| (Drug exposure through voluntary 2-choice food consumption; determine preference and effect on locomotion). | |||
| Fly group activity monitor assay (FlyGrAM; E) | [47,48] | ||
| (Drug exposure through vaporization and forced administration. Locomotor activity assay). | |||
| Drosophila activity monitor (DAM; M) | [45] | ||
| (Locomotor activity assay of variably drug-exposed flies). | |||
| DAM5M (M) | [45] | ||
| (Locomotor activity assay of variably drug-exposed flies). | |||
| Naïve sensitivity | Binge/Intoxication | Inebriometer (E) | [49,50] |
| Booze-o-mat (E) | [30,40] | ||
| Maples assay (E) | [42] | ||
| FlyGrAM (E) | [47,48] | ||
| Positive reinforcement | Binge/Intoxication | Fly liquid–food interaction counter (FLIC) | [51] |
| (Drug exposure through voluntary 2-choice food consumption; determine food interaction time and preference). | |||
| Fluorometric Reading Assay of Preference Primed by Ethanol (FRAPPE) | [52] | ||
| (Drug exposure through voluntary 2-choice food consumption; determine amount ingested and preference). | |||
| CAFÉ | [52,53] | ||
| FlyCafe (M) | [45] | ||
| Proboscis extension reflex (PER) assay (E) | [54] | ||
| (Consummatory reflex of variably drug-exposed flies). | |||
| T-maze (E) | [55] | ||
| (Olfactory choice assay, or variably drug-exposed flies). | |||
| DAM (M) | [45] | ||
| Bingeing | Binge/Intoxication | Not yet developed | |
| Dependence | Binge/Intoxication, Withdrawal/Negative affect | Drug feeding in combination with learning and memory assay (E) | [56] |
| Withdrawal | Withdrawal/Negative affect | Drug feeding in combination with learning and memory assay (E) | [56] |
| Negative reinforcement | Withdrawal/Negative affect | Not yet developed | |
| Abstinence | Withdrawal/Negative affect | Drug feeding in combination with learning and memory assay (E) | [56] |
| Cravings | Preoccupation/Anticipation | Not yet developed | |
| Impulsivity | Binge/Intoxication, Preoccupation/Anticipation | Not yet developed | |
| Relapse | Preoccupation/Anticipation | Not yet developed | |
2.1. Stage 1—Binge/Intoxication
2.1.1. Behaviors Underlying the Binge/Intoxication Stage
Sensitization
Positive Reinforcement
Bingeing
2.2. Stage 2—Withdrawal/Negative Affect
2.2.1. Behaviors Underlying the Withdrawal/Negative Affect Stage
Tolerance
Dependence
Withdrawal
Negative Reinforcement
2.3. Stage 3—Preoccupation/Anticipation
2.3.1. Behaviors Underlying the Preoccupation/Anticipation Stage
Abstinence
Cravings
Impulsivity
Relapse
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Verweij, K.J.H.; Gillespie, N.A.; Heath, A.C.; Lessov-Schlaggar, C.N.; Martin, N.G.; Nelson, E.C.; Slutske, W.S.; Whitfield, J.B.; Lynskey, M.T. The genetics of addiction—A translational perspective. Transl. Psychiatry 2012, 2, e140. [Google Scholar] [CrossRef] [Green Version]
- Potenza, M.N.; Balodis, I.M.; Derevensky, J.; Grant, J.E.; Petry, N.M.; Verdejo-Garcia, A.; Yip, S.W. Gambling disorder. Nat. Rev. Dis. Primer 2019, 5, 51. [Google Scholar] [CrossRef]
- Slutske, W.S.; Eisen, S.; True, W.R.; Lyons, M.J.; Goldberg, J.; Tsuang, M. Common genetic vulnerability for pathological gambling and alcohol dependence in men. Arch. Gen. Psychiatry 2000, 57, 666–673. [Google Scholar] [CrossRef]
- Johnson, E.C.; Demontis, D.; Thorgeirsson, T.E.; Walters, R.K.; Polimanti, R.; Hatoum, A.S.; Sanchez-Roige, S.; Paul, S.E.; Wendt, F.R.; Clarke, T.-K.; et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 2020, 7, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Zhou, H.; Kember, R.L.; Vickers Smith, R.; Justice, A.C.; Damrauer, S.; Tsao, P.S.; Klarin, D.; Baras, A.; Reid, J.; et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat. Commun. 2019, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Roige, S.; Palmer, A.A.; Clarke, T.-K. Recent Efforts to Dissect the Genetic Basis of Alcohol Use and Abuse. Biol. Psychiatry 2020, 87, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Rentsch, C.T.; Cheng, Z.; Kember, R.L.; Nunez, Y.Z.; Sherva, R.M.; Tate, J.P.; Dao, C.; Xu, K.; Polimanti, R.; et al. Association of OPRM1 Functional Coding Variant With Opioid Use Disorder: A Genome-Wide Association Study. JAMA Psychiatry 2020, 77, 1072–1080. [Google Scholar] [CrossRef]
