Anti-Inflammatory Effects of Nutritionally Relevant Concentrations of Oleuropein and Hydroxytyrosol on Peripheral Blood Mononuclear Cells: An Age-Related Analysis
Abstract
1. Introduction
2. Results
2.1. Characteristics of Adult and Senior Groups
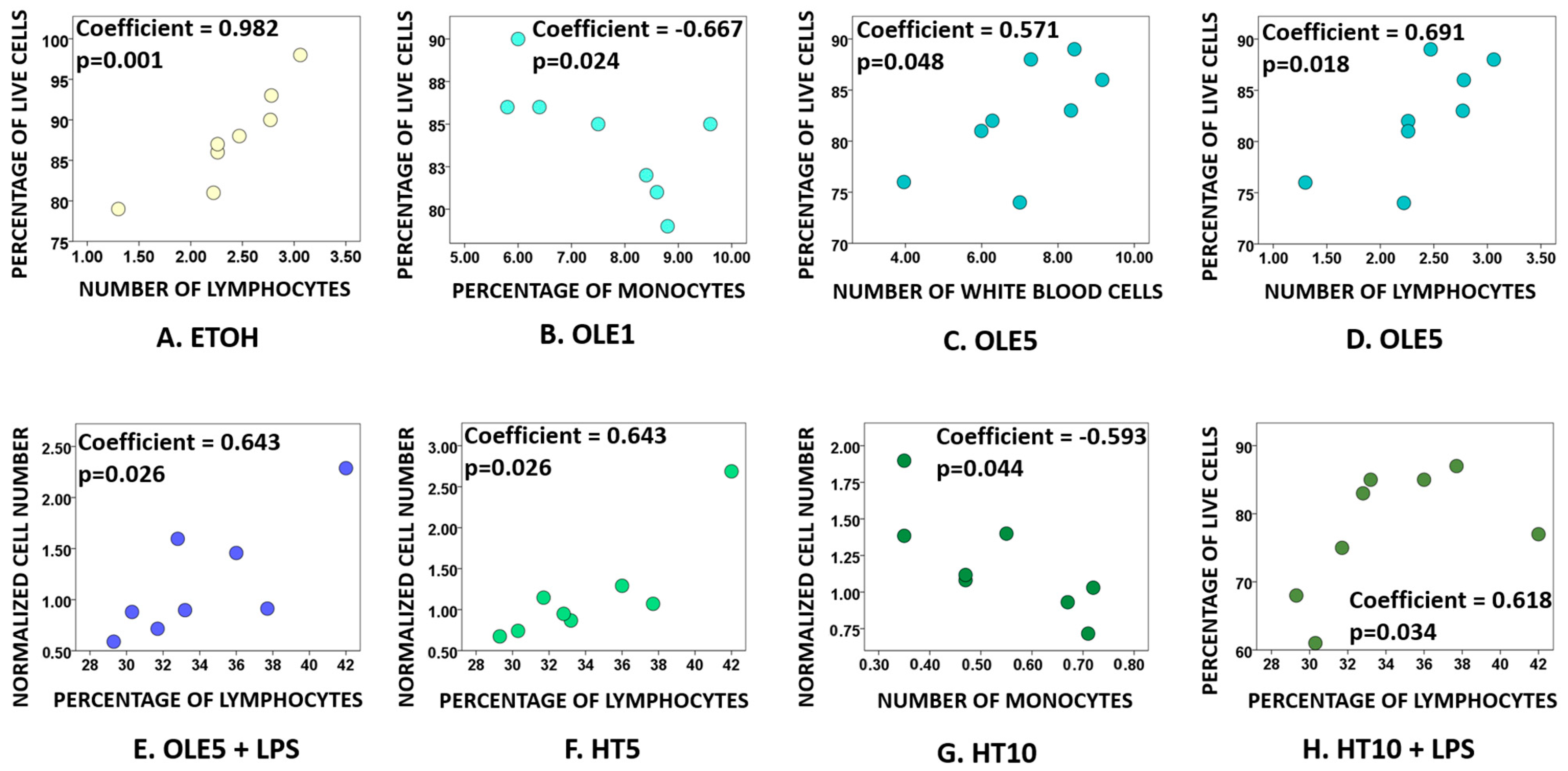
2.2. Effects of OLE and HT on Cell Viability
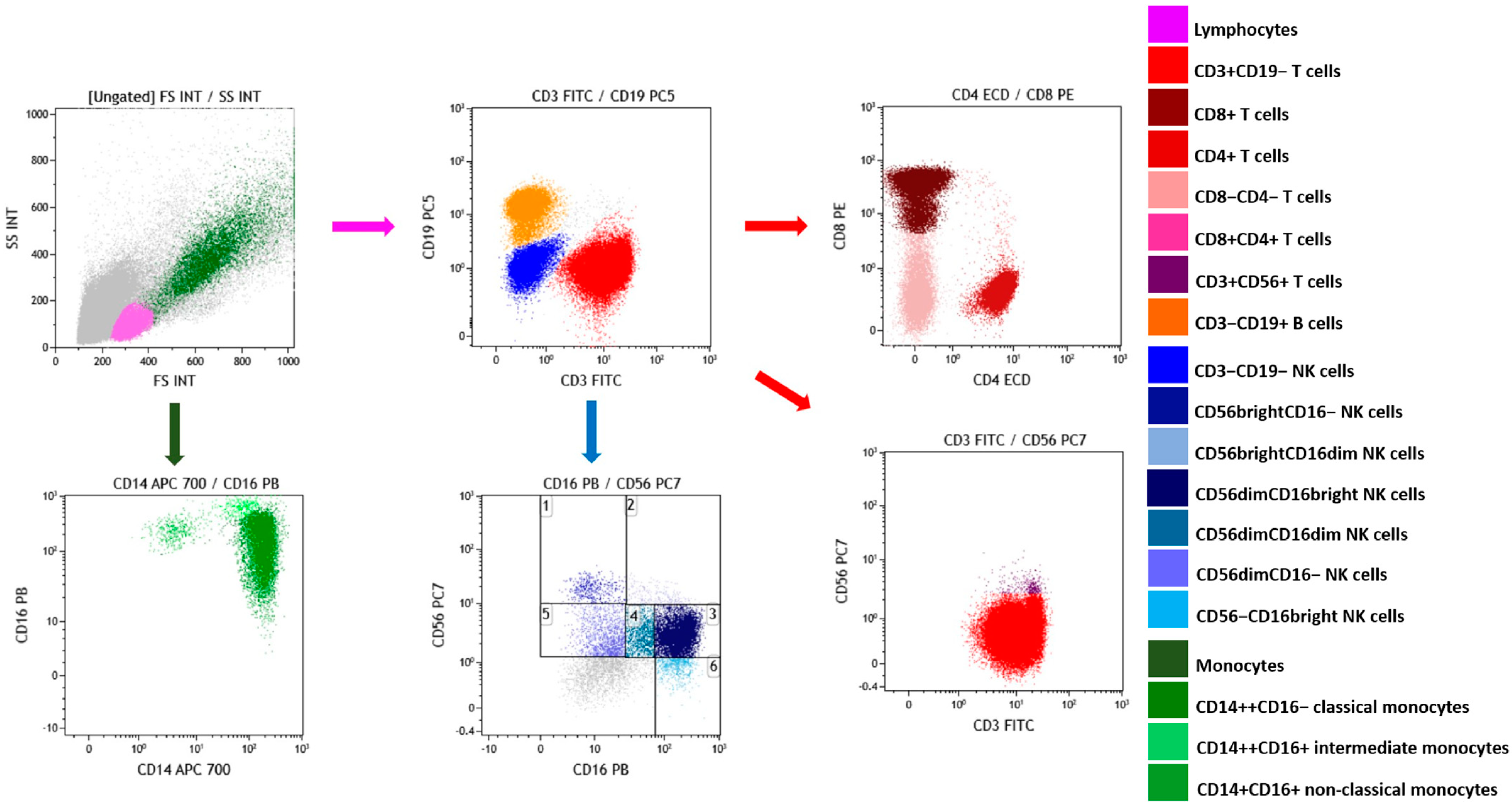
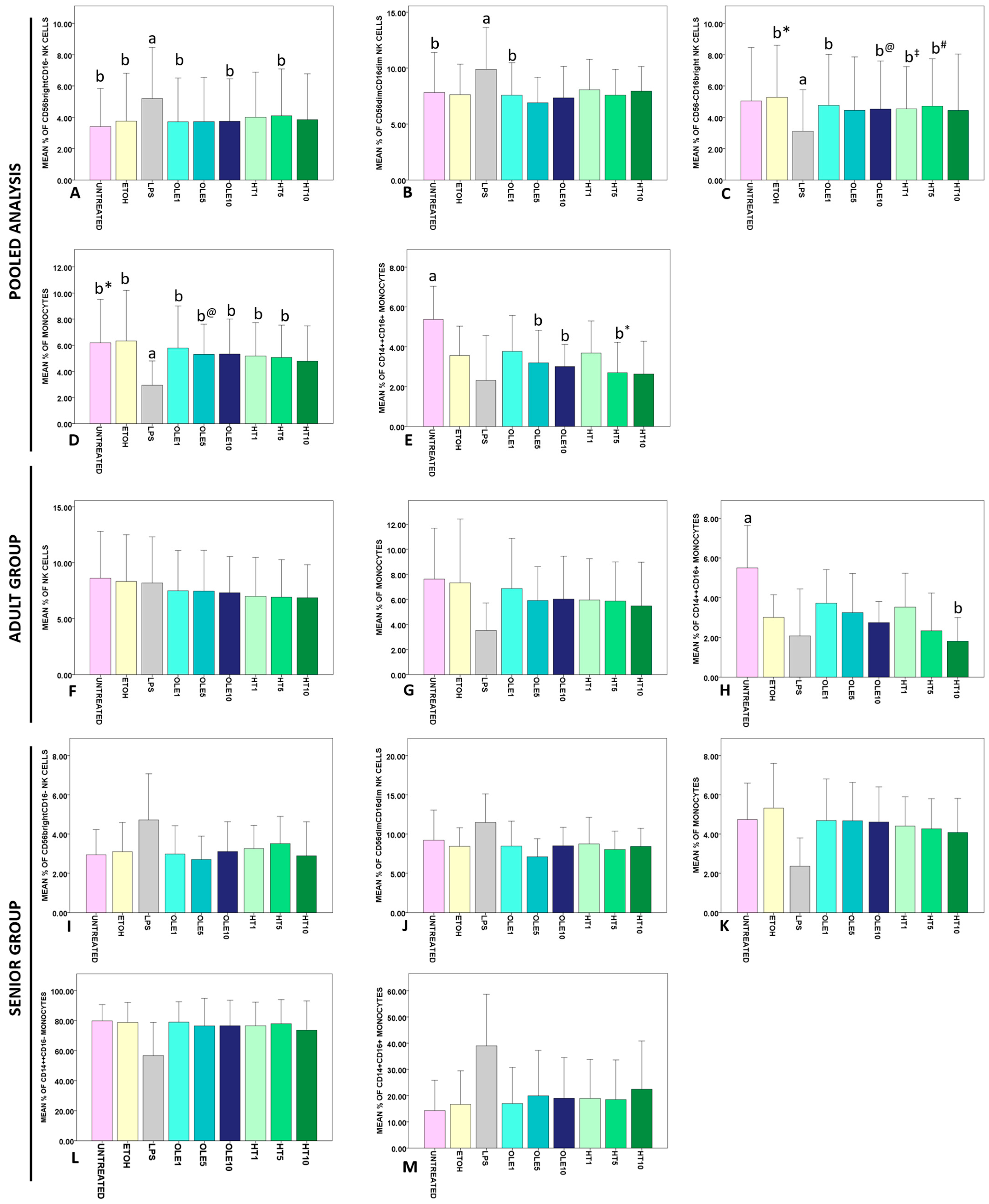
2.3. Effects of OLE and HT on the Frequency of Lymphocyte and Monocytes Subsets
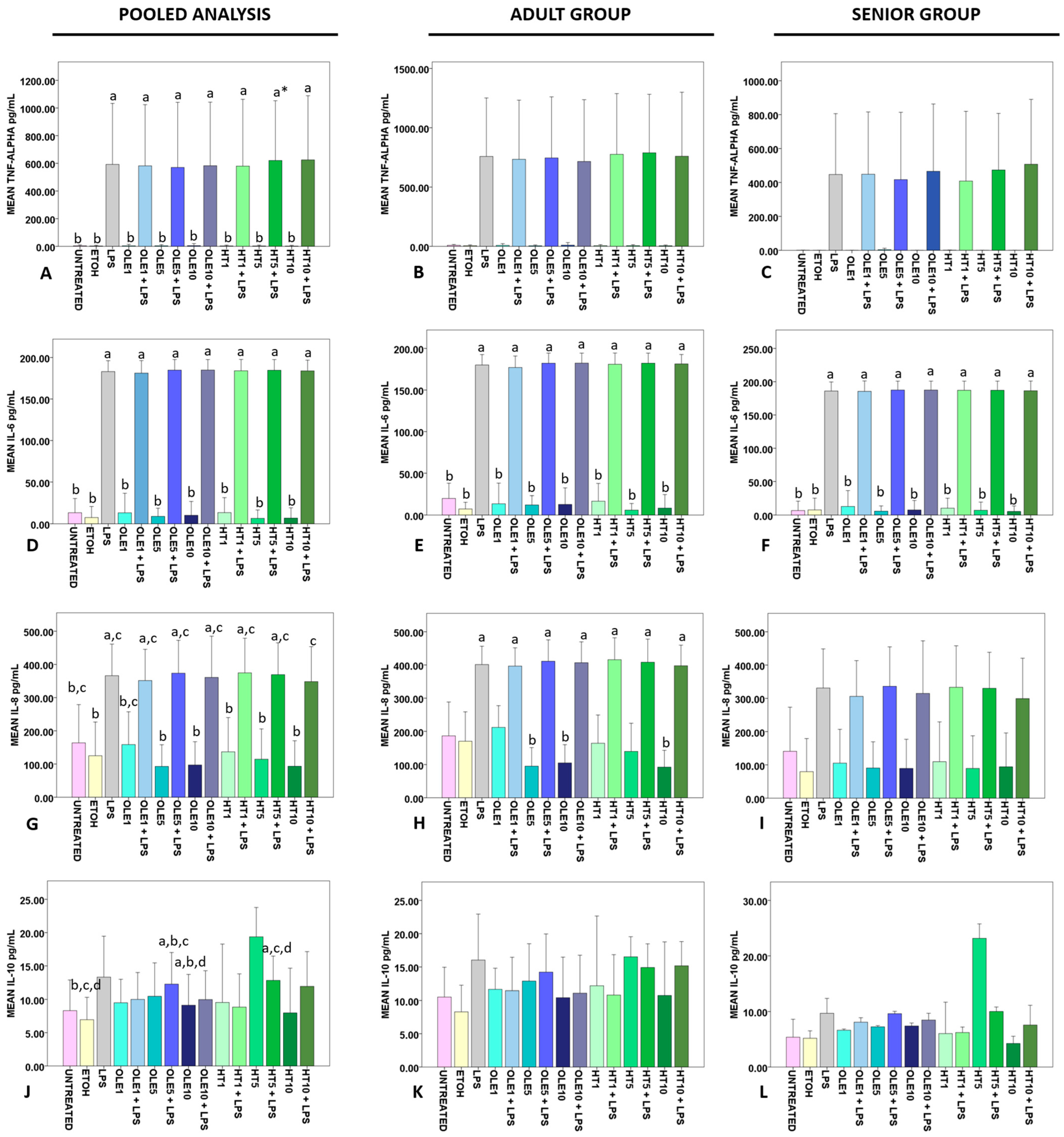
2.4. Effects of OLE and HT on the Release of Cytokines in the Extracellular Medium
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. OLE and HT
4.3. Materials
4.4. Blood Samples, PBMC Isolation and Treatment
4.5. Assessment of Cell Viability
4.6. Frequency of Lymphocyte and Monocyte Subsets
4.7. Measurement of Cytokine Release in the Extracellular Medium
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Tizazu, A.M.; Mengist, H.M.; Demeke, G. Aging, inflammaging and immunosenescence as risk factors of severe COVID-19. Immun. Ageing 2022, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, J.M.; Bryl, E.; Fulop, T. The role of inflammaging in the development of chronic diseases of older people. In Human Aging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 89–104. [Google Scholar] [CrossRef]
- Fülöp, T.; Larbi, A.; Pawelec, G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.A.; Calvo-Fortes, F.; Silveira-Nunes, G.; Camatta, G.C.; Speziali, E.; Turroni, S.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Neretti, N.; Maioli, T.U.; et al. Inflammaging in Endemic Areas for Infectious Diseases. Front. Immunol. 2020, 11, 579972. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Weinberger, B. Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives. Front. Immunol. 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 109–122. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, Inflammaging, and Frailty: Role of Myeloid Cells in Age-Related Diseases. Clin. Rev. Allergy Immunol. 2022, 64, 123–144. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.D.O.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- de Mol, J.; Kuiper, J.; Tsiantoulas, D.; Foks, A.C. The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 2021, 12, 733566. [Google Scholar] [CrossRef]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef] [PubMed]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.J.; Grant, M.D. The Immune Response against Human Cytomegalovirus Links Cellular to Systemic Senescence. Cells 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Serrano-López, J.; Martín-Antonio, B. Inflammaging, an Imbalanced Immune Response That Needs to Be Restored for Cancer Prevention and Treatment in the Elderly. Cells 2021, 10, 2562. [Google Scholar] [CrossRef]
- Govindasamy, V.; Rajendran, A.; Lee, Z.; Ooi, G.; Then, K.; Then, K.; Gayathri, M.; Das, A.K.; Cheong, S. The potential role of mesenchymal stem cells in modulating antiageing process. Cell Biol. Int. 2021, 45, 1999–2016. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Untersmayr, E.; Brandt, A.; Koidl, L.; Bergheim, I. The Intestinal Barrier Dysfunction as Driving Factor of Inflammaging. Nutrients 2022, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Jeong, J.-J.; Yoo, S.-Y.; Kim, D.-H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Gambino, C.M.; Accardi, G.; Aiello, A.; Candore, G.; Guccione, G.D.; Mirisola, M.; Procopio, A.; Taormina, G.; Caruso, C. Effect of Extra Virgin Olive Oil and Table Olives on the ImmuneInflammatory Responses: Potential Clinical Applications. Endocrine, Metab. Immune Disord. Drug Targets 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef]
