Developmental Neurotoxicity of Trichlorfon in Zebrafish Larvae
Abstract
:1. Introduction
2. Results
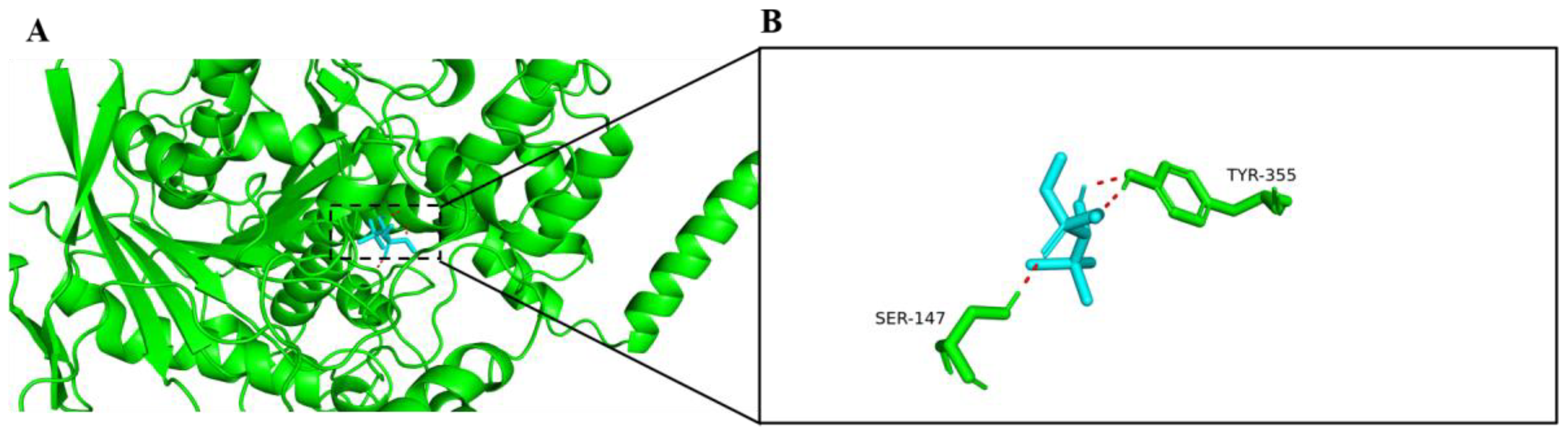
2.1. Molecular Docking with AChE
2.2. Developmental Parameters
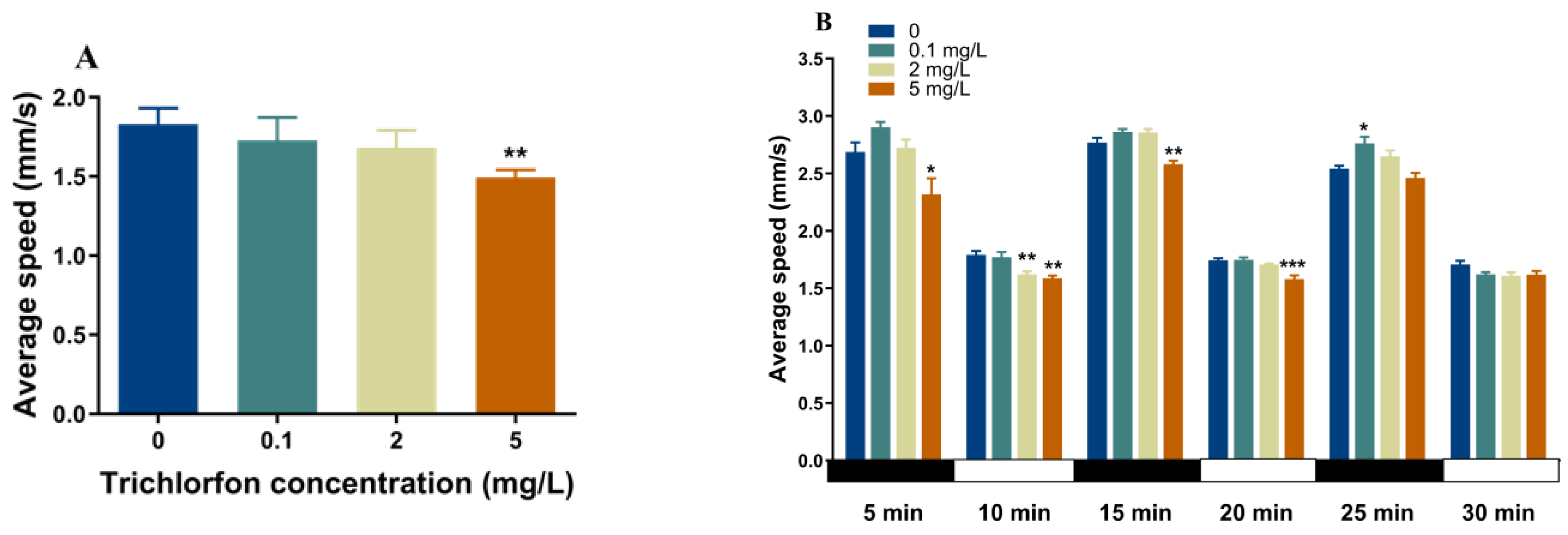
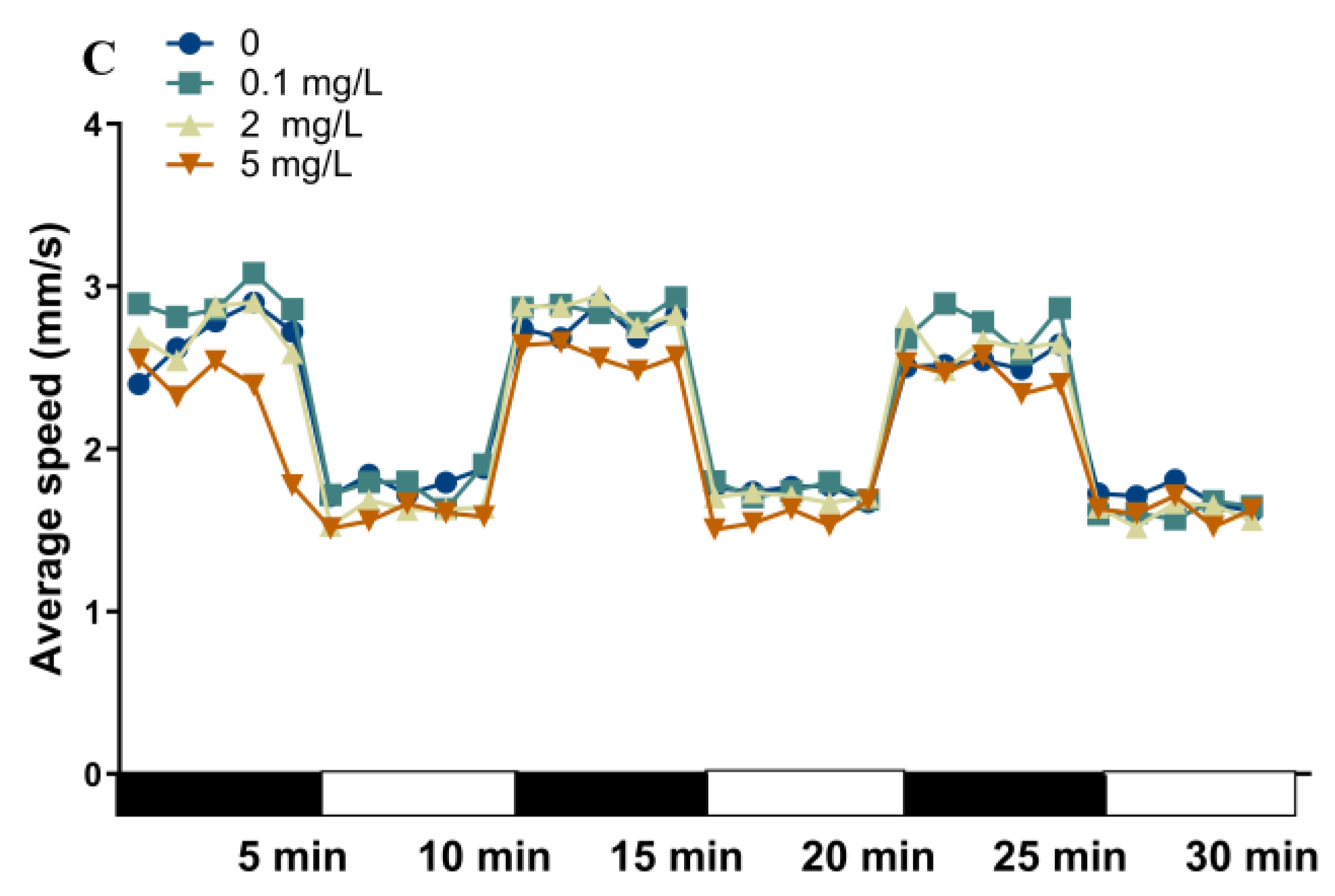
2.3. Locomotor Behavior of Zebrafish Larvae
2.4. Gene Expression
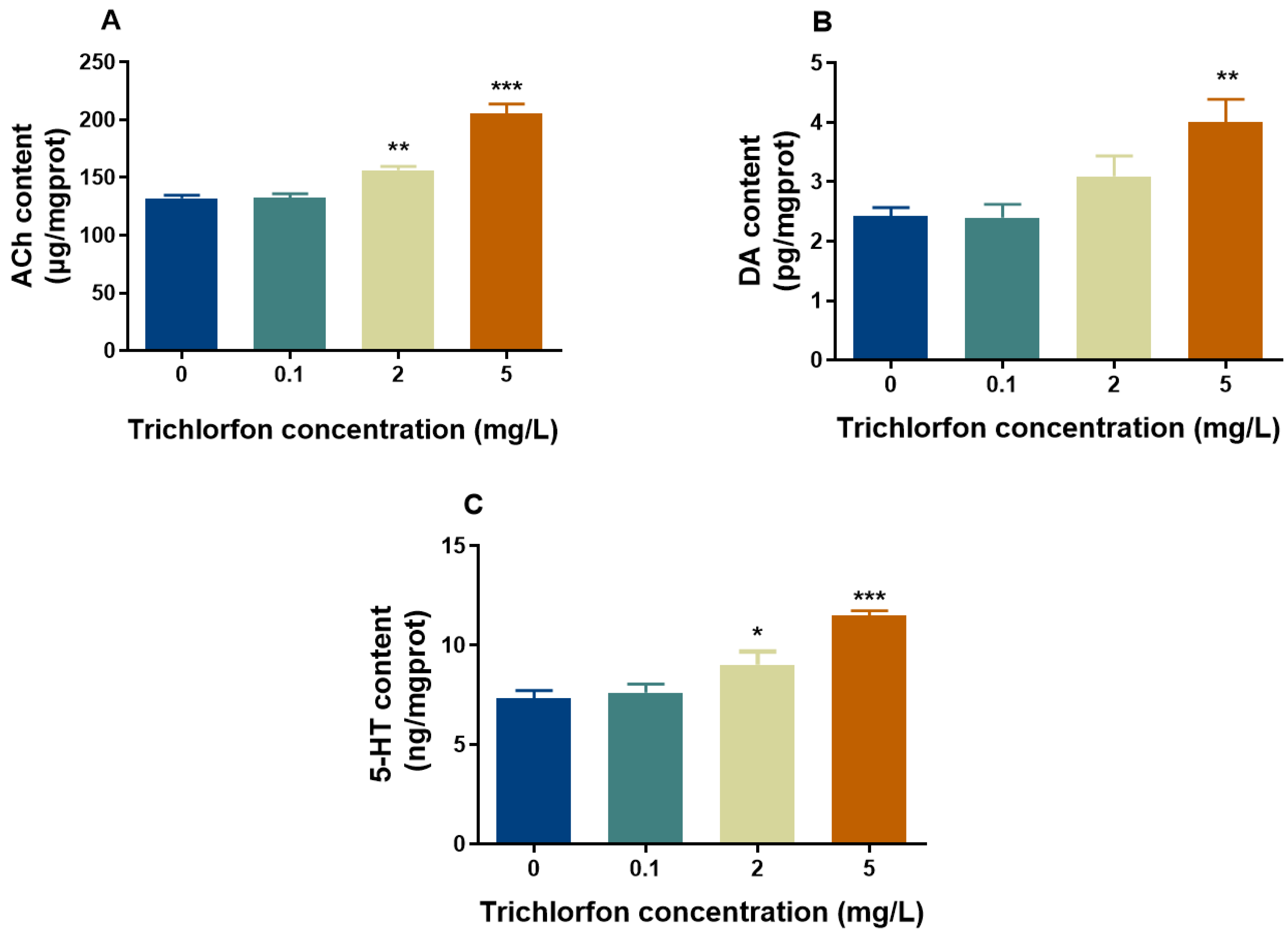
2.5. Neurotransmitter Content
2.6. AChE Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Zebrafish Culture and Embryo Exposure
4.3. Molecular Docking
4.4. Locomotor Behavior
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Neurotransmitter Measurements
4.7. Acetylcholinesterase Activity Measurements
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, R.B.; Paraiba, L.C.; Ceccarelli, P.S.; Tornisielo, V.L. Bioconcentration of trichlorfon insecticide in pacu (Piaractus mesopotamicus). Chemosphere 2006, 64, 56–62. [Google Scholar] [CrossRef]
- Zhang, X.P.; Li, W.X.; Ai, T.S.; Zou, H.; Wu, S.G.; Wang, G.T. The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea). Aquaculture 2014, 420, 302–307. [Google Scholar] [CrossRef]
- Dos Santos Costa, M.; da Silva, H.C.M.; Soares, S.C.; Favarato, R.M.; Feldbrg, E.; Gomes, A.L.S.; Artoni, R.F.; Matoso, D.A. A perspective of molecular cytogenomics, toxicology, and epigenetics for the increase of heterochromatic regions and retrotransposable elements in Tambaqui (Colossoma macropomum) exposed to the parasiticide trichlorfon. Animals 2022, 12, 1945. [Google Scholar] [CrossRef]
- Fernandes, L.S.; Emerick, G.L.; Ferreira, R.S.; dos Santos, N.A.G.; dos Santos, A.C. High concentration of trichlorfon (1 mM) disrupts axonal cytoskeleton and decreases the expression of plasticity-related proteins in SH-SY5Y cells. Toxicol. In Vitro 2017, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.L.; Wang, X.F.; Feng, J.C.; Su, X.; Liang, J.P.; Li, H.; Zhang, J.X. Impact of chronic exposure to trichlorfon on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in common carp (Cyprinus carpio L.). Environ. Pollut. 2020, 259, 113846. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Zanella, R.; Prestes, O.D.; da Matos, A.F.I.M.; da Silva, A.S.; Baldisserotto, B.; Gris, A.; Mendes, R.E. Disturbance of energetic homeostasis and oxidative damage provoked by trichlorfon as relevant toxicological mechanisms using silver catfish as experimental model. Chem. Biol. Interact. 2019, 299, 94–100. [Google Scholar] [CrossRef]