- Zhou, H.; Sealock, J.M.; Sanchez-Roige, S.; Clarke, T.-K.; Levey, D.F.; Cheng, Z.; Li, B.; Polimanti, R.; Kember, R.L.; Smith, R.V.; et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat. Neurosci. 2020, 23, 809–818. [Google Scholar] [CrossRef]
- Koob, G.F.; Moal, M.L. Drug Abuse: Hedonic Homeostatic Dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, A.Z.; Treistman, S.N. The Molecular Basis of Tolerance. Alcohol Res. Health 2008, 31, 298–309. [Google Scholar]
- Kalant, H. Research on tolerance: What can we learn from history? Alcohol. Clin. Exp. Res. 1998, 22, 67–76. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendler, K.S.; Bulik, C.M.; Silberg, J.; Hettema, J.M.; Myers, J.; Prescott, C.A. Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin control analysis. Arch. Gen. Psychiatry 2000, 57, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, C.E.; Agrawal, A.; McCutcheon, V.V.; Duncan, A.E.; Lynskey, M.T. Disentangling the Complex Association Between Childhood Sexual Abuse and Alcohol-Related Problems: A Review of Methodological Issues and Approaches. J. Stud. Alcohol Drugs 2008, 69, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, D.; Volkow, N.D. Associations of family income with cognition and brain structure in USA children: Prevention implications. Mol. Psychiatry 2021, 26, 6619–6629. [Google Scholar] [CrossRef]
- Veligati, S.; Howdeshell, S.; Beeler-Stinn, S.; Lingam, D.; Allen, P.C.; Chen, L.-S.; Grucza, R.A. Changes in alcohol and cigarette consumption in response to medical and recreational cannabis legalization: Evidence from U.S. state tax receipt data. Int. J. Drug Policy 2020, 75, 102585. [Google Scholar] [CrossRef]
- Goldman, D.; Oroszi, G.; Ducci, F. The genetics of addictions: Uncovering the genes. Nat. Rev. Genet. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [Green Version]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Pandey, U.B.; Nichols, C.D. Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Dev. Camb. Engl. 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Zirin, J.; Bosch, J.; Viswanatha, R.; Mohr, S.E.; Perrimon, N. State-of-the-art CRISPR for in vivo and cell-based studies in Drosophila. Trends Genet. TIG 2022, 38, 437–453. [Google Scholar] [CrossRef]
- Schumann, G.; Coin, L.J.; Lourdusamy, A.; Charoen, P.; Berger, K.H.; Stacey, D.; Desrivières, S.; Aliev, F.A.; Khan, A.A.; Amin, N.; et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc. Natl. Acad. Sci. USA 2011, 108, 7119–7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelou, E.; Gao, H.; Chu, C.; Ntritsos, G.; Blakeley, P.; Butts, A.R.; Pazoki, R.; Suzuki, H.; Koskeridis, F.; Yiorkas, A.M.; et al. New alcohol-related genes suggest shared genetic mechanisms with neuropsychiatric disorders. Nat. Hum. Behav. 2019, 3, 950–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andretic, R.; Chaney, S.; Hirsh, J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science 1999, 285, 1066–1068. [Google Scholar] [CrossRef]
- Abarca, C.; Albrecht, U.; Spanagel, R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl. Acad. Sci. USA 2002, 99, 9026–9030. [Google Scholar] [CrossRef] [Green Version]
