rAAV TGF-β and FGF-2 Overexpression via pNaSS-Grafted PCL Films Stimulates the Reparative Activities of Human ACL Fibroblasts
Abstract
:1. Introduction
2. Results
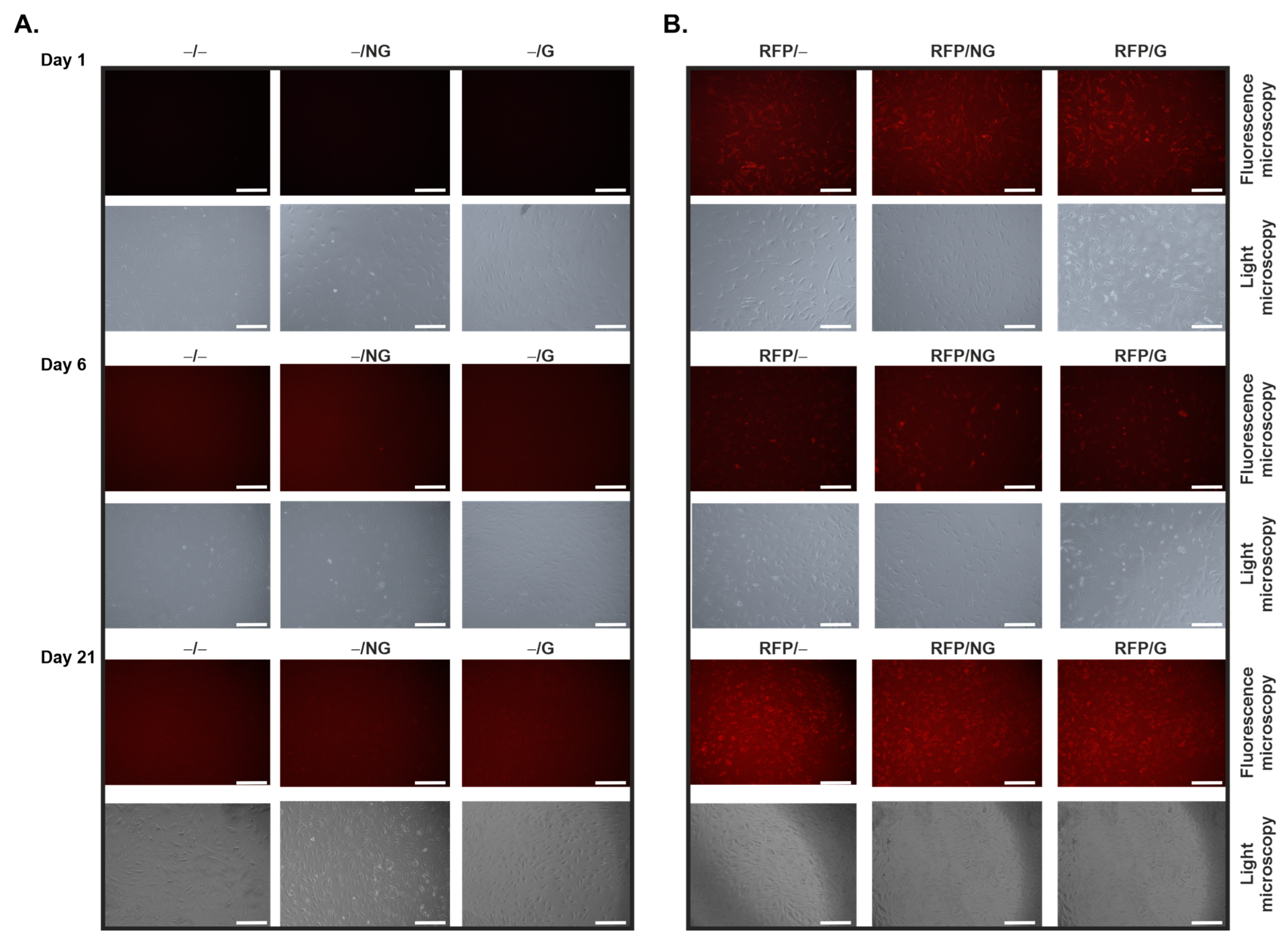
2.1. Effective, Sustained rAAV-Mediated Gene Expression in hACL Fibroblasts via PCL Film-Guided Vector Delivery
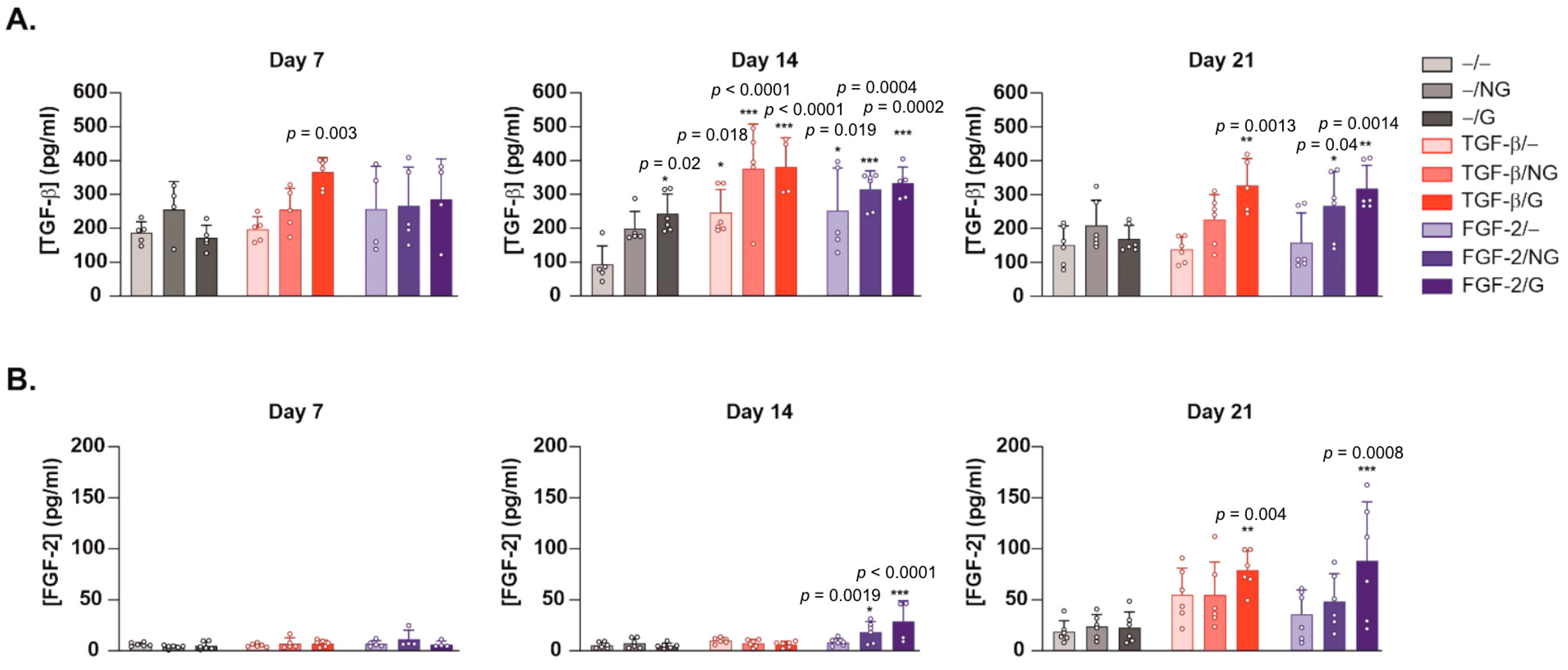
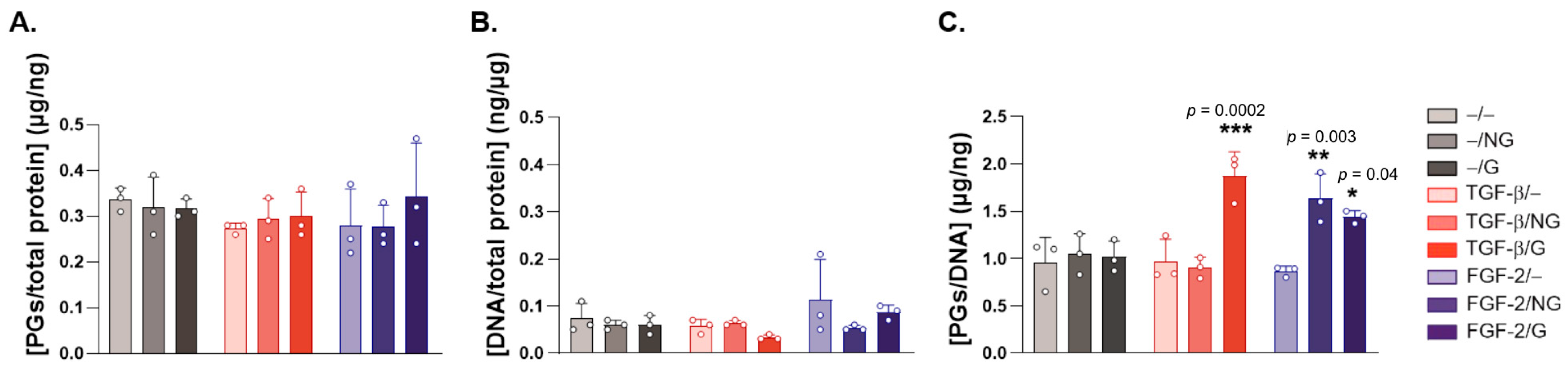
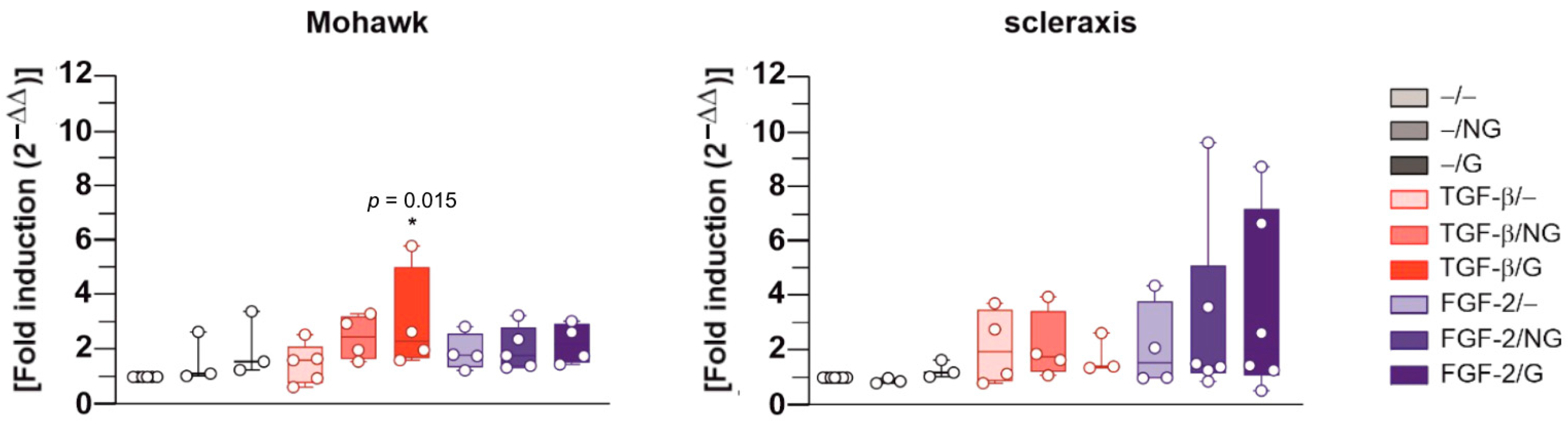
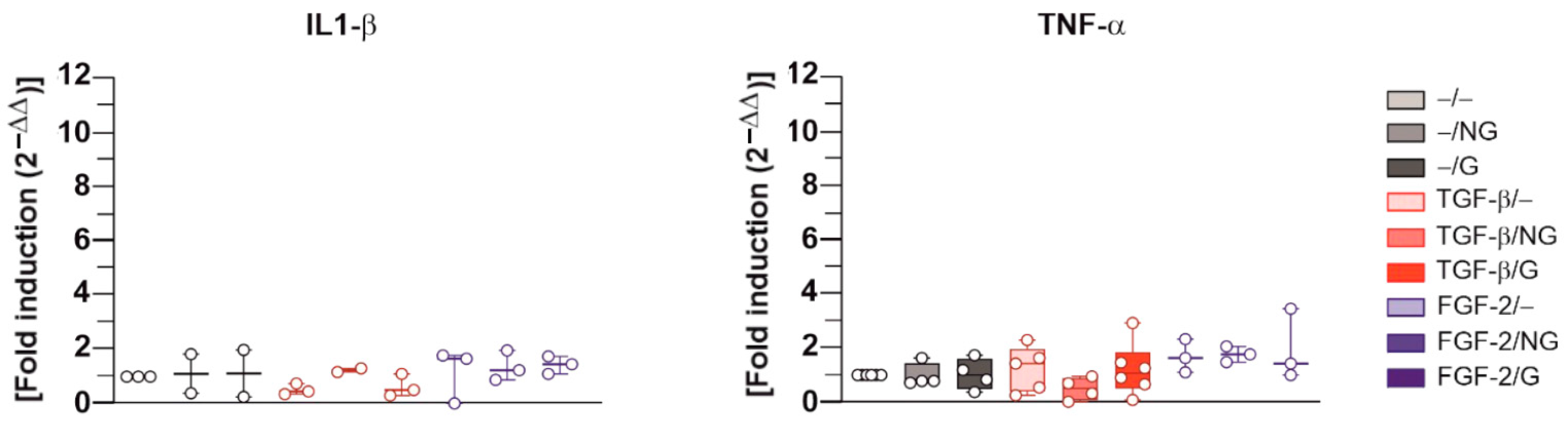
2.2. Effects of rAAV-Mediated TGF-β and FGF-2 Overexpression via PCL Film-Guided Vector Delivery on the Biological Activities of hACL Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation and Culture of Primary Human Anterior Cruciate Ligament (hACL) Fibroblasts
4.3. rAAV Vectors
4.4. Poly(ε-Caprolactone) Films
4.5. rAAV Immobilization on PCL Films
4.6. rAAV-Mediated Gene Transfer
4.7. Detection of Transgene Expression
4.8. Histomorphometric Analysis
4.9. Biological Analyses
4.10. Real-Time RT-PCR Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musahl, V.; Engler, I.D.; Nazzal, E.M.; Dalton, J.F.; Lucidi, G.A.; Hughes, J.D.; Zaffagnini, S.; Della Villa, F.; Irrgang, J.J.; Fu, F.H.; et al. Current trends in the anterior cruciate ligament part II: Evaluation, surgical technique, prevention, and rehabilitation. Knee Surg. Sports Traumatol. Arthrosc. 2022, 302, 34–51. [Google Scholar] [CrossRef]
- Musahl, V.; Karlsson, J. Anterior cruciate ligament tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Kiapour, A.M.; Murray, M.M. Basic science of anterior cruciate ligament injury and repair. Bone Jt. Res. 2014, 3, 20–31. [Google Scholar] [CrossRef]
- Werner, D.M.; Golightly, Y.M.; Tao, M.; Post, A.; Wellsandt, E. Environmental risk factors for osteoarthritis: The impact on individuals with knee joint injury. Rheum. Dis. Clin. N. Am. 2022, 48, 907–930. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P. Anatomy of the anterior cruciate ligament. Clin. Orthop. Relat. Res. 1983, 172, 19–25. [Google Scholar] [CrossRef]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.R.; Hewett, T.E. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am. J. Sports Med. 2014, 42, 1567–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerich, T.G.; Fu, F.H.; Robbins, P.D.; Evans, C.H. Prospects for gene therapy in sports medicine. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Huard, J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Amini, M.; Venkatesan, J.K.; Liu, W.; Leroux, A.; Nguyen, T.N.; Madry, H.; Migonney, V.; Cucchiarini, M. Advanced gene therapy strategies for the repair of ACL injuries. Int. J. Mol. Sci. 2022, 23, 14467–14499. [Google Scholar] [CrossRef]
- Pascher, A.; Steinert, A.F.; Palmer, G.D.; Betz, O.; Gouze, J.N.; Gouze, E.; Pilapil, C.; Ghivizzani, S.C.; Evans, C.H.; Murray, M.M. Enhanced repair of the anterior cruciate ligament by in situ gene transfer: Evaluation in an in vitro model. Mol. Ther. 2004, 10, 327–336. [Google Scholar] [CrossRef]
- Wei, X.; Mao, Z.; Hou, Y.; Lin, L.; Xue, T.; Chen, L.; Wang, H.; Yu, C. Local administration of TGFβ-1/VEGF165 gene-transduced bone mesenchymal stem cells for Achilles allograft replacement of the anterior cruciate ligament in rabbits. Biochem. Biophys. Res. Commun. 2011, 406, 204–210. [Google Scholar] [CrossRef]
- Madry, H.; Kohn, D.; Cucchiarini, M. Direct FGF-2 gene transfer via recombinant adeno-associated virus vectors stimulates cell proliferation, collagen production, and the repair of experimental lesions in the human ACL. Am. J. Sports Med. 2013, 41, 194–202. [Google Scholar] [CrossRef]
- Steinert, A.F.; Weber, M.; Kunz, M.; Palmer, G.D.; Nöth, U.; Evans, C.H.; Murray, M.M. In situ IGF-1 gene delivery to cells emerging from the injured anterior cruciate ligament. Biomaterials 2008, 29, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kunz, M.; Stehle, J.; Noth, U.; Steiner, A. BMP-12 transduced MSCs in collagen hydrogel for ligament reconstruction. J. Stem. Cells Regen Med. 2007, 2, 72–73. [Google Scholar]