- Losito, I.; Abbattista, R.; De Ceglie, C.; Castellaneta, A.; Calvano, C.D.; Cataldi, T.R. Bioactive Secoiridoids in Italian Extra-Virgin Olive Oils: Impact of Olive Plant Cultivars, Cultivation Regions and Processing. Molecules 2021, 26, 743. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; De Sancti, P.; Giovannini, C.; D‘archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocrine, Metab. Immune Disord. Drug Targets 2017, 18, 36–50. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Capurso, C.; Bellanti, F.; Buglio, A.L.; Vendemiale, G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients 2019, 12, 35. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A. Dietary Diversity and Healthy Aging: A Prospective Study. Nutrients 2021, 13, 1787. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Patrone, M.; Ranzato, E. Mediterranean Diet Polyphenols: Anthocyanins and Their Implications for Health. Mini-Reviews Med. Chem. 2021, 21, 1692–1700. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic Hydrolysis of Oleuropein from Olea europea (Olive) Leaf Extract and Antioxidant Activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Qabaha, K.; Al-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Rodriguez, J.V.; Tomić, S.; Kosalec, I. Protective effects of olive oil phenolics oleuropein and hydroxytyrosol against hydrogen peroxide-induced DNA damage in human peripheral lymphocytes. Acta Pharm. 2021, 71, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; Garcia-Perez, J.V.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol Disposition in Humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailability; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Oliveras-López, M.-J.; Berná, G.; Carneiro, E.M.; de la Serrana, H.L.-G.; Martín, F.; López, M.C. An Extra-Virgin Olive Oil Rich in Polyphenolic Compounds Has Antioxidant Effects in Of1 Mice. J. Nutr. 2008, 138, 1074–1078. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol Excretion Differs between Rats and Humans and Depends on the Vehicle of Administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-W.; Tuck, K.L.; Stupans, I.; Hayball, P.J. Simultaneous determination of oleuropein and hydroxytyrosol in rat plasma using liquid chromatography with fluorescence detection. J. Chromatogr. B 2003, 785, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Rodríguez-Morató, J.; Olesti, E.; Pujadas, M.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Covas, M.-I.; Solá, R.; Motilva, M.-J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Fitó, M.; de la Torre, R.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; Albaladejo, M.F.; Abanades, S.; Schroder, H.; et al. Olive Oils High in Phenolic Compounds Modulate Oxidative/Antioxidative Status in Men. J. Nutr. 2004, 134, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Sepporta, M.; Rosignoli, P. The hydroxytyrosol-dependent increase of TNF-α in LPS-activated human monocytes is mediated by PGE2 and adenylate cyclase activation. Toxicol. Vitr. 2015, 29, 933–937. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S422–S428. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.-J. Rejuvenating the Immune System: Insights for Anti-Neurodegeneration Strategies. Neurosci. Bull. 2022, 38, 107–109. [Google Scholar] [CrossRef]
- Nousis, L.; Doulias, P.-T.; Aligiannis, N.; Bazios, D.; Agalias, A.; Galaris, D.; Mitakou, S. DNA protecting and genotoxic effects of olive oil related components in cells exposed to hydrogen peroxide. Free. Radic. Res. 2005, 39, 787–795. [Google Scholar] [CrossRef]