- Rothenfluh, A.; Threlkeld, R.J.; Bainton, R.J.; Tsai, L.T.-Y.; Lasek, A.W.; Heberlein, U. Distinct Behavioral Responses to Ethanol Are Regulated by Alternate RhoGAP18B Isoforms. Cell 2006, 127, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Ojelade, S.A.; Acevedo, S.F.; Kalahasti, G.; Rodan, A.R.; Rothenfluh, A. RhoGAP18B Isoforms Act on Distinct Rho-Family GTPases and Regulate Behavioral Responses to Alcohol via Cofilin. PLoS ONE 2015, 10, e0137465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojelade, S.A.; Jia, T.; Rodan, A.R.; Chenyang, T.; Kadrmas, J.L.; Cattrell, A.; Ruggeri, B.; Charoen, P.; Lemaitre, H.; Banaschewski, T.; et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc. Natl. Acad. Sci. USA 2015, 112, E4085–E4093. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, D.A.; Jia, T.; Pinzón, J.H.; Acevedo, S.F.; Ojelade, S.A.; Xu, B.; Tay, N.; Desrivières, S.; Hernandez, J.L.; Banaschewski, T.; et al. The Arf6 activator Efa6/PSD3 confers regional specificity and modulates ethanol consumption in Drosophila and humans. Mol. Psychiatry 2018, 23, 621–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peru y Colón de Portugal, R.L.; Acevedo, S.F.; Rodan, A.R.; Chang, L.Y.; Eaton, B.A.; Rothenfluh, A. Adult Neuronal Arf6 Controls Ethanol-Induced Behavior with Arfaptin Downstream of Rac1 and RhoGAP18B. J. Neurosci. 2012, 32, 17706–17713. [Google Scholar] [CrossRef] [Green Version]
- Riley, B.P.; Kalsi, G.; Kuo, P.-H.; Vladimirov, V.; Thiselton, D.L.; Vittum, J.; Wormley, B.; Grotewiel, M.S.; Patterson, D.G.; Sullivan, P.F.; et al. Alcohol dependence is associated with the ZNF699 gene, a human locus related to Drosophila hangover, in the Irish Affected Sib Pair Study of Alcohol Dependence (IASPSAD) sample. Mol. Psychiatry 2006, 11, 1025–1031. [Google Scholar] [CrossRef] [Green Version]
- Scholz, H.; Franz, M.; Heberlein, U. The hangover gene defines a stress pathway required for ethanol tolerance development. Nature 2005, 436, 845–847. [Google Scholar] [CrossRef] [Green Version]
- Karnib, N.; Long, B.; van Staaden, M.; Sprague, J.E.; Hall, F.S.; Jacobson, D.; Huber, R. Opiate Sensitivity in Fruit Flies. Med. Res. Arch. 2023, 11, 3711. [Google Scholar] [CrossRef]
- Sanchez-Díaz, I.; Rosales-Bravo, F.; Reyes-Taboada, J.L.; Covarrubias, A.A.; Narvaez-Padilla, V.; Reynaud, E. The Esg Gene Is Involved in Nicotine Sensitivity in Drosophila melanogaster. PLoS ONE 2015, 10, e0133956. [Google Scholar] [CrossRef] [Green Version]
- Substance Abuse and Mental Health Services Administration (US); Office of the Surgeon General (US). Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health; US Department of Health and Human Services: Washington, DC, USA, 2016.
- Wolf, F.W.; Rodan, A.R.; Tsai, L.T.-Y.; Heberlein, U. High-Resolution Analysis of Ethanol-Induced Locomotor Stimulation in Drosophila. J. Neurosci. 2002, 22, 11035–11044. [Google Scholar] [CrossRef] [Green Version]
- Engel, G.L.; Marella, S.; Kaun, K.R.; Wu, J.; Adhikari, P.; Kong, E.C.; Wolf, F.W. Sir2/Sirt1 Links Acute Inebriation to Presynaptic Changes and the Development of Alcohol Tolerance, Preference, and Reward. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 5241–5251. [Google Scholar] [CrossRef] [Green Version]
- Maples, T.; Rothenfluh, A. A Simple Way to Measure Ethanol Sensitivity in Flies. J. Vis. Exp. JoVE 2011, 48, e2541. [Google Scholar] [CrossRef]
- Morozova, T.V.; Anholt, R.R.H.; Mackay, T.F.C. Transcriptional response to alcohol exposure in Drosophila melanogaster. Genome Biol. 2006, 7, R95. [Google Scholar] [CrossRef] [Green Version]
- Filošević, A.; Al-Samarai, S.; Andretić Waldowski, R. High Throughput Measurement of Locomotor Sensitization to Volatilized Cocaine in Drosophila melanogaster. Front. Mol. Neurosci. 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Rigo, F.; Filošević, A.; Petrović, M.; Jović, K.; Andretić Waldowski, R. Locomotor sensitization modulates voluntary self-administration of methamphetamine in Drosophila melanogaster. Addict. Biol. 2021, 26, e12963. [Google Scholar] [CrossRef]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; de la Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaplen, K.M.; Mei, N.J.; Bounds, H.A.; Song, S.L.; Azanchi, R.; Kaun, K.R. Automated real-time quantification of group locomotor activity in Drosophila melanogaster. Sci. Rep. 2019, 9, 4427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.Y.; Wachi, Y.; Engdorf, E.; Fumagalli, E.; Wang, Y.; Myers, J.; Massey, S.; Greiss, A.; Xu, S.; Roman, G. Normal Ethanol Sensitivity and Rapid Tolerance Require the G Protein Receptor Kinase 2 in Ellipsoid Body Neurons in Drosophila. Alcohol. Clin. Exp. Res. 2020, 44, 1686–1699. [Google Scholar] [CrossRef]