- Chang, C.; Lee, P.; Liu, C.; Cheng, W. Trichlorfon, an organophosphorus insecticide, depresses the immune responses and resistance to Lactococcus garvieae of the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish. Immunol. 2006, 20, 574–585. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotox. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef]
- Chang, C.; Rahmawaty, A.; Chang, Z. Molecular and immunological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to the organophosphorus insecticide, trichlorfon. Aquat. Toxicol. 2013, 130, 18–26. [Google Scholar] [CrossRef]
- Woo, S.J.; Chung, J.K. Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp. Biochem. Physiol. C 2020, 229, 108698. [Google Scholar] [CrossRef]
- Wang, X.; Chang, X.; Zhao, L.; Feng, J.; Li, H.; Liang, J. Trichlorfon exposure in common carp (Cyprinus carpio L.) leads to oxidative stress, neurotoxicity, and immune responses. Aquaculture 2022, 548, 737681. [Google Scholar] [CrossRef]
- Xu, W.N.; Liu, W.B.; Shao, X.P.; Jiang, G.Z.; Li, X.F. Effect of trichlorfon on hepatic lipid accumulation in crucian carp Carassius auratus gibelio. Aquat. Anim. Health 2012, 24, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekara, H.U.; Pathiratne, A. Influence of low concentrations of Trichlorfon on haematological parameters and brain acetylcholinesterase activity in common carp, Cyprinus carpio L. Aquacult. Res. 2005, 36, 144–149. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; Pala, A.; Silici, S.; Saglam, N. Trichlorfon-induced haematological and biochemical changes in Cyprinus carpio: Ameliorative effect of propolis. Dis. Aquat. Org. 2015, 114, 209–216. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, N.Y.; Kim, S.H.; Ahn, S.J.; Seo, J.S.; Jung, S.H.; Chung, J.K. Toxicological effects of trichlorfon on hematological and biochemical parameters in Cyprinus carpio L. Following thermal stress. Comp. Biochem. Physiol. C 2018, 209, 18–27. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Zanella, R.; Prestes, O.D.; da Silva, A.S.; Baldisserotto, B. Organophosphate pesticide trichlorfon induced neurotoxic effects in freshwater silver catfish Rhamdia quelen via disruption of blood–brain barrier: Implications on oxidative status, cell viability and brain neurotransmitters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 218, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.; Souza, C.F.; Zanella, R.; Prestes, O.D.; Meinhart, A.D.; Da Silva, A.S.; Baldisserotto, B. Behavioral impairment and neurotoxic responses of silver catfish Rhamdia quelen exposed to organophosphate pesticide trichlorfon: Protective effects of diet containing rutin. Comp. Biochem. Physiol. C 2021, 239, 108871. [Google Scholar] [CrossRef]
- Fu, H.; Xia, Y.; Chen, Y.; Xu, T.; Xu, L.; Guo, Z.; Xu, H.; Xie, H.; Zhao, B. Acetylcholinesterase is a potential biomarker for a broad spectrum of organic environmental pollutants. Environ. Sci. Technol. 2018, 52, 8065–8074. [Google Scholar] [CrossRef]
- Tilton, F.A.; Bammler, T.K.; Gallagher, E.P. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.K.; Vanparys, C.; De Boeck, G.; Kestemont, P.; Wang, N.; Nguyen, T.P.; Scippo, M.L.; De Coen, W.; Robbens, J. Expression characteristics of potential biomarker genes in Tra catfish, Pangasianodon hypophthalmus, exposed to trichlorfon. Comp. Biochem. Physiol. Part D Genom. Proteom. 2010, 5, 207–216. [Google Scholar] [CrossRef]
- Boix, J.; Cauli, O. Alteration of serotonin system by polychlorinated biphenylsexposure. Neurochem. Int. 2012, 60, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Ankarberg, E.; Viberg, H.; Fredriksson, A. The developing cholinergic system as target for environmental toxicants, nicotine and polychlorinated biphenyls (PCBs): Implications for neurotoxicological processes in mice. Neurotox. Res. 2001, 3, 37–51. [Google Scholar] [CrossRef]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; MacPhail, R.C.; Padilla, S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behavior. 2013, 103, 792–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufi, S.; Leonards, P.L.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in neurotransmitter profiles during early zebrafish (Danio rerio) development and after pesticide exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, M.; Shi, F.; Yang, L.; Guo, Y.; Feng, C.; Liu, J.; Zhou, B. Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquat. Toxicol. 2018, 203, 80–87. [Google Scholar] [CrossRef]