- Ilavarasi, K.; Kiruthiga, P.V.; Pandian, S.K.; Devi, K.P. Hydroxytyrosol, the phenolic compound of olive oil protects human PBMC against oxidative stress and DNA damage mediated by 2,3,7,8-TCDD. Chemosphere 2011, 84, 888–893. [Google Scholar] [CrossRef]
- Mao, X.; Xia, B.; Zheng, M.; Zhou, Z. Assessment of the anti-inflammatory, analgesic and sedative effects of oleuropein from Olea europaea L. Cell. Mol. Biol. 2019, 65, 52–55. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, H.; Han, S.; Yuan, R.; He, J.; Zhuo, Y.; Feng, Y.-L.; Tang, M.; Feng, J.; Yang, S. Oleuropein Attenuates Lipopolysaccharide-Induced Acute Kidney Injury In Vitro and In Vivo by Regulating Toll-Like Receptor 4 Dimerization. Front. Pharmacol. 2021, 12, 617314. [Google Scholar] [CrossRef]
- Ryu, S.-J.; Choi, H.-S.; Yoon, K.-Y.; Lee, O.-H.; Kim, K.-J.; Lee, B.-Y. Oleuropein Suppresses LPS-Induced Inflammatory Responses in RAW 264.7 Cell and Zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Mirsanei, Z.; Bitaraf, F.S.; Mahdavi, S.; Mirzaii, M.; Jafari, R. Dose-dependent effects of oleuropein administration on regulatory T-cells in patients with rheumatoid arthritis: An in vitro approach. Int. J. Immunopathol. Pharmacol. 2022, 36, 039463202210860. [Google Scholar] [CrossRef] [PubMed]
- Amani, F.; Farsani, M.A.; Gholami, M.; Aghamiri, S.M.R.; Bakhshandeh, M.; Mohammadi, M.H. The protective effect of oleuropein against radiation-induced cytotoxicity, apoptosis, and genetic damage in cultured human lymphocytes. Int. J. Radiat. Biol. 2020, 97, 179–193. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Ricelli, A.; Gionfra, F.; Percario, Z.; De Angelis, M.; Primitivo, L.; Bonfantini, V.; Antonioletti, R.; Bullitta, S.M.; Saso, L.; Incerpi, S.; et al. Antioxidant and Biological Activities of Hydroxytyrosol and Homovanillic Alcohol Obtained from Olive Mill Wastewaters of Extra-Virgin Olive Oil Production. J. Agric. Food Chem. 2020, 68, 15428–15439. [Google Scholar] [CrossRef]
- Bonura, A.; Vlah, S.; Longo, A.; Bulati, M.; Melis, M.R.; Cibella, F.; Colombo, P. Hydroxytyrosol modulates Par j 1-induced IL-10 production by PBMCs in healthy subjects. Immunobiology 2016, 221, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramírez-Tortosa, C.; Ochoa, J.; Perez-Lopez, P.; Pulido-Morán, M.; Ramirez-Tortosa, M. Squalene ameliorates atherosclerotic lesions through the reduction of CD36 scavenger receptor expression in macrophages. Mol. Nutr. Food Res. 2012, 56, 733–740. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, Y.; Jiang, H.; Zhang, D.; Yang, Y.; Geng, X.; Li, B. Functional differences and similarities in activated peripheral blood mononuclear cells by lipopolysaccharide or phytohemagglutinin stimulation between human and cynomolgus monkeys. Ann. Transl. Med. 2021, 9, 257. [Google Scholar] [CrossRef]
- von Haefen, C.; Mei, W.; Menk, M.; Klemz, R.; Jones, A.; Wernecke, K.-D.; Spies, C.D. Ethanol Changes Gene Expression of Transcription Factors and Cytokine Production of CD4+ T-Cell Subsets in PBMCs Stimulated With LPS. Alcohol. Clin. Exp. Res. 2010, 35, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Castilla, R.; González, R.; Fouad, D.; Fraga, E.; Muntané, J. Dual Effect of Ethanol on Cell Death in Primary Culture of Human and Rat Hepatocytes. Alcohol Alcohol. 2004, 39, 290–296. [Google Scholar] [CrossRef]