- Berger, K.H.; Kong, E.C.; Dubnau, J.; Tully, T.; Moore, M.S.; Heberlein, U. Ethanol Sensitivity and Tolerance in Long-Term Memory Mutants of Drosophila melanogaster. Alcohol. Clin. Exp. Res. 2008, 32, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Morozova, T.V.; Anholt, R.R.H.; Mackay, T.F.C. Phenotypic and transcriptional response to selection for alcohol sensitivity in Drosophila melanogaster. Genome Biol. 2007, 8, R231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ro, J.; Harvanek, Z.M.; Pletcher, S.D. FLIC: High-Throughput, Continuous Analysis of Feeding Behaviors in Drosophila. PLoS ONE 2014, 9, e101107. [Google Scholar] [CrossRef] [Green Version]
- Peru y Colón de Portugal, R.L.; Ojelade, S.A.; Penninti, P.S.; Dove, R.J.; Nye, M.J.; Acevedo, S.F.; Lopez, A.; Rodan, A.R.; Rothenfluh, A. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict. Biol. 2014, 19, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Devineni, A.V.; Heberlein, U. Preferential Ethanol Consumption in Drosophila Models Features of Addiction. Curr. Biol. CB 2009, 19, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Cadieu, N.; Cadieu, J.-C.; El Ghadraoui, L.; Grimal, A.; Lamboeuf, Y. Conditioning to ethanol in the fruit fly-a study using an inhibitor of ADH. J. Insect Physiol. 1999, 45, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kaun, K.R.; Azanchi, R.; Maung, Z.; Hirsh, J.; Heberlein, U. A Drosophila model for alcohol reward. Nat. Neurosci. 2011, 14, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.G.; Khurana, S.; Kuperman, A.; Atkinson, N.S. Neural Adaptation Leads to Cognitive Ethanol Dependence. Curr. Biol. 2012, 22, 2338–2341. [Google Scholar] [CrossRef] [Green Version]
- Wise, R.A. Roles for nigrosriatal—Not just mesocorticolimbic—Dopamine in reward and addiction. Trends Neurosci. 2009, 32, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chvilicek, M.M.; Titos, I.; Rothenfluh, A. The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors. Front. Behav. Neurosci. 2020, 14, 607700. [Google Scholar] [CrossRef]
- Philyaw, T.J.; Rothenfluh, A.; Titos, I. The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines 2022, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Baler, R.; Telang, F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 2009, 56, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Hummel, M.; Unterwald, E.M. D1 dopamine receptor: A putative neurochemical and behavioral link to cocaine action. J. Cell. Physiol. 2002, 191, 17–27. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulzer, D.; Chen, T.K.; Lau, Y.Y.; Kristensen, H.; Rayport, S.; Ewing, A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 4102–4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; O’Neil, J.P.; Janabi, M.; Marks, S.M.; Jagust, W.J.; Fields, H.L. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci. Transl. Med. 2012, 4, 116ra6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 12700–12706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deadwyler, S.A. Electrophysiological correlates of abused drugs: Relation to natural rewards. Ann. N. Y. Acad. Sci. 2010, 1187, 140–147. [Google Scholar] [CrossRef]