- Heyer, D.B.; Meredith, R.M. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 2017, 58, 23–41. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Coelho, S.; Oliveira, R.; Pereira, S.; Musso, C.; Domingues, I.; Bhujel, R.C.; Soares, A.M.; Nogueira, A.J. Assessing lethal and sublethal effects of trichlorfon on different trophic levels. Aquat. Toxicol. 2011, 103, 191–198. [Google Scholar] [CrossRef]
- Shi, Q.; Guo, W.; Shen, Q.; Han, J.; Lei, L.; Chen, L.; Yang, L.; Feng, C.; Zhou, B. In vitro biolayer interferometry analysis of acetylcholinesterase as a potential target of aryl-organophosphorus flame-retardants. J. Hazard. Mater. 2021, 409, 124999. [Google Scholar] [CrossRef] [PubMed]
- Altenhofen, S.; Nabinger, D.D.; Bitencourt, P.E.R.; Bonan, C.D. Dichlorvos alters morphology and behavior in zebrafish (Danio rerio) larvae. Environ. Pollut. 2019, 245, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, K.; Huang, C.; Yu, L.; Zhu, B.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2012, 46, 9727–9734. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; McManus, M.; Kumar, N.; Awoyemi, O.; Crago, J. Comparative analyses of the neurobehavioral, molecular, and enzymatic effects of organophosphates on embryo-larval zebrafish (Danio rerio). Neurotoxicol. Teratol. 2019, 73, 67–75. [Google Scholar] [CrossRef]
- Serafini, S.; Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Segat, J.C.; Baretta, D.; Zanella, R.; da Silva, A.S. Fish exposed to water contaminated with eprinomectin show inhibition of the activities of AChE and Na+/K+-ATPase in the brain, and changes in natural behavior. Chemosphere 2019, 223, 124–130. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Lei, L.; Guo, Y.; Yang, L.; Han, J.; Zhou, B. Bioconcentration and developmental neurotoxicity of novel brominated flame retardants, hexabromobenzene and pentabromobenzene in zebrafish. Environ. Pollut. 2021, 268, 115895. [Google Scholar] [CrossRef]
- Velki, M.; Meyer-Alert, H.; Seiler, T.B.; Hollert, H. Enzymatic activity and gene expression changes in zebrafish embryos and larvae exposed to pesticides diazinon and diuron. Aquat. Toxicol. 2017, 193, 187–200. [Google Scholar] [CrossRef]
- Yen, J.; Donerly, S.; Levin, E.D.; Linney, E.A. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol. Teratol. 2011, 33, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, A.T.B.; de Assis, H.C.S.; Boeger, W. The effect of trichlorfon on acetylcholinesterase activity and histopathology of cultivated fish Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2007, 68, 57–62. [Google Scholar] [CrossRef]