- Chen, H.; George, I.; Sperber, K. Effect of Ethanol on Monocytic Function in Human Immunodeficiency Virus Type 1 Infection. Clin. Diagn. Lab. Immunol. 1998, 5, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Nguyen, H.T.-L.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Kar, N.; Gupta, D.; Bellare, J. Ethanol affects fibroblast behavior differentially at low and high doses: A comprehensive, dose-response evaluation. Toxicol. Rep. 2021, 8, 1054–1066. [Google Scholar] [CrossRef]
- Levallois, C.; Rouahi, N.; Balmes, J.-L.; Mani, J.-C. Effects of ethanol in vitro on some parameters of the immune response. Drug Alcohol Depend. 1989, 24, 239–244. [Google Scholar] [CrossRef]
- Spinozzi, F.; Agea, E.; Florucci, G.; Gerli, R.; Muscat, C.; Belia, S.; Bertotto, A. Ethanol-induced CD3 and CD2 hyporesponsiveness of peripheral blood T lymphocytes. Immunopharmacol. Immunotoxicol. 1992, 14, 939–953. [Google Scholar] [CrossRef]
- Tapani, E.; Taavitsainen, M.; Lindros, K.; Vehmas, T.; Lehtonen, E. Toxicity of Ethanol in Low Concentrations. Acta Radiol. 1996, 37, 923–926. [Google Scholar] [CrossRef]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv. Biobank. 2016, 14, 410–415. [Google Scholar] [CrossRef]
- Golke, T.; Mucher, P.; Schmidt, P.; Radakovics, A.; Repl, M.; Hofer, P.; Perkmann, T.; Fondi, M.; Schmetterer, K.G.; Haslacher, H. Delays during PBMC isolation have a moderate effect on yield, but severly compromise cell viability. Clin. Chem. Lab. Med. 2022, 60, 701–706. [Google Scholar] [CrossRef]
- Kleeberger, C.A.; Lyles, R.H.; Margolick, J.B.; Rinaldo, C.R.; Phair, J.P.; Giorgi, J.V. Viability and Recovery of Peripheral Blood Mononuclear Cells Cryopreserved for up to 12 Years in a Multicenter Study. Clin. Diagn. Lab. Immunol. 1999, 6, 14–19. [Google Scholar] [CrossRef]
- Hope, C.M.; Huynh, D.; Wong, Y.Y.; Oakey, H.; Perkins, G.B.; Nguyen, T.; Binkowski, S.; Bui, M.; Choo, A.Y.L.; Gibson, E.; et al. Optimization of Blood Handling and Peripheral Blood Mononuclear Cell Cryopreservation of Low Cell Number Samples. Int. J. Mol. Sci. 2021, 22, 9129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Wang, L.; He, H.; Mu, H.; Sun, W.; Zhou, Y.; Liu, Y.; Ma, W.; Zhang, W.; et al. Bioactivity-guided isolation of immunomodulatory compounds from the fruits of Ligustrum lucidum. J. Ethnopharmacol. 2021, 274, 114079. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Malek, T.R. Redundant and unique regulation of activated mouse B lymphocytes by IL-4 and IL-21. J. Leukoc. Biol. 2006, 80, 1416–1423. [Google Scholar] [CrossRef]
- Ellmeier, W.; Jung, S.; Sunshine, M.J.; Hatam, F.; Xu, Y.; Baltimore, D.; Mano, H.; Littman, D.R. Severe B Cell Deficiency in Mice Lacking the Tec Kinase Family Members Tec and Btk. J. Exp. Med. 2000, 192, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.; Aubin, E.; Proulx, D.P.; Lemieux, R.; Bazin, R. Increased secretion of hyperimmune antibodies following lipopolysaccharide stimulation of CD40-activated human B cells in vitro. Immunology 2009, 126, 588–595. [Google Scholar] [CrossRef]
- Parekh, V.V.; Prasad, D.V.R.; Banerjee, P.P.; Joshi, B.N.; Kumar, A.; Mishra, G.C. B Cells Activated by Lipopolysaccharide, But Not By Anti-Ig and Anti-CD40 Antibody, Induce Anergy in CD8+ T Cells: Role of TGF-β1. J. Immunol. 2003, 170, 5897–5911. [Google Scholar] [CrossRef]
- Poujol, F.; Monneret, G.; Pachot, A.; Textoris, J.; Venet, F. Altered T Lymphocyte Proliferation upon Lipopolysaccharide Challenge Ex Vivo. PLoS ONE 2015, 10, e0144375. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Ledderose, C.; Shen, Y.; Lee, A.H.; Shapiro, N.I.; Junger, W.G. Lipopolysaccharide suppresses T cells by generating extracellular ATP that impairs their mitochondrial function via P2Y11 receptors. J. Biol. Chem. 2019, 294, 6283–6293. [Google Scholar] [CrossRef]