- Adinoff, B. Neurobiologic Processes in Drug Reward and Addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.C.; Woo, K.; Li, H.; Lebestky, T.; Mayer, N.; Sniffen, M.R.; Heberlein, U.; Bainton, R.J.; Hirsh, J.; Wolf, F.W. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 2010, 5, e9954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andretic, R.; van Swinderen, B.; Greenspan, R.J. Dopaminergic modulation of arousal in Drosophila. Curr. Biol. CB 2005, 15, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.; Hirsh, J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr. Biol. CB 1998, 8, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-G.; Kim, Y.-C.; Dunning, J.S.; Han, K.-A. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS ONE 2008, 3, e1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalivas, P.W.; Duffy, P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 1990, 5, 48–58. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Becker, J.B. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986, 396, 157–198. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ferreira, J.C.B.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrés, J.; Wang, X.; Takahashi, K.; Cunningham, S.J.; Wang, T.T.; Weiner, H. Effects of changing glutamate 487 to lysine in rat and human liver mitochondrial aldehyde dehydrogenase. A model to study human (Oriental type) class 2 aldehyde dehydrogenase. J. Biol. Chem. 1994, 269, 13854–13860. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.N.; Weiner, H.; Hurley, T.D. Disruption of the coenzyme binding site and dimer interface revealed in the crystal structure of mitochondrial aldehyde dehydrogenase “Asian” variant. J. Biol. Chem. 2005, 280, 30550–30556. [Google Scholar] [CrossRef] [Green Version]
- Schuckit, M.A. Low level of response to alcohol as a predictor of future alcoholism. Am. J. Psychiatry 1994, 151, 184–189. [Google Scholar] [CrossRef]
- Devineni, A.V.; McClure, K.D.; Guarnieri, D.J.; Corl, A.B.; Wolf, F.W.; Eddison, M.; Heberlein, U. The genetic relationships between ethanol preference, acute ethanol sensitivity and ethanol tolerance in Drosophila melanogaster. Fly 2011, 5, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Bainton, R.J.; Tsai, L.T.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. CB 2000, 10, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Milán, M.; Diaz-Benjumea, F.J.; Cohen, S.M. Beadex encodes an LMO protein that regulates Apterous LIM–homeodomain activity in Drosophila wing development: A model for LMO oncogene function. Genes Dev. 1998, 12, 2912–2920. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Justice, N.J.; Abdelilah, S.; Chan, Y.M.; Jan, L.Y.; Jan, Y.N. The Drosophila LIM-only gene, dLMO, is mutated in Beadex alleles and might represent an evolutionarily conserved function in appendage development. Proc. Natl. Acad. Sci. USA 1998, 95, 10637–10642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, L.T.-Y.; Bainton, R.J.; Blau, J.; Heberlein, U. Lmo mutants reveal a novel role for circadian pacemaker neurons in cocaine-induced behaviors. PLoS Biol. 2004, 2, e408. [Google Scholar] [CrossRef] [PubMed]
- Renn, S.C.; Park, J.H.; Rosbash, M.; Hall, J.C.; Taghert, P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 1999, 99, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasek, A.W.; Kapfhamer, D.; Kharazia, V.; Gesch, J.; Giorgetti, F.; Heberlein, U. Lmo4 in the nucleus accumbens regulates cocaine sensitivity. Genes Brain Behav. 2010, 9, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aso, Y.; Herb, A.; Ogueta, M.; Siwanowicz, I.; Templier, T.; Friedrich, A.B.; Ito, K.; Scholz, H.; Tanimoto, H. Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability. PLoS Genet. 2012, 8, e1002768. [Google Scholar] [CrossRef] [Green Version]
- Burke, C.J.; Huetteroth, W.; Owald, D.; Perisse, E.; Krashes, M.J.; Das, G.; Gohl, D.; Silies, M.; Certel, S.; Waddell, S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 2012, 492, 433–437. [Google Scholar] [CrossRef] [Green Version]
- Huetteroth, W.; Perisse, E.; Lin, S.; Klappenbach, M.; Burke, C.; Waddell, S. Sweet Taste and Nutrient Value Subdivide Rewarding Dopaminergic Neurons in Drosophila. Curr. Biol. 2015, 25, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, T.; Aso, Y.; Yamagata, N.; Abe, A.; Rubin, G.M.; Tanimoto, H. Reward signal in a recurrent circuit drives appetitive long-term memory formation. eLife 2015, 4, e10719. [Google Scholar] [CrossRef]