- Allebrandt, K.V.; Rajesh, V.; Layer, P.G. Expression of acetylcholinesterase (AChE) and aryl acylamidase (AAA) during early zebrafish embryogenesis. Chem. Biol. Interact. 2005, 157–158, 353–355. [Google Scholar] [CrossRef]
- Behra, M.; Cousin, X.; Bertrand, C.; Vonesch, J.L.; Biellmann, D.; Chatonnet, A.; Strahle, U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Persp. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, B.; Sun, Q.; Zhang, Y.; Ren, M.; Zhang, X.; Li, A.; Yuan, J.; Madisen, L.; Luo, Q.; et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangas, I.; Estevez, J.; Vilanova, E.; França, T.C. New insights on molecular interactions of organophosphorus pesticides with esterases. Toxicology 2017, 376, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I.; Burket, J.A.; Benson, A.D.; Urbano, M.R. The 15q13.3 deletion syndrome: Defificient alpha (7)-containing nicotinic acetylcholine receptor mediated neurotransmission in the pathogenesis of neurodevelopmental disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 64, 109–117. [Google Scholar] [CrossRef]
- Iversen, S.D.; Iversen, L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2017, 30, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Faria, M.; Bedrossiantz, J.; Ramírez, J.R.R.; Mayol, M.; García, G.H.; Bellot, M.; Prats, E.; Garcia-Reyero, N.; Gómez-Canela, C.; Gómez-Oliván, L.M.; et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ. Int. 2021, 146, 106253. [Google Scholar] [CrossRef]
- Oliveri, A.N.; Ortiz, E.; Levin, E.D. Developmental exposure to an organophosphate flame retardant alters later behavioral responses to dopamine antagonism in zebrafish larvae. Neurotoxicol. Teratol. 2018, 67, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lai, N.L.; Wang, X.; Guo, Y.; Lam, P.K.; Lam, J.C.; Zhou, B. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2015, 49, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Boehmler, W.; Carr, T.; Thisse, C.; Thisse, B.; Canfield, V.A.; Levenson, R. D4 Dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007, 6, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, L.; Kong, X.; Liu, K.; Wu, H. Different exercise time on 5-HT and anxiety-like behavior in the rat with vascular dementia. Am. J. Alzheimers Dis. Other Demen. 