- Haddad-Weber, M.; Prager, P.; Kunz, M.; Seefried, L.; Jakob, F.; Murray, M.M.; Evans, C.H.; Nöth, U.; Steinert, A.F. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 2010, 12, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, Y.; Takayama, K.; Matsumoto, T.; Tang, Y.; Wang, B.; Mifune, Y.; Cummins, J.H.; Warth, R.J.; Kuroda, R.; Kurosaka, M.; et al. Anterior cruciate ligament-derived stem cells transduced with BMP2 accelerate graft-bone integration after ACL reconstruction. Am. J. Sports Med. 2017, 45, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Bez, M.; Kremen, T.J.; Tawackoli, W.; Avalos, P.; Sheyn, D.; Shapiro, G.; Giaconi, J.C.; Ben David, S.; Snedeker, J.G.; Gazit, Z.; et al. Ultrasound-mediated gene delivery enhances tendon allograft integration in mini-pig ligament reconstruction. Mol. Ther. 2018, 26, 1746–1755. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Shino, K.; Natsuume, T.; Horibe, S.; Matsumoto, N.; Kaneda, Y.; Ochi, T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998, 5, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Alberton, P.; Popov, C.; Prägert, M.; Kohler, J.; Shukunami, C.; Schieker, M.; Docheva, D. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem. Cells Dev. 2012, 21, 846–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, A.E.C.; Were, S.R.; Dahlgren, L.A. Transient scleraxis overexpression combined with cyclic strain enhances ligament cell differentiation. Tissue Eng. Part A 2018, 24, 1444–1455. [Google Scholar] [CrossRef]
- Otabe, K.; Nakahara, H.; Hasegawa, A.; Matsukawa, T.; Ayabe, F.; Onizuka, N.; Inui, M.; Takada, S.; Ito, Y.; Sekiya, I.; et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res. 2015, 33, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Li, J.; Samulski, R.J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996, 70, 8098–8108. [Google Scholar] [CrossRef] [Green Version]