- Tincati, C.; Bellistrì, G.M.; Ancona, G.; Merlini, E.; Monforte, A.D.; Marchetti, G. Role of In Vitro Stimulation with Lipopolysaccharide on T-Cell Activation in HIV-Infected Antiretroviral-Treated Patients. Clin. Dev. Immunol. 2012, 2012, 935425. [Google Scholar] [CrossRef]
- Goodier, M.R.; Londei, M. Lipopolysaccharide Stimulates the Proliferation of Human CD56+CD3− NK Cells: A Regulatory Role of Monocytes and IL-10. J. Immunol. 2000, 165, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, I.W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef]
- Amand, M.; Iserentant, G.; Poli, A.; Sleiman, M.; Fievez, V.; Sanchez, I.P.; Sauvageot, N.; Michel, T.; Aouali, N.; Janji, B.; et al. Human CD56dimCD16dim Cells as an Individualized Natural Killer Cell Subset. Front. Immunol. 2017, 8, 699. [Google Scholar] [CrossRef]
- Zimmer, J. CD56dimCD16dim Natural Killer (NK) Cells: The Forgotten Population. Hemasphere 2020, 4, e348. [Google Scholar] [CrossRef]
- Forconi, C.S.; Oduor, C.I.; Oluoch, P.; Ong’Echa, J.M.; Münz, C.; Bailey, J.A.; Moormann, A.M. A New Hope for CD56negCD16pos NK Cells as Unconventional Cytotoxic Mediators: An Adaptation to Chronic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 162. [Google Scholar] [CrossRef]
- Mavilio, D.; Lombardo, G.; Benjamin, J.; Kim, D.; Follman, D.; Marcenaro, E.; O’Shea, M.A.; Kinter, A.; Kovacs, C.; Moretta, A.; et al. Characterization of CD56-/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 2005, 102, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, R.S.; Read, S.A.; Schibeci, S.; Han, S.; Azardaryany, M.K.; van der Poorten, D.; Lin, R.; Yuen, L.; Lam, V.; Douglas, M.W.; et al. Expansion of dysfunctional CD56-CD16+ NK cells in chronic hepatitis B patients. Liver Int. 2021, 41, 969–981. [Google Scholar] [CrossRef]
- Vitale, M.; Della Chiesa, M.; Carlomagno, S.; Romagnani, C.; Thiel, A.; Moretta, L.; Moretta, A. The small subset of CD56brightCD16– natural killer cells is selectively responsible for both cell proliferation and interferon-γ production upon interaction with dendritic cells. Eur. J. Immunol. 2004, 34, 1715–1722. [Google Scholar] [CrossRef]
- Chidrawar, S.M.; Khan, N.; Chan, Y.L.T.; Nayak, L.; Moss, P.A. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006, 3, 10. [Google Scholar] [CrossRef]
- Caligiuri, M.A.; Murray, C.; Robertson, M.J.; Wang, E.; Cochran, K.; Cameron, C.; Schow, P.; Ross, M.E.; Klumpp, T.R.; Soiffer, R.J. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J. Clin. Investig. 1993, 91, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.; Zamai, L.; Rezzani, R.; Artico, M.; Peri, G.; Falconi, M.; Facchini, A.; Pelusi, G.; Vitale, M. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br. J. Haematol. 2001, 115, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Chikosi, S.-J.; Yanik, S.; Rupp, J.; Jungck, D.; Koch, A. A systemic defect in Toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L24–L39. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E. Cytokines and Cytokine Receptors. In The Immune Response; Elsevier: Amsterdam, The Netherlands, 2006; pp. 463–516. [Google Scholar] [CrossRef]
- Carson, W.E.; Giri, J.G.; Lindemann, M.J.; Linett, M.L.; Ahdieh, M.; Paxton, R.; Anderson, D.; Eisenmann, J.; Grabstein, K.; Caligiuri, M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994, 180, 1395–1403. [Google Scholar] [CrossRef]
- Gangemi, S.; Basile, G.; Monti, D.; Merendino, R.A.; Di Pasquale, G.; Bisignano, U.; Nicita-Mauro, V.; Franceschi, C. Age-Related Modifications in Circulating IL-15 Levels in Humans. Mediat. Inflamm. 2005, 2005, 245–247. [Google Scholar] [CrossRef]