2022, 37, 15333175221082743. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.G.; Guimarães, F.S.; De Andrade, T.G.; Deakin, J.F. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Wu, Y.; Huang, C.; Wang, Q.; Han, J.; Guo, Y.; Shi, X.; Zhou, B. The developmental neurotoxicity of polybrominated diphenyl ethers: Effect of DE-71 on dopamine in zebrafish larvae. Environ. Toxicol. Chem. 2015, 34, 1119–1126. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, G.; Sandtner, W. Serotonin transport in the 21st century. J. Gen. Physiol. 2019, 151, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Takai, R.; Yoshioka, H.; Shirabe, K. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J. Exp. Med. 2006, 208, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Bengel, D.; Murphy, D.L.; Andrews, A.M.; Wichems, C.H.; Feltner, D.; Heils, A.; Mossner, R.; Westphal, H.; Lesch, K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998, 53, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Olivier, J.D.A.; Van Der Hart, M.G.C.; Van Swelm, R.P.L.; Dederen, P.J.; Homberg, J.R.; Cremers, T.; Deen, P.M.T.; Cuppen, E.; Cools, A.R.; Ellenbroek, B.A. A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience 2008, 152, 573–584. [Google Scholar] [CrossRef]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. WIREs Membr. Transp. Signal 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Norton, W.H.; Folchert, A.; Bally Cuif, L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp. Neurol. 2008, 511, 521–542. [Google Scholar] [CrossRef]
- Brosamle, C.; Halpern, M. Characterization of myelination in the developing zebrafish. Glia 2002, 39, 47–57. [Google Scholar] [CrossRef]
- Muller, F.; Chang, B.; Albert, S.; Fischer, N.; Tora, L.; Strahle, U. Intronic enhancers control expression of zebrafish sonic hedgehog in floor plate and notochord. Development 1999, 126, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Costa, S.; Pestarino, M.; Candiani, S. Differential expressions of synapsin genes during early zebrafish development. Neuroscience 2014, 280, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.T.; Porton, B.; Czernik, A.J.; Feng, J.; Yiu, G.; Haring, M.; Benfenati, F.; Greengard, P. A third member of the synapsin gene family. Proc. Natl. Acad. Sci. USA 1988, 95, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gamby, C.; Waage, M.C.; Allen, R.G.; Baizer, L. Analysis of the role of calmodulin binding and sequestration in neuromodulin (GAP-43) function. J. Biol. Chem. 1996, 271, 26698–26705. [Google Scholar] [CrossRef] [Green Version]