- Schrenker, S.; Cucchiarini, M.; Goebel, L.; Oláh, T.; Venkatesan, J.K.; Schmitt, G.; Speicher-Mentges, S.; Maihöfer, J.; Gao, L.; Zurakoswski, D.; et al. In vivo rAAV-mediated human TGF-beta overexpression reduces perifocal osteoarthritis and improves osteochondral repair in a large animal model at one year. Osteoarthr. Cartil. 2023, 31, 467–481. [Google Scholar] [CrossRef]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.C.; Bessis, N. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]
- Leroux, A.; Egles, C.; Migonney, V. Impact of chemical and physical treatments on the mechanical properties of poly(epsilon-caprolactone) fibers bundles for the anterior cruciate ligament reconstruction. PLoS ONE 2018, 13, e0205722. [Google Scholar] [CrossRef]
- Leroux, A.; Venkatesan, J.K.; Castner, D.G.; Cucchiarini, M.; Migonney, V. Analysis of early cellular responses of anterior cruciate ligament fibroblasts seeded on different molecular weight polycaprolactone films functionalized by a bioactive poly(sodium styrene sulfonate) polymer. Biointerphases 2019, 14, 041004–041016. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly(sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 061006–061020. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Falentin-Daudré, C.; Leroux, A.; Migonney, V.; Cucchiarini, M. Biomaterial-guided recombinant adeno-associated virus delivery from poly(sodium styrene sulfonate)-grafted poly(epsilon-caprolactone) films to target human bone marrow aspirates. Tissue Eng. Part A 2020, 26, 450–459. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Cai, X.; Meng, W.; Rey-Rico, A.; Schmitt, G.; Speicher-Mentges, S.; Falentin-Daudré, C.; Leroux, A.; Madry, H.; Migonney, V.; et al. pNaSS-grafted PCL film-guided rAAV TGF-beta gene therapy activates the chondrogenic activities in human bone marrow aspirates. Hum. Gene Ther. 2021, 32, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Growth factor regulation of growth factors in articular chondrocytes. J. Biol. Chem. 2009, 284, 6697–6704. [Google Scholar] [CrossRef] [Green Version]