- Cooley, S.; Xiao, F.; Pitt, M.; Gleason, M.; McCullar, V.; Bergemann, T.L.; McQueen, K.L.; Guethlein, L.A.; Parham, P.; Miller, J.S. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood 2007, 110, 578–586. [Google Scholar] [CrossRef]
- Dubois, S.; Conlon, K.C.; Müller, J.R.; Hsu-Albert, J.; Beltran, N.; Bryant, B.R.; Waldmann, T.A. IL15 Infusion of Cancer Patients Expands the Subpopulation of Cytotoxic CD56bright NK Cells and Increases NK-Cell Cytokine Release Capabilities. Cancer Immunol. Res. 2017, 5, 929–938. [Google Scholar] [CrossRef]
- Magrone, T.; Spagnoletta, A.; Salvatore, R.; Magrone, M.; Dentamaro, F.; Russo, M.A.; Difonzo, G.; Summo, C.; Caponio, F.; Jirillo, E. Olive Leaf Extracts Act as Modulators of the Human Immune Response. Endocr. Metab. Immune Disord. Drug Targets 2017, 18, 85–93. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Ammann, S.; Fuchs, S.; Martin-Martin, L.; Castro, C.N.; Spielberger, B.; Klemann, C.; Elling, R.; Heeg, M.; Speckmann, C.; Hainmann, I.; et al. Functional flow cytometry of monocytes for routine diagnosis of innate primary immunodeficiencies. J. Allergy Clin. Immunol. 2020, 145, 434–437.e4. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Barman, P.K.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.; James, C.; De Jong, A.; Blackmore, L.; Ma, Y.; Stagg, A.; Kelsell, D.; O’Dwyer, M.; Hutchins, R.; Alazawi, W. ADAM17-Mediated Reduction in CD14++CD16+ Monocytes ex vivo and Reduction in Intermediate Monocytes with Immune Paresis in Acute Pancreatitis and Acute Alcoholic Hepatitis. Front. Immunol. 2019, 10, 1902. [Google Scholar] [CrossRef]
- Zhou, L.; Ge, M.; Zhang, Y.; Wu, X.; Leng, M.; Gan, C.; Mou, Y.; Zhou, J.; Valencia, C.A.; Hao, Q.; et al. Centenarians Alleviate Inflammaging by Changing the Ratio and Secretory Phenotypes of T Helper 17 and Regulatory T Cells. Front. Pharmacol. 2022, 13, 877709. [Google Scholar] [CrossRef] [PubMed]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, Peripheral Blood Mononuclear Cells, and THP-1 Cells Exhibit Different Cytokine Expression Patterns following Stimulation with Lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Chaiwut, R.; Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef]
- Agarwal, S.; Piesco, N.; Johns, L.; Riccelli, A. Differential Expression of IL-1β, TNF-α, IL-6, and IL-8 in Human Monocytes in Response to Lipopolysaccharides from Different Microbes. J. Dent. Res. 1995, 74, 1057–1065. [Google Scholar] [CrossRef]
- Miles, E.A.; Zoubouli, P.; Calder, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef]
- Martin, M.E.; Millan-Linares, M.C.; Naranjo, M.C.; Toscano, R.; Abia, R.; Muriana, F.J.G.; Bermudez, B.; la Paz, S.M. Minor compounds from virgin olive oil attenuate LPS-induced inflammation via visfatin-related gene modulation on primary human monocytes. J. Food Biochem. 2019, 43, e12941. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Zhuang, H.-Z.; Ji, X.-J.; Dong, L.; Duan, M.-L. Hydroxytyrosol suppresses LPS-induced intrahepatic inflammatory responses via inhibition of ERK signaling pathway activation in acute liver injury. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6455–6462. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Vismara, L.; Lucchi, L.; Viviani, B.; Govoni, S.; Galli, C.L.; Marinovich, M.; Racchi, M. High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J. Leukoc. Biol. 2006, 80, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Almanan, M.; Raynor, J.; Ogunsulire, I.; Malyshkina, A.; Mukherjee, S.; Hummel, S.A.; Ingram, J.T.; Saini, A.; Xie, M.M.; Alenghat, T.; et al. IL-10–producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci. Adv. 2020, 6, eabb0806. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fulop, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.Y.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V.; Iacovino, S. Delivery Systems for Hydroxytyrosol Supplementation: State of the Art. Colloids Interfaces 2020, 4, 25. [Google Scholar] [CrossRef]