- Alm, H.; Kultima, K.; Scholz, B.; Nilsson, A.; Andren, P.E. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology 2008, 29, 628–637. [Google Scholar] [CrossRef]
- Lee, I.; Eriksson, P.; Fredriksson, A.; Buratovic, S.; Viberg, H. Developmental neurotoxic effects of two pesticides: Behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol. Appl. Pharmacol. 2015, 288, 429–438. [Google Scholar] [CrossRef]


| Trichlorfon (mg/L) | Survival Rate (%) | Hatching Rate (%) | Malformation Rate (%) | Heart Rate (Beats/min) | Body Length (mm) | Body Weight (mg/Larvae) |
|---|---|---|---|---|---|---|
| 0 | 90.31 ± 0.79 | 90.31 ± 0.79 | 2.83 ± 0.56 | 170.20 ± 2.59 | 3.93 ± 0.11 | 0.56 ± 0.01 |
| 0.1 | 89.56 ± 0.67 | 88.75 ± 0.88 | 3.28 ± 0.42 | 169.00 ± 1.98 | 3.84 ± 1.63 | 0.56 ± 0.01 |
| 2 | 88.13 ± 0.36 | 88.13 ± 0.36 | 6.28 ± 0.73 * | 164.80 ± 1.45 | 3.72 ± 0.12 | 0.55 ± 0.01 |
| 5 | 84.69 ± 1.39 ** | 83.75 ± 0.88 *** | 21.29 ± 1.37 *** | 156.3 ± 1.37 *** | 3.53 ± 0.13 *** | 0.50 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Yang, H.; Chen, Y.; Zheng, N.; Li, X.; Wang, X.; Ding, W.; Zhang, B. Developmental Neurotoxicity of Trichlorfon in Zebrafish Larvae. Int. J. Mol. Sci. 2023, 24, 11099. https://doi.org/10.3390/ijms241311099
Shi Q, Yang H, Chen Y, Zheng N, Li X, Wang X, Ding W, Zhang B. Developmental Neurotoxicity of Trichlorfon in Zebrafish Larvae. International Journal of Molecular Sciences. 2023; 24(13):11099. https://doi.org/10.3390/ijms241311099
Chicago/Turabian StyleShi, Qipeng, Huaran Yang, Yangli Chen, Na Zheng, Xiaoyu Li, Xianfeng Wang, Weikai Ding, and Bangjun Zhang. 2023. "Developmental Neurotoxicity of Trichlorfon in Zebrafish Larvae" International Journal of Molecular Sciences 24, no. 13: 11099. https://doi.org/10.3390/ijms241311099
APA StyleShi, Q., Yang, H., Chen, Y., Zheng, N., Li, X., Wang, X., Ding, W., & Zhang, B. (2023). Developmental Neurotoxicity of Trichlorfon in Zebrafish Larvae. International Journal of Molecular Sciences, 24(13), 11099. https://doi.org/10.3390/ijms241311099