- Liu, C.; Plaçais, P.-Y.; Yamagata, N.; Pfeiffer, B.D.; Aso, Y.; Friedrich, A.B.; Siwanowicz, I.; Rubin, G.M.; Preat, T.; Tanimoto, H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 2012, 488, 512–516. [Google Scholar] [CrossRef]
- Mao, Z. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owald, D.; Waddell, S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr. Opin. Neurobiol. 2015, 35, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perisse, E.; Owald, D.; Barnstedt, O.; Talbot, C.B.; Huetteroth, W.; Waddell, S. Aversive Learning and Appetitive Motivation Toggle Feed-Forward Inhibition in the Drosophila Mushroom Body. Neuron 2016, 90, 1086–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagata, N.; Hiroi, M.; Kondo, S.; Abe, A.; Tanimoto, H. Suppression of Dopamine Neurons Mediates Reward. PLOS Biol. 2016, 14, e1002586. [Google Scholar] [CrossRef] [Green Version]
- Butts, A.R.; Ojelade, S.A.; Pronovost, E.D.; Seguin, A.; Merrill, C.B.; Rodan, A.R.; Rothenfluh, A. Altered Actin Filament Dynamics in the Drosophila Mushroom Bodies Lead to Fast Acquisition of Alcohol Consumption Preference. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 8877–8884. [Google Scholar] [CrossRef]
- Kanno, M.; Hiramatsu, S.; Kondo, S.; Tanimoto, H.; Ichinose, T. Voluntary intake of psychoactive substances is regulated by the dopamine receptor Dop1R1 in Drosophila. Sci. Rep. 2021, 11, 3432. [Google Scholar] [CrossRef]
- Belovich, A.N.; Aguilar, J.I.; Mabry, S.J.; Cheng, M.H.; Zanella, D.; Hamilton, P.J.; Stanislowski, D.J.; Shekar, A.; Foster, J.D.; Bahar, I.; et al. A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol. Psychiatry 2021, 26, 4417–4430. [Google Scholar] [CrossRef]
- Philyaw, T.J.; Titos, I.; Cummins, P.N.; Rodan, A.R.; Rothenfluh, A. Drosophila Cocaine Avoidance is Mediated by Peripheral Bitter Gustatory Neurons. bioRxiv 2022, 2022.06.22.497211. [Google Scholar] [CrossRef]
- Drinking Levels Defined|National Institute on Alcohol Abuse and Alcoholism (NIAAA). Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 3 May 2023).
- Harzke, A.J.; Williams, M.L.; Bowen, A.M. Binge Use of Crack Cocaine and Sexual Risk Behaviors Among African-American, HIV-Positive Users. AIDS Behav. 2009, 13, 1106–1118. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.S.; Garfein, R.S.; Semple, S.J.; Strathdee, S.A.; Zians, J.K.; Patterson, T.L. Binge use and sex and drug use behaviors among HIV(−), heterosexual methamphetamine users in San Diego. Subst. Use Misuse 2010, 45, 116–133. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.K.; Melega, W.P. Patterns of methamphetamine abuse and their consequences. J. Addict. Dis. 2002, 21, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.J.; Patterson, T.L.; Grant, I. Binge use of methamphetamine among HIV-positive men who have sex with men: Pilot data and HIV prevention implications. AIDS Educ. Prev. Off. Publ. Int. Soc. AIDS Educ. 2003, 15, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Richardson, K.; Dacey, J.; Glynn, S.; Domier, C.P.; Rawson, R.A.; Ling, W. A comparison of patterns of methamphetamine and cocaine use. J. Addict. Dis. 2002, 21, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sommers, I.; Baskin, D.; Baskin-Sommers, A. Methamphetamine use among young adults: Health and social consequences. Addict. Behav. 2006, 31, 1469–1476. [Google Scholar] [CrossRef]
- Cservenka, A.; Brumback, T. The Burden of Binge and Heavy Drinking on the Brain: Effects on Adolescent and Young Adult Neural Structure and Function. Front. Psychol. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Scaife, J.C.; Duka, T. Behavioural measures of frontal lobe function in a population of young social drinkers with binge drinking pattern. Pharmacol. Biochem. Behav. 2009, 93, 354–362. [Google Scholar] [CrossRef]
- Gimenez-Gomez, P.; Le, T.; Martin, G.E. Modulation of neuronal excitability by binge alcohol drinking. Front. Mol. Neurosci. 2023, 16, 1098211. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat. Neurosci. 2005, 8, 1442–1444. [Google Scholar] [CrossRef]