- Nardi, M.; Brocchini, S.; Somavarapu, S.; Procopio, A. Hydroxytyrosol oleate: A promising neuroprotective nanocarrier delivery system of oleuropein and derivatives. Int. J. Pharm. 2023, 631, 122498. [Google Scholar] [CrossRef] [PubMed]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef]
- Zygouri, P.; Athinodorou, A.M.; Spyrou, K.; Simos, Y.V.; Subrati, M.; Asimakopoulos, G.; Vasilopoulos, K.C.; Vezyraki, P.; Peschos, D.; Tsamis, K.; et al. Oxidized-Multiwalled Carbon Nanotubes as Non-Toxic Nanocarriers for Hydroxytyrosol Delivery in Cells. Nanomaterials 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Gambacorta, A.; Tofani, D.; Bernini, R.; Migliorini, A. High-Yielding Preparation of a Stable Precursor of Hydroxytyrosol by Total Synthesis and from the Natural Glycoside Oleuropein. J. Agric. Food Chem. 2007, 55, 3386–3391. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Akter, S.; Addepalli, R.; Netzel, M.E.; Tinggi, U.; Fletcher, M.T.; Sultanbawa, Y.; Osborne, S.A. Antioxidant-Rich Extracts of Terminalia ferdinandiana Interfere with Estimation of Cell Viability. Antioxidants 2019, 8, 191. [Google Scholar] [CrossRef]
- Lemieux, J.; Jobin, C.; Simard, C.; Néron, S. A global look into human T cell subsets before and after cryopreservation using multiparametric flow cytometry and two-dimensional visualization analysis. J. Immunol. Methods 2016, 434, 73–82. [Google Scholar] [CrossRef]
- Hønge, B.L.; Petersen, M.S.; Olesen, R.; Møller, B.K.; Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLoS ONE 2017, 12, e0187440. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, C.; Jia, G.; Liu, Y.; Na Wang, N.; Yang, F.; Su, R.; Shang, Y.; Han, Y. Comprehensive evaluation of the effects of long-term cryopreservation on peripheral blood mononuclear cells using flow cytometry. BMC Immunol. 2022, 23, 30. [Google Scholar] [CrossRef] [PubMed]

| Adult | Senior | p | |||||
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female | Male | Total | Female | Male | Total | ||
| 13 | 10 | 23 | 7 | 8 | 15 | n.s. | |
| Age | |||||||
| Median | Minimum | Maximum | Median | Minimum | Maximum | ||
| 25 | 19 | 59 | 67 | 65 | 83 | <0.0005 | |
| WBC × 103/μL | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 7.08 | 1.56 | 6.40–7.75 | 7.27 | 1.34 | 6.53–8.01 | n.s. | |
| Lymphocytes × 103/μL | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 2.41 | 0.47 | 2.20–2.61 | 2.27 | 0.37 | 2.06–2.47 | n.s. | |
| Monocytes × 103/μL | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 0.53 | 0.13 | 0.47–0.59 | 0.56 | 0.15 | 0.48–0.64 | n.s. | |
| Neutrophils × 103/μL | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 3.87 | 1.30 | 3.31–4.44 | 4.22 | 1.14 | 3.59–4.86 | n.s. | |
| %Lymphocytes | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 34.95 | 6.38 | 32.19–37.71 | 31.84 | 6.27 | 28.37–35.31 | n.s. | |
| %Monocytes | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 7.58 | 1.13 | 7.09–8.07 | 7.77 | 1.98 | 6.67–8.87 | n.s. | |
| %Neutrophils | |||||||
| Mean | S.D. | 95% C. I. | Mean | S.D. | 95% C. I. | ||