- DesRosiers, E.A.; Yahia, L.; Rivard, C.H. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J. Orthop. Res. 1996, 14, 200–208. [Google Scholar] [CrossRef]
- Marui, T.; Niyibizi, C.; Georgescu, H.I.; Cao, M.; Kavalkovich, K.W.; Levine, R.E.; Woo, S.L. Effect of growth factors on matrix synthesis by ligament fibroblasts. J. Orthop. Res. 1997, 15, 18–23. [Google Scholar] [CrossRef]
- Meaney Murray, M.; Rice, K.; Wright, R.J.; Spector, M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J. Orthop. Res. 2003, 21, 238–244. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef]
- Nakahara, H.; Hasegawa, A.; Otabe, K.; Ayabe, F.; Matsukawa, T.; Onizuka, N.; Ito, Y.; Ozaki, T.; Lotz, M.K.; Asahara, H. Transcription factor Mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 2013, 65, 2081–2089. [Google Scholar] [CrossRef] [Green Version]
- Farhat, Y.M.; Al-Maliki, A.A.; Chen, T.; Jujena, S.C.; Schwarz, E.M.; O`Keefe, R.J.; Awad, H.A. Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing. PLoS ONE 2012, 7, e51411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, S.Y.; Lee, J.H.; Kang, K.J.; Jang, Y.J. Effect of FGF-2, TGF-beta-1, and BMPs on teno/ligamentogenesis and osteo/cementogenesis of human periodontal ligament stem cells. Mol. Cells 2017, 40, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Redini, F.; Mauviel, A.; Pronost, S.; Loyau, G.; Pujol, J.P. Transforming growth factor beta exerts opposite effects from interleukin-1 beta on cultured rabbit articular chondrocytes through reduction of interleukin-1 receptor expression. Arthritis Rheum. 1993, 36, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, C.; Kanai, Y.; Masumoto, R.; Kitagaki, J.; Matsumoto, M.; Yamada, S.; Kajikawa, T.; Murakami, S. Fibroblast growth factor-2 inhibits CD40-mediated periodontal inflammation. J. Cell. Physiol. 2019, 234, 7149–7160. [Google Scholar] [CrossRef] [PubMed]