- Koob, G.F.; Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef]
- Weiss, F.; Markou, A.; Lorang, M.T.; Koob, G.F. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992, 593, 314–318. [Google Scholar] [CrossRef]
- Davidson, M.; Shanley, B.; Wilce, P. Increased NMDA-induced excitability during ethanol withdrawal: A behavioural and histological study. Brain Res. 1995, 674, 91–96. [Google Scholar] [CrossRef]
- Dahchour, A.; De Witte, P.; Bolo, N.; Nédélec, J.-F.; Muzet, M.; Durbin, P.; Macher, J.-P. Central effects of acamprosate: Part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res. Neuroimaging 1998, 82, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Addiction and the Brain Antireward System. Annu. Rev. Psychol. 2008, 59, 29–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koob, G.F.; Buck, C.L.; Cohen, A.; Edwards, S.; Park, P.E.; Schlosburg, J.E.; Schmeichel, B.; Vendruscolo, L.F.; Wade, C.L.; Whitfield, T.W.; et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014, 76, 370–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Scholz, H.; Ramond, J.; Singh, C.M.; Heberlein, U. Functional Ethanol Tolerance in Drosophila. Neuron 2000, 28, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Lathen, D.R.; Merrill, C.B.; Rothenfluh, A. Flying Together: Drosophila as a Tool to Understand the Genetics of Human Alcoholism. Int. J. Mol. Sci. 2020, 21, 6649. [Google Scholar] [CrossRef]
- Ghezzi, A.; Krishnan, H.R.; Atkinson, N.S. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict. Biol. 2014, 19, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Bayard, M.; McIntyre, J.; Hill, K.R.; Woodside, J. Alcohol withdrawal syndrome. Am. Fam. Physician 2004, 69, 1443–1450. [Google Scholar]
- Cowmeadow, R.B.; Krishnan, H.R.; Ghezzi, A.; Al’Hasan, Y.M.; Wang, Y.Z.; Atkinson, N.S. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol. Clin. Exp. Res. 2006, 30, 745–753. [Google Scholar] [CrossRef]
- Lepack, A.E.; Werner, C.T.; Stewart, A.F.; Fulton, S.L.; Zhong, P.; Farrelly, L.A.; Smith, A.C.W.; Ramakrishnan, A.; Lyu, Y.; Bastle, R.M.; et al. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science 2020, 368, 197–201. [Google Scholar] [CrossRef]
- Der-Avakian, A.; Markou, A. The Neurobiology of Anhedonia and Other Reward-Related Deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heshmati, M.; Russo, S.J. Anhedonia and the brain reward circuitry in depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.; Robbins, T.W. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006, 73, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Jentsch, J.D.; Taylor, J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology 1999, 146, 373–390. [Google Scholar] [CrossRef]
- Peck, J.A.; Ranaldi, R. Drug abstinence: Exploring animal models and behavioral treatment strategies. Psychopharmacology 2014, 231, 2045–2058. [Google Scholar] [CrossRef]
- Sinha, R. The clinical neurobiology of drug craving. Curr. Opin. Neurobiol. 2013, 23, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Goeders, N.E. Stress and Drug Craving. In Encyclopedia of Behavioral Neuroscience; Koob, G.F., Moal, M.L., Thompson, R.F., Eds.; Academic Press: Oxford, UK, 2010; pp. 310–315. ISBN 978-0-08-045396-5. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Volkow, N.D. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine Cues and Dopamine in Dorsal Striatum: Mechanism of Craving in Cocaine Addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.F.; Kuwabara, H.; Schretlen, D.J.; Bonson, K.R.; Zhou, Y.; Nandi, A.; Brasic, J.R.; Kimes, A.S.; Maris, M.A.; Kumar, K.; et al. Increased Occupancy of Dopamine Receptors in Human Striatum during Cue-Elicited Cocaine Craving|EndNote Click. Available online: https://click.endnote.com/viewer?doi=10.1038%2Fsj.npp.1301194&token=WzM2OTY5ODUsIjEwLjEwMzgvc2oubnBwLjEzMDExOTQiXQ.2qZVlm-hy24E4EwqjfUMyZ_mGqI (accessed on 18 April 2023).