| 54.13 | 7.54 | 50.88–57.39 | 57.25 | 7.01 | 53.36–61.13 | n.s. | |
| Serum IL-6 | |||||||
| Median | Minimum | Maximum | Median | Minimum | Maximum | ||
| 1.5 | 1.5 | 3.41 | 2.52 | 1.5 | 13.6 | 0.002 | |
| Adult (n = 4) | Senior (n = 4) | Student’s t-Test | ||
|---|---|---|---|---|
| Percentage of live cells | ||||
| Untreated | 86.25 ± 3.59 | 86 ± 3.65 | n.s. | |
| ETOH | 87 ± 6.06 | 88.5 ± 7.05 | n.s. | |
| LPS | 90 ± 8.12 | 77.5 ± 14.2 | n.s. | |
| OLE1 | 83.75 ± 4.86 | 84.75 ± 1.89 | n.s. | |
| OLE1 + LPS | 86.5 ± 6.56 | 90.75 ± 1.71 | n.s. | |
| OLE5 | 81.75 ± 4.19 | 83 ± 6.98 | n.s. | |
| OLE5 + LPS | 77 ± 13.44 | 83.75 ± 3.69 | n.s. | |
| OLE10 | 82.5 ± 7.19 | 82.5 ± 5.07 | n.s. | |
| OLE10 + LPS | 81.5 ± 6.76 | 84 ± 6.68 | n.s. | |
| HT1 | 90.75 ± 3.78 | 77.5 ± 9.47 | 0.041 | |
| HT1 + LPS | 78.25 ± 6.24 | 76.5 ± 10.72 | n.s. | |
| HT5 | 78.25 ± 6.4 | 76.25 ± 20.27 | n.s. | |
| HT5 + LPS | 87.5 ± 9.68 | 81.5 ± 10.15 | n.s. | |
| HT10 | 83.25 ± 7.27 | 78 ± 8.04 | n.s. | |
| HT10 + LPS | 78.5 ± 11.71 | 76.75 ± 7.85 | n.s. | |
| Repeated measures ANOVA | n.s. | n.s. | ||
| Normalized cell number | ||||
| ETOH | 1.04 ± 0.27 | 1.06 ± 0.37 | n.s. | |
| LPS | 0.85 ± 0.31 | 0.77 ± 0.51 | n.s. | |
| OLE1 | 0.97 ± 0.24 | 0.66 ± 0.41 | n.s. | |
| OLE1 + LPS | 0.92 ± 0.48 | 0.98 ± 0.54 | n.s. | |
| OLE5 | 0.77 ± 0.16 | 1.08 ± 0.46 | n.s. | |
| OLE5 + LPS | 1.21 ± 0.37 | 1.13 ± 0.79 | n.s. | |
| OLE10 | 1.09 ± 0.39 | 0.9 ± 0.23 | n.s. | |
| OLE10 + LPS | 1.06 ± 0.16 | 0.85 ± 0.51 | n.s. | |
| HT1 | 1.12 ± 0.65 | 1.04 ± 0.73 | n.s. | |
| HT1 + LPS | 1.04 ± 0.29 | 1.26 ± 0.58 | n.s. | |
| HT5 | 0.96 ± 0.24 | 1.39 ± 0.89 | n.s. | |
| HT5 + LPS | 1.26 ± 0.42 | 1 ± 0.61 | n.s. | |
| HT10 | 1.35 ± 0.4 | 1.04 ± 0.28 | n.s. | |
| HT10 + LPS | 1.08 ± 0.6 | 1.3 ± 0.7 | n.s. | |
| Repeated measures ANOVA | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pojero, F.; Gervasi, F.; Fiore, S.D.; Aiello, A.; Bonacci, S.; Caldarella, R.; Attanzio, A.; Candore, G.; Caruso, C.; Ligotti, M.E.; et al. Anti-Inflammatory Effects of Nutritionally Relevant Concentrations of Oleuropein and Hydroxytyrosol on Peripheral Blood Mononuclear Cells: An Age-Related Analysis. Int. J. Mol. Sci. 2023, 24, 11029. https://doi.org/10.3390/ijms241311029
Pojero F, Gervasi F, Fiore SD, Aiello A, Bonacci S, Caldarella R, Attanzio A, Candore G, Caruso C, Ligotti ME, et al. Anti-Inflammatory Effects of Nutritionally Relevant Concentrations of Oleuropein and Hydroxytyrosol on Peripheral Blood Mononuclear Cells: An Age-Related Analysis. International Journal of Molecular Sciences. 2023; 24(13):11029. https://doi.org/10.3390/ijms241311029
Chicago/Turabian StylePojero, Fanny, Francesco Gervasi, Salvatore Davide Fiore, Anna Aiello, Sonia Bonacci, Rosalia Caldarella, Alessandro Attanzio, Giuseppina Candore, Calogero Caruso, Mattia Emanuela Ligotti, and et al. 2023. "Anti-Inflammatory Effects of Nutritionally Relevant Concentrations of Oleuropein and Hydroxytyrosol on Peripheral Blood Mononuclear Cells: An Age-Related Analysis" International Journal of Molecular Sciences 24, no. 13: 11029. https://doi.org/10.3390/ijms241311029
APA StylePojero, F., Gervasi, F., Fiore, S. D., Aiello, A., Bonacci, S., Caldarella, R., Attanzio, A., Candore, G., Caruso, C., Ligotti, M. E., Procopio, A., Restivo, I., Tesoriere, L., Allegra, M., & Accardi, G. (2023). Anti-Inflammatory Effects of Nutritionally Relevant Concentrations of Oleuropein and Hydroxytyrosol on Peripheral Blood Mononuclear Cells: An Age-Related Analysis. International Journal of Molecular Sciences, 24(13), 11029. https://doi.org/10.3390/ijms241311029