- Mengsteab, P.Y.; Otsuka, T.; McClinton, A.; Shemshaki, N.S.; Shah, S.; Kan, H.M.; Obopilwe, E.; Vella, A.T.; Nair, L.S.; Laurencin, C.T. Mechanically superior matrices promote osteointegration and regeneration of anterior cruciate ligament tissue in rabbits. Proc. Natl. Acad. Sci. USA 2020, 117, 28655–28666. [Google Scholar] [CrossRef] [PubMed]
- Morscheid, S.; Rey-Rico, A.; Schmitt, G.; Madry, H.; Cucchiarini, M.; Venkatesan, J.K. Therapeutic effects of rAAV-mediated concomittant gene transfer and overexpression of TGF-beta and IGF-I on the chondrogenesis of human bone-marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2019, 20, 2591. [Google Scholar] [CrossRef] [Green Version]
- Maurice, E.; Rangel, A.L.R.; Venkatesan, J.K.; Leroux, A.; El Hafci, H.; Pichard, D.; Manassero, M.; Godineau, T.; Vial, J.; Schmitt, G.; et al. The effect of pNaSS grafting of knitted poly(ε-caprolactone) artificial ligaments on in vitro mineralization and in vivo osseointegration. Materialia 2022, 21, 101331. [Google Scholar] [CrossRef]
- Leong, N.L.; Kabir, N.; Arshi, A.; Nazemi, A.; Wu, B.; Petrigliano, F.A.; McAllister, D.R. Evaluation of polycaprolactone scaffold with basic fibroblast growth factor and fibroblasts in an athymic rat model for anterior cruciate ligament reconstruction. Tissue Eng. Part A 2015, 21, 1859–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispim, J.; Fernandes, H.A.M.; Fu, S.C.; Lee, Y.W.; Jonkheijm, P.; Saris, D.B.F. TGF-beta1 activation in human hamstring cells through growth factor binding peptides on polycaprolactone surfaces. Acta Biomater. 2017, 53, 165–178. [Google Scholar] [CrossRef]
- Samulski, R.J.; Chang, L.S.; Shenk, T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 1987, 61, 3096–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [CrossRef] [Green Version]