- Grüsser, S.M.; Wrase, J.; Klein, S.; Hermann, D.; Smolka, M.N.; Ruf, M.; Weber-Fahr, W.; Flor, H.; Mann, K.; Braus, D.F.; et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 2004, 175, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Wrase, J.; Grüsser, S.M.; Klein, S.; Diener, C.; Hermann, D.; Flor, H.; Mann, K.; Braus, D.F.; Heinz, A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatry 2002, 17, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.T.; Yager, L.M.; Robinson, T.E. Cue-Evoked Cocaine “Craving”: Role of Dopamine in the Accumbens Core. J. Neurosci. 2013, 33, 13989–14000. [Google Scholar] [CrossRef] [Green Version]
- Felsenberg, J. Changing memories on the fly: The neural circuits of memory re-evaluation in Drosophila melanogaster. Curr. Opin. Neurobiol. 2021, 67, 190–198. [Google Scholar] [CrossRef]
- Berlin, G.S.; Hollander, E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr. 2014, 19, 62–68. [Google Scholar] [CrossRef]
- Sanchez-Roige, S.; Barnes, S.A.; Mallari, J.; Wood, R.; Polesskaya, O.; Palmer, A.A. A mutant allele of glycoprotein M6-B (GPM6B) facilitates behavioral flexibility but increases delay discounting. Genes Brain Behav. 2022, 21, e12800. [Google Scholar] [CrossRef]
- Mishra, P.; Yang, S.E.; Montgomery, A.B.; Reed, A.R.; Rodan, A.R.; Rothenfluh, A. The fly liquid-food electroshock assay (FLEA) suggests opposite roles for neuropeptide F in avoidance of bitterness and shock. BMC Biol. 2021, 19, 31. [Google Scholar] [CrossRef]
- Svensson, A.I. The aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) reduces disinhibitory behavior in intact adult male rats treated with a high dose of testosterone. Behav. Brain Res. 2010, 206, 216–222. [Google Scholar] [CrossRef]
- Beardsley, P.M.; Shelton, K.L. Prime-, Stress- and Cue-Induced Reinstatement of Extinguished Drug-Reinforced Responding in Rats: Cocaine as the Prototypical Drug of Abuse. Curr. Protoc. Neurosci. 2012, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B.J.; Belin, D.; Economidou, D.; Pelloux, Y.; Dalley, J.W.; Robbins, T.W. Neural Mechanisms Underlying the Vulnerability to Develop Compulsive Drug-Seeking Habits and Addiction. Philos. Trans. Biol. Sci. 2008, 363, 3125–3135. [Google Scholar] [CrossRef] [Green Version]
- Vanderschuren, L.J.M.J.; Ciano, P.D.; Everitt, B.J. Involvement of the Dorsal Striatum in Cue-Controlled Cocaine Seeking. J. Neurosci. 2005, 25, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorel, S.R.; Liu, X.; Hayes, R.J.; Spector, J.A.; Gardner, E.L. Relapse to Cocaine-Seeking After Hippocampal Theta Burst Stimulation. Science 2001, 292, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- De Witte, P.; Littleton, J.; Parot, P.; Koob, G. Neuroprotective and abstinence-promoting effects of acamprosate: Elucidating the mechanism of action. CNS Drugs 2005, 19, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.R.; Roberts, A.J.; Chan, K.; Davis, H.; Brennan, M.; Zorrilla, E.P.; Koob, G.F. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002, 26, 1494–1501. [Google Scholar] [CrossRef]
- Zhao, Y.; Dayas, C.V.; Aujla, H.; Baptista, M.A.S.; Martin-Fardon, R.; Weiss, F. Activation of Group II Metabotropic Glutamate Receptors Attenuates Both Stress and Cue-Induced Ethanol-Seeking and Modulates c-fos Expression in the Hippocampus and Amygdala. J. Neurosci. 2006, 26, 9967–9974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wang, Z.; Xu, K.; Zhang, X.; Amei, A.; Gelernter, J.; Zhao, H.; Justice, A.C.; Wang, Z. Retrospective Association Analysis of Longitudinal Binary Traits Identifies Important Loci and Pathways in Cocaine Use. Genetics 2019, 213, 1225–1236. [Google Scholar] [CrossRef]
- Spanagel, R. Animal models of addiction. Dialogues Clin. Neurosci. 2017, 19, 247–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummins-Beebee, P.N.; Chvilicek, M.M.; Rothenfluh, A. The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses. Int. J. Mol. Sci. 2023, 24, 10909. https://doi.org/10.3390/ijms241310909
Cummins-Beebee PN, Chvilicek MM, Rothenfluh A. The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses. International Journal of Molecular Sciences. 2023; 24(13):10909. https://doi.org/10.3390/ijms241310909
Chicago/Turabian StyleCummins-Beebee, Pearl N., Maggie M. Chvilicek, and Adrian Rothenfluh. 2023. "The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses" International Journal of Molecular Sciences 24, no. 13: 10909. https://doi.org/10.3390/ijms241310909
APA StyleCummins-Beebee, P. N., Chvilicek, M. M., & Rothenfluh, A. (2023). The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses. International Journal of Molecular Sciences, 24(13), 10909. https://doi.org/10.3390/ijms241310909