- Cucchiarini, M.; Schetting, S.; Terwilliger, E.F.; Kohn, D.; Madry, H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009, 16, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.K.; Rey-Rico, A.; Schmitt, G.; Wezel, A.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta stably restructures human osteoarthritic articular cartilage in situ. J. Transl. Med. 2013, 11, 211–224. [Google Scholar] [CrossRef] [Green Version]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| COL1A1 | 5′-ACGTCCTGGTGAAG TTGGTC-3′ | 5′-ACCAGGGAAGCCTCTCTCTC-3′ |
| COL3A1 | 5′-CACAAGGAGTCTGCATGTCT-3′ | 5′-GTTCACCAGGCTCACCAGCA-3′ |
| decorin | 5′-ACCCACTGAAGAGCTCAGGA-3′ | 5′-GCCATTGTCAACAGCAGAGA-3′ |
| tenascin-C | 5′-TCACATCCAGGTGCTTATTCC-3′ | 5′-CTAGAGTGTCTCACTATCAGG-3′ |
| Mohawk | 5′-AAGATACTCTTGGCGCTCGG-3′ | 5′-ACACTAAGCCGCTCAGCATT-3′ |
| scleraxis | 5′-TACCTGGGTTTTCTTCTGGTCACT-3′ | 5′-TATCAAAGACACAAGATGCCAGC-3′ |
| IL-1β | 5′- CCGTGCCTACGAACATGTC-3′ | 5′-CACACAGAAGCTCATCGGAG-3′ |
| TNF-α | 5′-AGAACCCCCTGGAGATAACC-3′ | 5′-AAGTGCAGCAGGCAGAAGAG-3′ |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGTC-3′ | 5′-GAAGATGGTGATGGGATTTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amini, M.; Venkatesan, J.K.; Nguyen, T.N.; Liu, W.; Leroux, A.; Madry, H.; Migonney, V.; Cucchiarini, M. rAAV TGF-β and FGF-2 Overexpression via pNaSS-Grafted PCL Films Stimulates the Reparative Activities of Human ACL Fibroblasts. Int. J. Mol. Sci. 2023, 24, 11140. https://doi.org/10.3390/ijms241311140
Amini M, Venkatesan JK, Nguyen TN, Liu W, Leroux A, Madry H, Migonney V, Cucchiarini M. rAAV TGF-β and FGF-2 Overexpression via pNaSS-Grafted PCL Films Stimulates the Reparative Activities of Human ACL Fibroblasts. International Journal of Molecular Sciences. 2023; 24(13):11140. https://doi.org/10.3390/ijms241311140
Chicago/Turabian StyleAmini, Mahnaz, Jagadeesh K. Venkatesan, Tuan N. Nguyen, Wei Liu, Amélie Leroux, Henning Madry, Véronique Migonney, and Magali Cucchiarini. 2023. "rAAV TGF-β and FGF-2 Overexpression via pNaSS-Grafted PCL Films Stimulates the Reparative Activities of Human ACL Fibroblasts" International Journal of Molecular Sciences 24, no. 13: 11140. https://doi.org/10.3390/ijms241311140






